Understanding Mn-Based Intercalation Cathodes from Thermodynamics and Kinetics
Abstract
:1. Introduction
2. Thermodynamics
2.1. Before Electrochemical Cycles
2.1.1. Theoretical Capacity
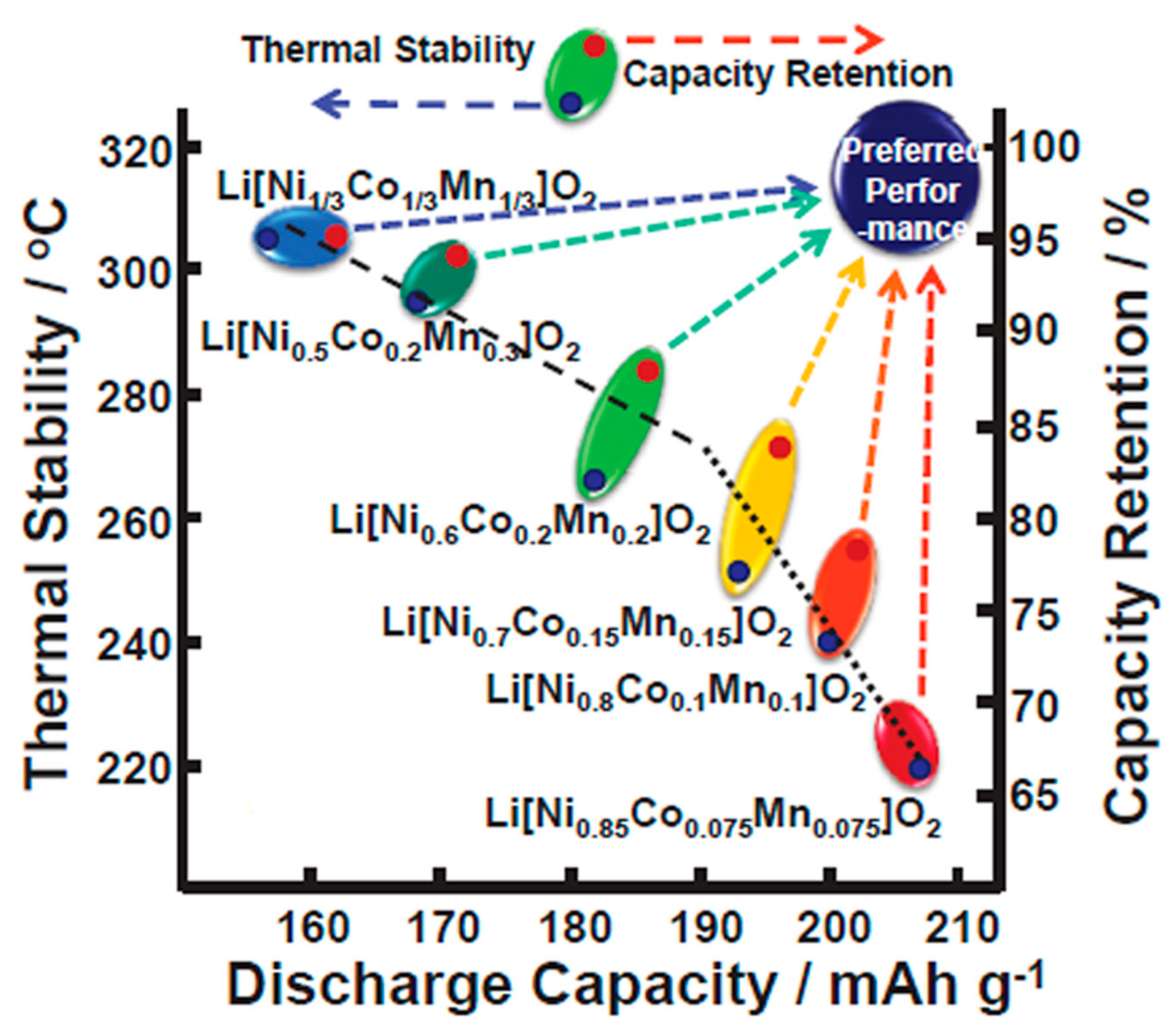
2.1.2. Thermal Stability
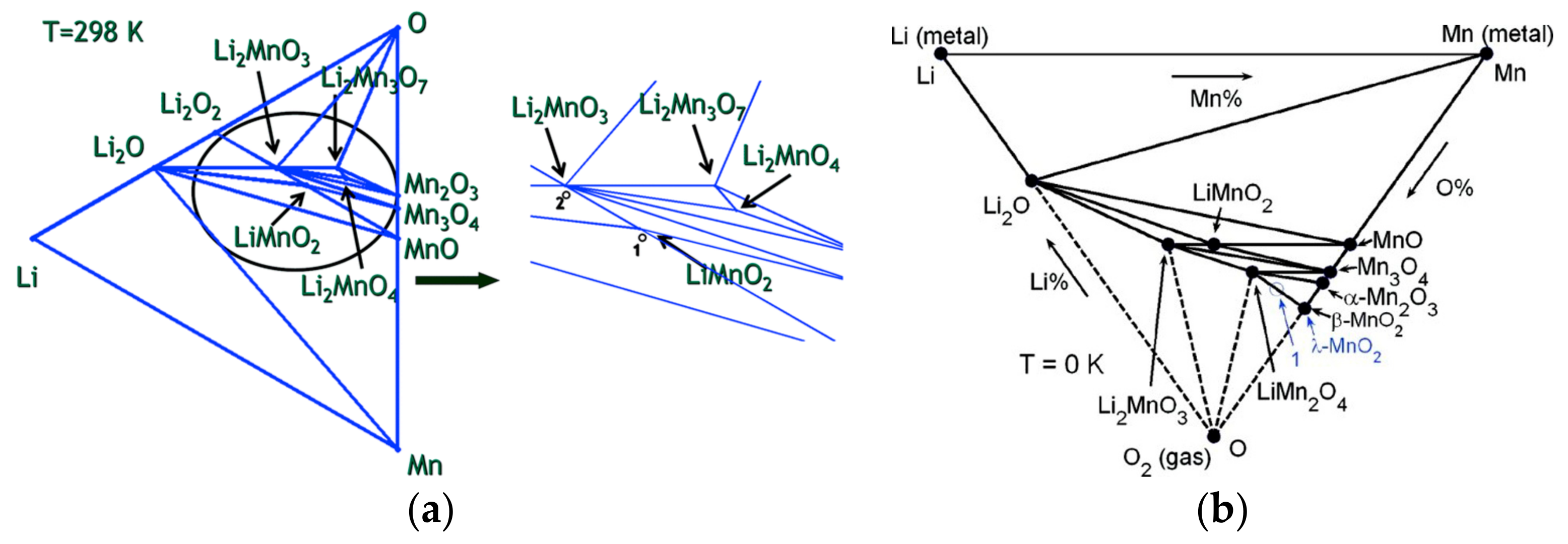
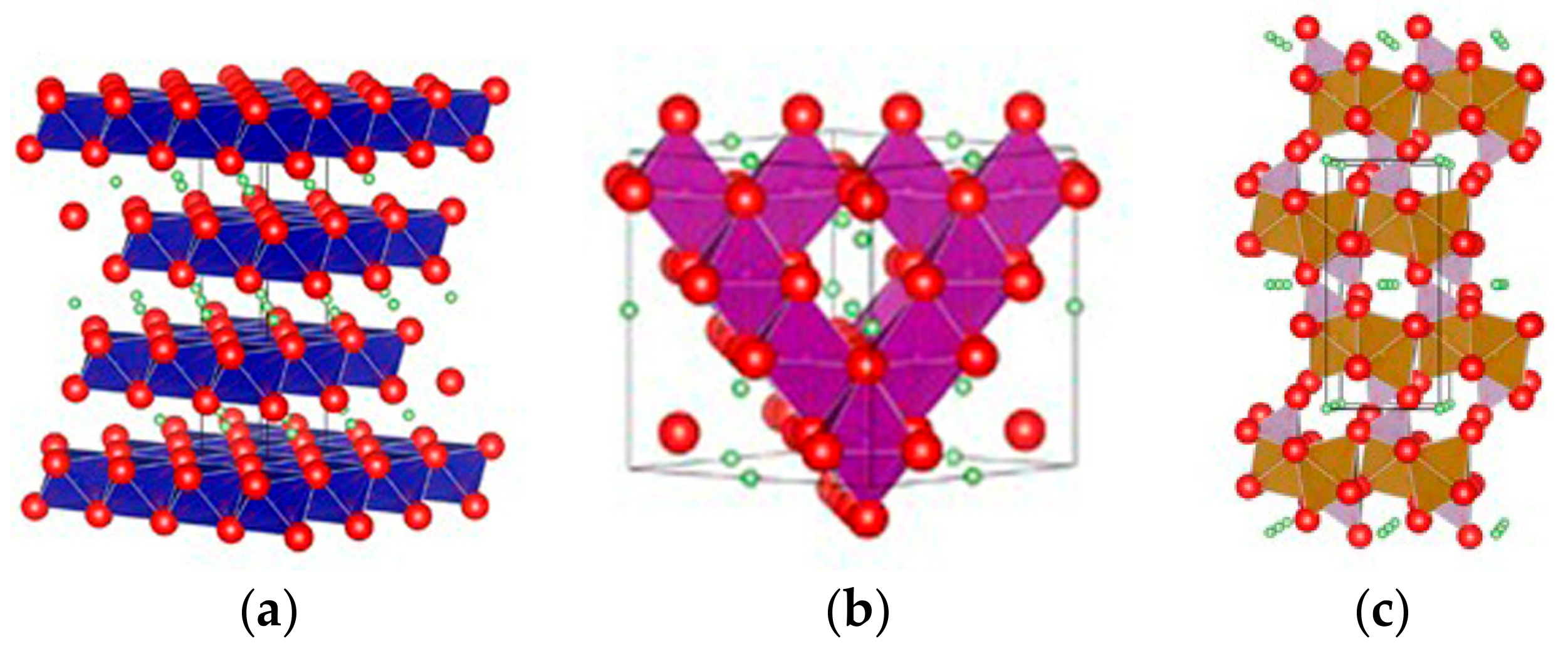
2.1.3. Pristine Structures and Their Formation
2.2. During Electrochemical Cycles
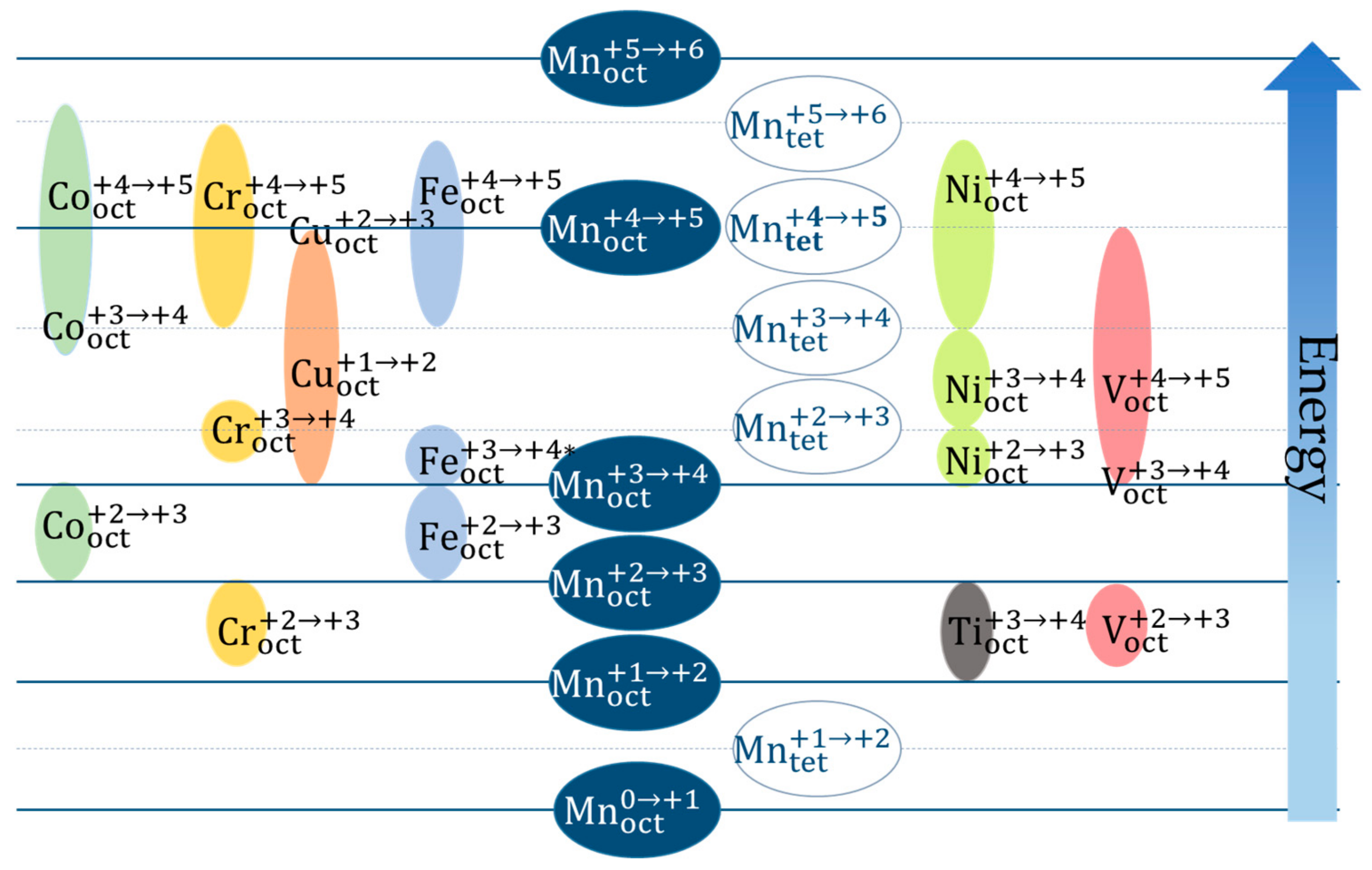
2.2.1. Involved Redox Couples and Voltage Profiles
2.2.2. Phase Changes during Cycling
3. Kinetics
3.1. Electrical Conductivity
3.2. Ion Diffusion
3.2.1. Ion Transport Mechanisms
3.2.2. Li+ Diffusion in Different Structures
3.2.3. The Mobility of Cations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Freire, M.; Kosova, N.V.; Jordy, C.; Chateigner, D.; Lebedev, O.I.; Maignan, A.; Pralong, V. A new active Li-Mn-O compound for high energy density Li-ion batteries. Nat. Mater. 2016, 15, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 2004, 104, 4513–4534. [Google Scholar] [CrossRef] [PubMed]
- Ammundsen, B.; Paulsen, J. Novel lithium-ion cathode materials based on layered manganese oxides. Adv. Mater. 2001, 13, 943–956. [Google Scholar] [CrossRef]
- Yu, X.; Hu, E.; Bak, S.; Zhou, Y.-N.; Yang, X.-Q. Strategies to curb structural changes of lithium/transition metal oxide cathode materials & the changes’ effects on thermal & cycling stability. Chin. Phys. B 2016, 25, 018205. [Google Scholar]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Source 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.-T.; Oh, S.-M.; Sun, Y.-K. Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater. 2016, 6, 1501010. [Google Scholar] [CrossRef]
- Peng, J.Y.; Zu, C.; Li, H. Fundamental scientific aspects of lithium batteries (I) -thermodynamic calculations of theoretical energy densities of chemical energy storage systems. Energy Storage Sci. Technol. 2013, 2, 55–62. [Google Scholar]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: an automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Kim, J.-S.; Johnson, C.S.; Vaughey, J.T.; Thackeray, M.M.; Hackney, S.A.; Yoon, W.; Grey, C.P. Electrochemical and structural properties of xLi2M’O3·(1−x)LiMn0.5Ni0.5O2 electrodes for lithium batteries (M‘ = Ti, Mn, Zr; 0 ≤ x ≤ 0.3). Chem. Mater. 2004, 16, 1996–2006. [Google Scholar] [CrossRef]
- Gummow, R.J.; de Kock, A.; Thackeray, M.M. Improved capacity retention in rechargeable 4 V lithium/lithium-manganese oxide (spinel) cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Markovsky, B.; Talyossef, Y.; Salitra, G.; Aurbach, D.; Kim, H.-J.; Choi, S. Cycling and storage performance at elevated temperatures of LiNi0.5Mn1.5O4 positive electrodes for advanced 5 V Li-ion batteries. Electrochem. Commun. 2004, 6, 821–826. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. A first-principles approach to studying the thermal stability of oxide cathode materials. Chem. Mater. 2007, 19, 543–552. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223. [Google Scholar] [CrossRef]
- Stroukoff, K.R.; Manthiram, A. Thermal stability of spinel Li1.1Mn1.9−yMyO4−zFz (M = Ni, Al, and Li, 0 ≤y≤ 0.3, and 0 ≤ z ≤ 0.2) cathodes for lithium ion batteries. J. Mater. Chem. 2011, 21, 10165. [Google Scholar] [CrossRef]
- Belharouak, I.; Lu, W.; Vissers, D.; Amine, K. Safety characteristics of Li(Ni0.8Co0.15Al0.05)O2 and Li(Ni1/3Co1/3Mn1/3)O2. Electrochem. Commun. 2006, 8, 329–335. [Google Scholar] [CrossRef]
- Nam, K.-W.; Bak, S.-M.; Hu, E.; Yu, X.; Zhou, Y.; Wang, X.; Wu, L.; Zhu, Y.; Chung, K.-Y.; Yang, X.-Q. Combining in situ synchrotron X-ray diffraction and absorption techniques with transmission electron microscopy to Study the origin of thermal instability in overcharged cathode materials for lithium-Ion batteries. Adv. Funct. Mater. 2013, 23, 1047–1063. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wen, L.; Liu, Y.M. Spinel LiNi0.5Mn1.5O4 and its derivatives as cathodes for high-voltage Li-ion batteries. J. Solid State Electrochem. 2010, 14, 2191–2202. [Google Scholar] [CrossRef]
- Park, S.B.; Eom, W.S.; Cho, W.I.; Jang, H. Electrochemical properties of LiNi0.5Mn1.5O4 cathode after Cr doping. J. Power Source 2006, 159, 679–684. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Higher, stronger, better... A review of 5 Volt cathode materials for advanced lithium-ion batteries. Adv. Energy Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Wang, Q.S.; Sun, J.H.; Chen, C.H. Thermal stability of delithiated LiMn2O4 with electrolyte for lithium-ion batteries. J. Electrochem. Soc. 2007, 154, A263–A267. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Z.; Zhang, C.; Cui, G.; Chen, L. Strategies for improving the cyclability and thermo-stability of LiMn2O4-based batteries at elevated temperatures. J. Mater. Chem. A 2015, 3, 4092–4123. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ceder, G. Structural stability of lithium manganese oxides. Phys. Rev. B 1999, 59, 6120–6130. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499. [Google Scholar] [CrossRef]
- Yin, S.C.; Rho, Y.H.; Swainson, I.; Nazar, L.F. X-ray/Neutron diffraction and electrochemical studies of lithium de/re-intercalation in Li1-xCo1/3Ni1/3Mn1/3O2 (x = 0 → 1). Chem. Mater. 2006, 18, 1901–1910. [Google Scholar] [CrossRef]
- Doeff, M.M. Battery Cathodes. In Batteries for Sustainability: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Brodd, R.J., Ed.; Springer: New York, NY, USA, 2013; pp. 5–49. [Google Scholar]
- Ma, C.; Lv, Y.C.; Li, H. Fundamental scientific aspects of lithium batteries (VII)—Positive electrode materials. Energy Storage Sci. Technol. 2014, 3, 53–65. [Google Scholar]
- Rozier, P.; Tarascon, J.M. Review-Li-rich layered oxide cathodes for next-generation Li-Ion batteries: Chances and challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Shi-xi, Z.; Han-xing, L.; Shi-xi, O.; Qiang, L. Synthesis and performance of LiMnO2 as cathodes for Li-ion batteries. J. Wuhan Univ. Technol.Mater. Sci. Ed. 2003, 18, 5–8. [Google Scholar] [CrossRef]
- Wu, E.J.; Tepesch, P.D.; Ceder, G. Size and charge effects on the structural stability of LiMO2 (M = transition metal) compounds. Philos. Mag. Part B 1998, 77, 1039–1047. [Google Scholar] [CrossRef]
- Goodenough, J.B. On the influence of 3d4 ions on the magnetic and crystallographic properties of magnetic oxides. J. Phys. Radium 1959, 20, 155–159. [Google Scholar] [CrossRef]
- Jang, Y.I.; Moorehead, W.D.; Chiang, Y.M. Synthesis of the monoclinic and orthorhombic phases of LiMnO2 in oxidizing atmosphere. Solid State Ion. 2002, 149, 201–207. [Google Scholar] [CrossRef]
- Jang, Y.-I.; Chiang, Y.-M. Stability of the monoclinic and orthorhombic phases of LiMnO2 with temperature, oxygen partial pressure, and Al doping. Solid State Ion. 2000, 130, 53–59. [Google Scholar] [CrossRef]
- Whitfield, P.; Davidson, I.; Cranswick, L.; Swainson, I.; Stephens, P. Investigation of possible superstructure and cation disorder in the lithium battery cathode material LiMn1/3Ni1/3Co1/3O2 using neutron and anomalous dispersion powder diffraction. Solid State Ion. 2005, 176, 463–471. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Hwang, B.J.; Ceder, G.; Sheu, H.S.; Liu, D.G.; Lee, J.F. In-situ X-ray absorption spectroscopic study on variation of electronic transitions and local structure of LiNi1/3Co1/3Mn1/3O2 cathode material during electrochemical cycling. Chem. Mater. 2005, 17, 3191–3199. [Google Scholar] [CrossRef]
- Zeng, D.; Cabana, J.; Bréger, J.; Yoon, W.-S.; Grey, C.P. Cation ordering in Li[NixMnxCo(1–2x)]O2-layered cathode materials: A nuclear magnetic resonance (NMR), pair distribution function, X-ray absorption spectroscopy, and electrochemical study. Chem. Mater. 2007, 19, 6277–6289. [Google Scholar] [CrossRef]
- Cahill, L.S.; Yin, S.C.; Samoson, A.; Heinmaa, I.; Nazar, L.F.; Goward, G.R. 6Li NMR studies of cation disorder and transition metal ordering in Li[Ni1/3Mn1/3Co1/3]O2 using ultrafast magic angle spinning. Chem. Mater. 2005, 17, 6560–6566. [Google Scholar] [CrossRef]
- Koyama, Y.; Tanaka, I.; Adachi, H.; Makimura, Y.; Ohzuku, T. Crystal and electronic structures of superstructural Li1−x[Co1/3Ni1/3Mn1/3]O2 (0≤x≤1). J. Power Source 2003, 119–121, 644–648. [Google Scholar] [CrossRef]
- Yabuuchi, N. Solid-state redox reaction of oxide ions for rechargeable batteries. Chem. Lett. 2017, 46, 412–422. [Google Scholar] [CrossRef]
- Strobel, P.; Lambert-Andron, B. Crystallographic and magnetic structure of Li2MnO3. J. Solid State Chem. 1988, 75, 90–98. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Thackeray, M.M. Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res. Bull. 1991, 26, 463–473. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Liles, D.C.; Thackeray, M.M. Synthesis and structural characterization of a novel layered lithium manganese oxide, Li0.36Mn0.91O2, and Its lithiated derivative, Li1.09Mn0.91O2. J. Solid State Chem. 1993, 104, 464–466. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Genc, A.; Xiao, J.; Xu, P.; Chen, X.; Zhu, Z.; Zhao, W.; Pullan, L.; Wang, C.; et al. Mitigating Voltage Fade in Cathode Materials by Improving the Atomic Level Uniformity of Elemental Distribution. Nano Lett. 2014, 14, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Knight, J.C.; Li, W.; Manthiram, A. Understanding the influence of composition and synthesis temperature on oxygen loss, reversible capacity, and electrochemical behavior of xLi2MnO3-(1 – x)LiCoO2 cathodes in the first cycle. J. Phys. Chem. C 2014, 118, 23553–23558. [Google Scholar] [CrossRef]
- Nakayama, M.; Kaneko, M.; Wakihara, M. First-principles study of lithium ion migration in lithium transition metal oxides with spinel structure. Phys. Chem. Chem. Phys. 2012, 14, 13963–13970. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.C.; Kong, F.T.; Kc, S.; Park, M.S.; Yoon, J.; Yeon, D.H.; Park, J.H.; Doo, S.G.; Cho, K. Phase stability of Li-Mn-O oxides as cathode materials for Li-ion batteries: insights from ab initio calculations. Phys. Chem. Chem. Phys. 2014, 16, 11218–11227. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-W.; Zhang, D.-W.; Zang, Y.; Sun, X.; Cheng, B.; Ding, C.-X.; Yu, Y.; Chen, C.-H. One-step synthesis and effect of heat-treatment on the structure and electrochemical properties of LiNi0.5Mn1.5O4 cathode material for lithium-ion batteries. Electrochim. Acta 2014, 133, 515–521. [Google Scholar] [CrossRef]
- Van der Ven, A.; Bhattacharya, J.; Belak, A.A. Understanding Li diffusion in Li-intercalation compounds. Acc. Chem. Res. 2013, 46, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Meng, S. Factors affecting Li mobility in spinel LiMn2O4—A first-principles study by GGA and GGA+U methods. J. Power Source 2010, 195, 4971–4976. [Google Scholar] [CrossRef]
- Ceder, G.; Aydinol, M.K.; Kohan, A.F. Application of first-principles calculations to the design of rechargeable Li-batteries. Comput. Mater. Sci. 1997, 8, 161–169. [Google Scholar] [CrossRef]
- Vitins, G.; West, K. Lithium intercalation into layered LiMnO2. J. Electrochem. Soc. 1997, 144, 2587–2592. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Zhang, Z.; Li, J. Correlation of intercalation potential with d-electron configurations for cathode compounds of lithium-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 13255–13261. [Google Scholar] [CrossRef] [PubMed]
- Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef] [PubMed]
- Saubanère, M.; McCalla, E.; Tarascon, J.M.; Doublet, M.L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 2016, 9, 984–991. [Google Scholar] [CrossRef]
- Hy, S.; Liu, H.; Zhang, M.; Qian, D.; Hwang, B.-J.; Meng, Y.S. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 2016, 9, 1931–1954. [Google Scholar] [CrossRef]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Roberts, M.R.; Hao, R.; Guerrini, N.; Pickup, D.M.; Liu, Y.S.; Edstrom, K.; Guo, J.; Chadwick, A.V.; Duda, L.C.; et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 2016, 8, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Ceder, G.; Van der Ven, A. Layered-to-spinel phase transition in LixMnO2. Electrochem. Solid St. 2001, 4, A78–A81. [Google Scholar] [CrossRef]
- Zheng, J.; Kan, W.H.; Manthiram, A. Role of Mn content on the electrochemical properties of nickel-rich layered LiNi0.8-xCo0.1Mn0.1+xO2 (0.0 ≤ x ≤ 0.08) cathodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 6926–6934. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Edit. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ouyang, C.; Wang, Z.; Huang, X.; Chen, L. Effect of Co content on rate performance of LiMn0.5 − xCo2xNi0.5 − xO 2 cathode materials for lithium-Ion batteries. J. Electrochem. Soc. 2004, 151, A504–A508. [Google Scholar] [CrossRef]
- Ngala, J.K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalij, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214–220. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Holzapfel, M.; Novák, P.; Johnson, C.S.; Kang, S.-H.; Thackeray, M.M.; Bruce, P.G. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Croguennec, L.; Menetrier, M.; Douhil, K.; Belin, S.; Bourgeois, L.; Suard, E.; Weill, F.; Delmas, C. Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J. Electrochem. Soc. 2013, 160, A786–A792. [Google Scholar] [CrossRef]
- Lu, Z.H.; Dahn, J.R. Understanding the anomalous capacity of Li/Li[NixLi1/3-2x/3Mn2/3-x/3]O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem. Soc. 2002, 149, A815–A822. [Google Scholar] [CrossRef]
- Boulineau, A.; Simonin, L.; Colin, J.F.; Bourbon, C.; Patoux, S. First evidence of manganese-nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett. 2013, 13, 3857–3863. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Croguennec, L.; Ménétrier, M.; Weill, F.; Biensan, P.; Jordy, C.; Delmas, C. Mechanisms associated with the “plateau” observed at high voltage for the overlithiated Li1.12(Ni0.425Mn0.425Co0.15)0.88O2 System. Chem. Mater. 2008, 20, 4815–4825. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshii, K.; Myung, S.-T.; Nakai, I.; Komaba, S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3−LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 2011, 133, 4404–4419. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Xu, B.; Chi, M.; Meng, Y.S. Uncovering the roles of oxygen vacancies in cation migration in lithium excess layered oxides. Phys. Chem. Chem. Phys. 2014, 16, 14665–14668. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Manthiram, A. Influence of cationic substitutions on the first charge and reversible capacities of lithium-rich layered oxide cathodes. J. Mater. Chem. A 2013, 1, 10209. [Google Scholar] [CrossRef]
- Lee, E.-S.; Manthiram, A. Smart design of lithium-rich layered oxide cathode compositions with suppressed voltage decay. J. Mater. Chem. A 2014, 2, 3932. [Google Scholar] [CrossRef]
- Croy, J.R.; Gallagher, K.G.; Balasubramanian, M.; Long, B.R.; Thackeray, M.M. Quantifying hysteresis and voltage fade in xLi2MnO3·(1-x)LiMn0.5Ni0.5O2 electrodes as a function of Li2MnO3 content. J. Electrochem. Soc. 2014, 161, A318–A325. [Google Scholar] [CrossRef]
- Erickson, E.M.; Schipper, F.; Penki, T.R.; Shin, J.-Y.; Erk, C.; Chesneau, F.-F.; Markovsky, B.; Aurbach, D. Review-Recent advances and remaining challenges for lithium ion battery cathodes: II. lithium-rich, xLi2MnO3⋅(1-x)LiNiaCobMncO2. J. Electrochem. Soc. 2017, 164, A6341–A6348. [Google Scholar] [CrossRef]
- Dogan, F.; Long, B.R.; Croy, J.R.; Gallagher, K.G.; Iddir, H.; Russell, J.T.; Balasubramanian, M.; Key, B. Re-entrant lithium local environments and defect driven electrochemistry of Li- and Mn-rich Li-ion battery cathodes. J. Am. Chem. Soc. 2015, 137, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Zhang, M.; Xia, Y.; Liu, Z.; Meng, Y.S. Understanding and controlling anionic electrochemical activity in high-capacity oxides for next generation Li-Ion batteries. Chem. Mater. 2017, 29, 908–915. [Google Scholar] [CrossRef]
- Ates, M.N.; Jia, Q.; Shah, A.; Busnaina, A.; Mukerjee, S.; Abraham, K.M. Mitigation of layered to spinel conversion of a Li-rich layered metal oxide cathode material for Li-Ion batteries. J. Electrochem. Soc. 2014, 161, A290–A301. [Google Scholar] [CrossRef]
- Ates, M.N.; Mukerjee, S.; Abraham, K.M. A Li-rich layered cathode material with enhanced structural stability and rate capability for Li-on batteries. J. Electrochem. Soc. 2014, 161, A355–A363. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Xiao, J.; Polzin, B.J.; Yan, P.; Chen, X.; Wang, C.; Zhang, J.-G. Functioning mechanism of AlF3 coating on the Li- and Mn-rich cathode materials. Chem. Mater. 2014, 26, 6320–6327. [Google Scholar] [CrossRef]
- Kim, D.; Sandi, G.; Croy, J.R.; Gallagher, K.G.; Kang, S.-H.; Lee, E.; Slater, M.D.; Johnson, C.S.; Thackeray, M.M. Composite ‘layered-layered-spinel’ cathode structures for lithium-ion batteries. J. Electrochem. Soc. 2013, 160, A31–A38. [Google Scholar] [CrossRef]
- Long, B.R.; Croy, J.R.; Park, J.S.; Wen, J.; Miller, D.J.; Thackeray, M.M. Advances in stabilizing ‘layered-layered’ xLi2MnO3·(1-x)LiMO2 (M=Mn, Ni, Co) electrodes with a spinel component. J. Electrochem. Soc. 2014, 161, A2160–A2167. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, M.; Wu, L.; Wang, J.; Xia, Y.; Qian, D.; Liu, H.; Hy, S.; Chen, Y.; An, K.; et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 2016, 7, 12108. [Google Scholar] [CrossRef] [PubMed]
- Du Pasquier, A.; Blyr, A.; Courjal, P.; Larcher, D.; Amatucci, G.; Gérand, B.; Tarascon, J.M. Mechanism for limited 55°C storage performance of Li1.05Mn1.95O4 electrodes. J. Electrochem. Soc. 1999, 146, 428–436. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, Y.; Yoshio, M. Capacity Fading on Cycling of 4 V Li / LiMn2O4 Cells. J. Electrochem. Soc. 1997, 144, 2593–2600. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Liu, Z.; Lu, P.; Olson, K.L.; Moote, J.; Powell, B.R.; Kim, J.-H. Understanding transition-metal dissolution behavior in LiNi0.5Mn1.5O4 high-voltage spinel for lithium ion batteries. J. Phys. Chem. C 2013, 117, 15947–15957. [Google Scholar] [CrossRef]
- Gao, J.; Lv, Y.; Li, H. Fundamental scientific aspects of lithium batteries (IV): Phase transition and phase diagram (2). Energy Storage Sci. Technol. 2013, 2, 383–401. [Google Scholar]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Source 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klopsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.C.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef] [PubMed]
- Aydinol, M.K.; Kohan, A.F.; Ceder, G.; Cho, K.; Joannopoulos, J. Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Am. Phys. Soc. 1997, 56, 1354–1365. [Google Scholar]
- Liu, Y.; Fujiwara, T.; Yukawa, H.; Morinaga, M. Electronic structures of lithium manganese oxides for rechargeable lithium battery electrodes. Solid State Ion. 1999, 126, 209–218. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Gao, Y.; Dahn, J.R. Explanation for the 4.8V plateau in LiCrxMn2−xO4. Phys. Rev. B 1998, 57, 5728–5733. [Google Scholar] [CrossRef]
- Iguchi, E.; Nakamura, N.; Aoki, A. Electrical transport properties in LiMn2O4. Philos. Mag. Part B 2009, 78, 65–77. [Google Scholar] [CrossRef]
- Molenda, J. The effect of 3d substitutions in the manganese sublattice on the charge transport mechanism and electrochemical properties of manganese spinel. Solid State Ion. 2004, 171, 215–227. [Google Scholar] [CrossRef]
- Yang, M.-C.; Xu, B.; Cheng, J.-H.; Pan, C.-J.; Hwang, B.-J.; Meng, Y.S. Electronic, structural, and electrochemical properties of LiNixCuyMn2–x–yO4(0 <x< 0.5, 0 <y< 0.5) high-voltage spinel materials. Chem. Mater. 2011, 23, 2832–2841. [Google Scholar] [CrossRef]
- Mackrodt, W.C.; Williamson, E.A. First-principles Hartree-Fock description of the electronic structure of monoclinic C2/m LixMnO2(1 ≥ x ≥ 0). Philos. Mag. Part B 1998, 77, 1077–1092. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Van der Ven, A.; Ceder, G. Lithium diffusion mechanisms in layered intercalation compounds. J. Power Source 2001, 97–98, 529–531. [Google Scholar] [CrossRef]
- Morgan, D.; Van der Ven, A.; Ceder, G. Li conductivity in LixMPO4 ( M = Mn , Fe , Co , Ni ) olivine materials. Electrochem. Solid State Lett. 2004, 7, A30–A32. [Google Scholar] [CrossRef]
- Deng, Z.; Mo, Y.; Ong, S.P. Computational studies of solid-state alkali conduction in rechargeable alkali-ion batteries. NPG Asia Mater. 2016, 8, e254. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakumoto, K.; Okamoto, M.; Seki, S.; Kobayashi, Y.; Takeuchi, T.; Tabuchi, M.; Yamada, Y. Electrochemical study on Mn2+-substitution in LiFePO4 olivine compound. J. Power Source 2007, 174, 435–441. [Google Scholar] [CrossRef]
- Urban, A.; Seo, D.-H.; Ceder, G. Computational understanding of Li-ion batteries. npj Comput. Mater. 2016, 2, 16002. [Google Scholar] [CrossRef]
- Kang, K.; Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Urban, A.; Lee, J.; Ceder, G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes. Adv. Energy Mater. 2014, 4, 1400478. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Belharouak, I. Part-II: Exchange current density and ionic diffusivity studies on the ordered and disordered spinel LiNi0.5Mn1.5O4 cathode. J. Power Source 2017, 348, 318–325. [Google Scholar] [CrossRef]
- Pang, W.K.; Lin, H.-F.; Peterson, V.K.; Lu, C.-Z.; Liu, C.-E.; Liao, S.-C.; Chen, J.-M. Enhanced rate-capability and cycling-stability of 5V SiO2- and polyimide-coated cation ordered LiNi0.5Mn1.5O4 lithium-ion battery positive electrodes. J. Phys. Chem. C 2017, 121, 3680–3689. [Google Scholar] [CrossRef]
- Laskar, M.R.; Jackson, D.H.; Xu, S.; Hamers, R.J.; Morgan, D.; Kuech, T.F. Atomic layer deposited MgO: A lower overpotential coating for Li[Ni0.5Mn0.3Co0.2]O2 cathode. ACS Appl. Mater Interfaces 2017, 9, 11231–11239. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yao, Q.; Zhu, H.; Chen, Z.; Song, L.; Duan, J. Effect of Al substitution sites on Li1−xAlx(Ni0.5Co0.2Mn0.3)1−yAlyO2 cathode materials for lithium ion batteries. J. Alloys Compd. 2016, 686, 30–37. [Google Scholar] [CrossRef]
- Yang, S.; Yan, B.; Wu, J.; Lu, L.; Zeng, K. Temperature-dependent lithium-ion diffusion and activation energy of Li1.2Co0.13Ni0.13Mn0.54O2 thin-film cathode at nanoscale by using electrochemical strain microscopy. ACS Appl. Mater. Interfaces 2017, 9, 13999–14005. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Zhong, H.; Yu, H.-T.; Fan, S.-S.; Xie, Y.; Yi, T.-F. Li1.2Mn0.54Ni0.13Co0.13O2 hollow hierarchical microspheres with enhanced electrochemical performances as cathode material for lithium-ion battery application. Electrochim. Acta 2017, 237, 217–226. [Google Scholar] [CrossRef]
- Yi, T.F.; Li, Y.M.; Yang, S.Y.; Zhu, Y.R.; Xie, Y. Improved cycling stability and fast charge-discharge performance of cobalt-free lithium-rich oxides by magnesium-doping. ACS Appl. Mater. Interfaces 2016, 8, 32349–32359. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Hou, X.; Li, Y.; Ru, Q.; Hu, S.; Lam, K.-H. Performance and mechanism research of hierarchically structured Li-rich cathode materials for advanced lithium–ion batteries. J. Mater. Sci. Mater. Electron. 2016, 28, 2705–2715. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.-B.; Yu, F.-D.; Liu, B.-S.; Zhang, Y.; Zhou, Y.-X. Investigation on performances of Li1.2Co0.4Mn0.4O2 prepared by self-combustion reaction as stable cathode for lithium-ion batteries. Ceram. Int. 2016, 42, 14818–14825. [Google Scholar] [CrossRef]
- Ma, X.; Kang, B.; Ceder, G. High rate micron-sized ordered LiNi0.5Mn1.5O4. J. Electrochem. Soc. 2010, 157, A925–A931. [Google Scholar] [CrossRef]
- Mohamedi, M.; Makino, M.; Dokko, K.; Itoh, T.; Uchida, I. Electrochemical investigation of LiNi0.5Mn1.5O4 thin film intercalation electrodes. Electrochim. Acta 2002, 48, 79–84. [Google Scholar] [CrossRef]
- Xia, H.; Lu, L. Li diffusion in spinel LiNi0.5Mn1.5O4 thin films prepared by pulsed laser deposition. Phys. Scr. 2007, T129, 43–48. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Lu, L.; Ceder, G. Electrochemical properties of nonstoichiometric LiNi0.5Mn1.5O4 − δ thin-film electrodes prepared by pulsed laser deposition. J. Electrochem. Soc. 2007, 154, A737–A743. [Google Scholar] [CrossRef]
- Kondracki, Ł.; Kulka, A.; Milewska, A.; Molenda, J. In-situ structural studies of manganese spinel-based cathode materials. Electrochim. Acta 2017, 227, 294–302. [Google Scholar] [CrossRef]
- Mao, J.; Ma, M.; Liu, P.; Hu, J.; Shao, G.; Battaglia, V.; Dai, K.; Liu, G. The effect of cobalt doping on the morphology and electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathode material. Solid State Ion. 2016, 292, 70–74. [Google Scholar] [CrossRef]
- Liu, W.; Shi, Q.; Qu, Q.; Gao, T.; Zhu, G.; Shao, J.; Zheng, H. Improved Li-ion diffusion and stability of a LiNi0.5Mn1.5O4 cathode through in situ co-doping with dual-metal cations and incorporation of a superionic conductor. J. Mater. Chem. A 2017, 5, 145–154. [Google Scholar] [CrossRef]

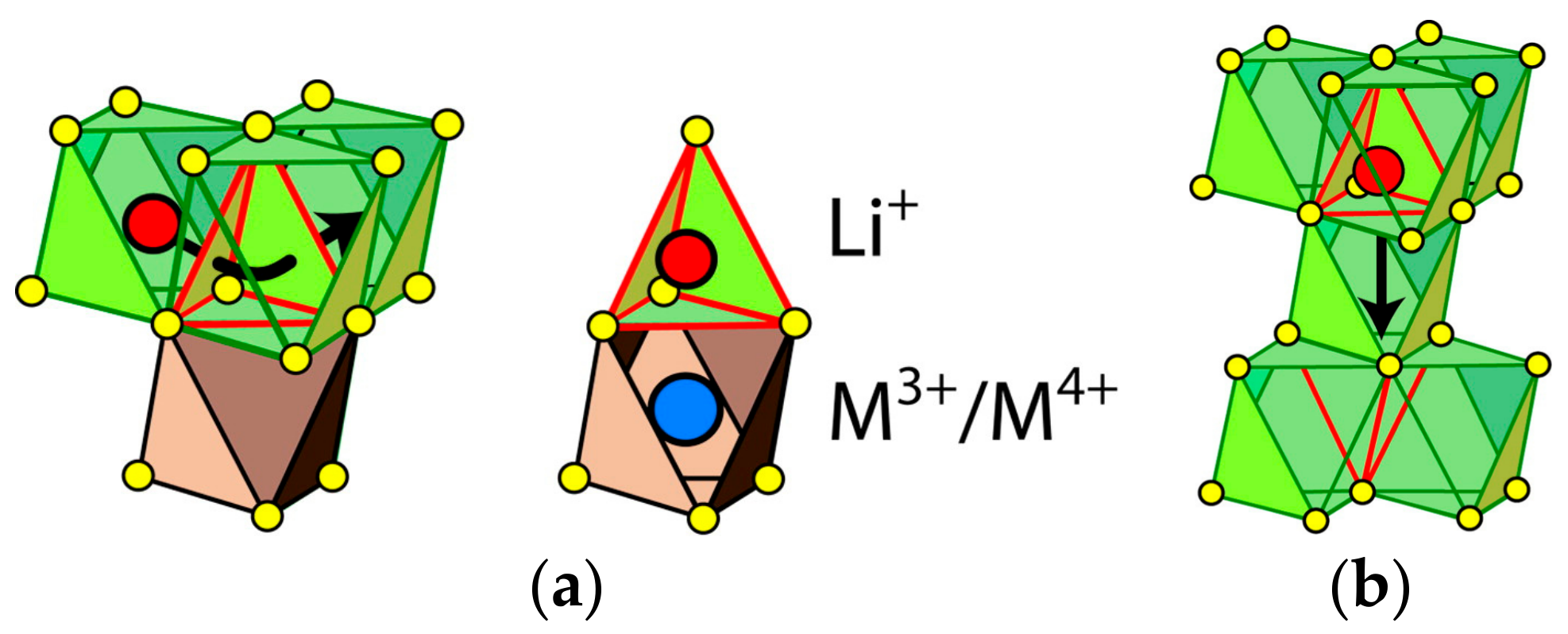
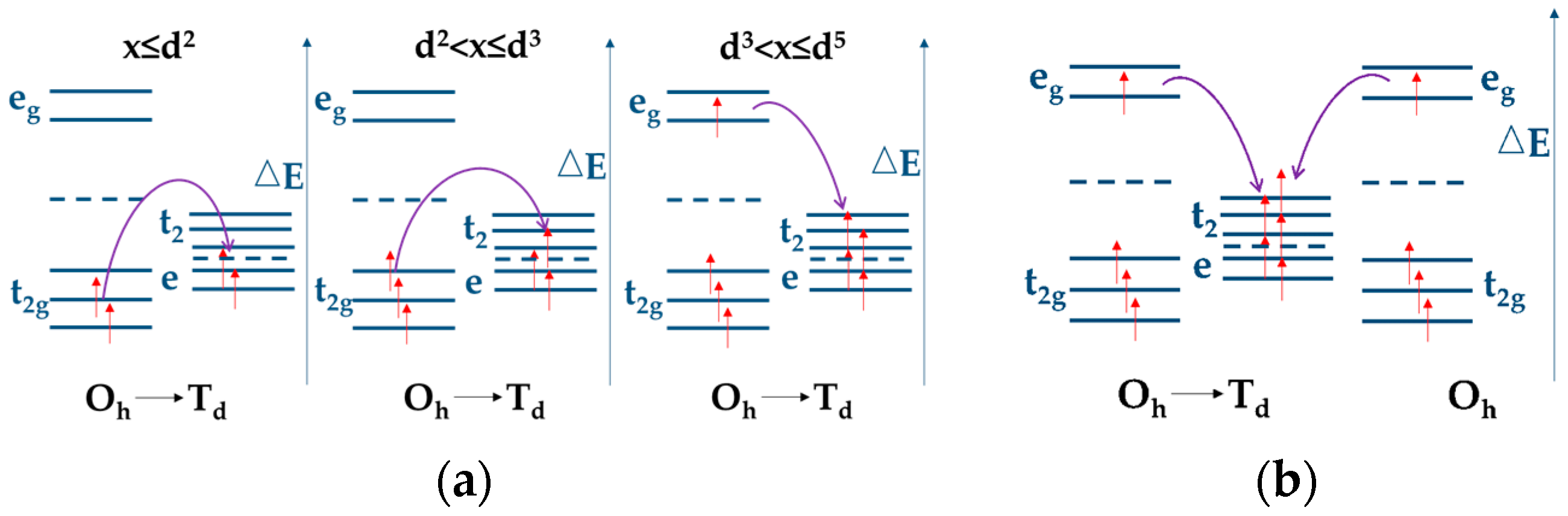
| Compounds | Theoretical Capacity (mA h g−1) | Experimental Capacity (mA h g−1) | References |
|---|---|---|---|
| Li(Ni1−x−yCoxMny)O2 | ~285 | 150~210 | [5] |
| xLi2MnO3∙(1 − x)LiMO2 | ~280 | 250~300 | [8,9] |
| LiMn2O4 | 148 | 120 (C/10) | [10] |
| LiNi0.5Mn1.5O4 | 147 | 146 (C/20) | [11] |
| Li4Mn2O5 | 492 | 355 (C/20) | [1] |
| Compounds | Onset Temperatures of Decomposition (°C) | Reference |
|---|---|---|
| Li0.45(Ni0.8Co0.15Al0.05)O2 | 190 | [16] |
| Li0.55(Ni1/3Co1/3Mn1/3)O2 | 250 | [16] |
| 0.22Li2MnO3∙0.78Li(Mn0.143Ni0.429Co0.429)O2 | 275 | [20] |
| LiNi0.5Mn1.5O4 (ordered) | 300 | [4] |
| LiNi0.5Mn1.5O4 (disordered) | 240 | [4] |
| LiMn2O4 | 600 | [21] |
| LixMn2O4 | 152~200 | [22] |
| Type | Compounds | Symmetry | Space Group | Reference |
|---|---|---|---|---|
| Layered | LiMnO2 | orthorhombic | Pmmn | [24] |
| Monoclinic | C2/m | [25] | ||
| Li(Ni1−x−yCoxMny)O2 | Hexagonal | Rm | [26] | |
| LiMn2O3 | monoclinic | C2/m | [27] | |
| xLi2MnO3∙(1 − x)LiMO2 | Hexagonal & monoclinic | Rm & C2/m | [27] | |
| Spinel | LiMn2O4 | Cubic | Fdm | [28] |
| LiNi0.5Mn1.5O4 | Cubic | Fdm | [18] | |
| Cubic | P4332 | [18] |
| Cathode Material | Band Gap (eV) | Electrical Conductivity (S cm−1) | References |
|---|---|---|---|
| orthorhombic-LiMnO2 | ~1.9 | - | [95] |
| LiCoO2 | 0.5~2.7 | ~10−4 | [87] |
| Li(Ni1/3Co1/3Mn1/3)O2 | - | 5.2 × 10−8 | [5] |
| Li(Ni0.5Co0.2Mn0.3)O2 | - | 4.9 × 10−7 | [5] |
| Li(Ni0.6Co0.2Mn0.2)O2 | - | 1.6 × 10−6 | [5] |
| Li(Ni0.8Co0.1Mn0.1)O2 | - | 1.7 × 10−5 | [5] |
| LiMn2O4 | 0.28~2.2 | ~10−6 | [87] |
| LiMnPO4 | 3.8~4.0 | ~10−14 | [96] |
| Mechanisms | Descriptions | Examples |
|---|---|---|
| Direct interstitial | An interstitial solid solution can diffuse by jumping from one interstitial site to one of its neighboring sites. | The diffusion of small foreign atoms such as H, C, N, and O in metals. |
| Collective | Simultaneous motion of several atoms in a chain-like or caterpillar-like fashion. | The motion of alkali ions in ion-conducting oxide glasses. |
| Vacancy | An atom jumps into a neighboring vacancy, making a series of exchanges with vacancies. | The diffusion of substitutional solutes and of matrix atoms in metals. |
| Divacancy | When a binding energy exists, which tends to create agglomerates of vacancies, diffusion occur via aggregates of vacancies. | The diffusion of Li+ in LiCoO2. |
| Indirect interstitial | A lattice atom is knocked out by a neighboring interstitial atom from its lattice positions under irradiating/ heating, and then deposited in the lattice as a self-interstitial. | Radiation-induced diffusion. |
| Interstitial-substitutional exchange | Some solute atoms may be dissolved on both interstitial and substitutional sites of a host crystal. | The diffusion of Au, Pt, Zn in silicon. |
| Category | Materials | Diffusivity (cm2 s−1) | Preparation Methods | Measurement Techniques | References |
|---|---|---|---|---|---|
| Layered | Li[Ni0.5Co0.2Mn0.3]O2 | ~1 × 10−8 | Atomic layer deposition (ALD) | CV | [108] |
| Li0.99Al0.01Ni0.5Co0.2Mn0.3O2 | 2.41 × 10−11 | Solid-state reaction | EIS | [109] | |
| Al2O3-coated Li[Ni0.5 Co0.2Mn0.3]O2 | ~0.5 × 10−8 | ALD | CV | [108] | |
| ZrO2-coated Li[Ni0.5 Co0.2Mn0.3]O2 | 1.5~2.5 × 10−8 | ALD | CV | [108] | |
| MgO-coated Li[Ni0.5 Co0.2Mn0.3]O2 | 3~4 × 10−8 | ALD | CV | [108] | |
| Li1.2Ni0.13Co0.13Mn0.54O2 | ~10−16 | Pulse laser deposition (PLD) method | DART−ESM | [110] | |
| Hollow hierarchical microspheres Li1.2Ni0.13Co0.13Mn0.54O2 | 1.34 × 10−15 | Molten salt method | EIS | [111] | |
| Li1.17Ni0.23Mn0.58Mg0.02O2 | 2.10 × 10−15 | Co-precipitation method | EIS | [112] | |
| Graphene@CNTs-modified Li1.165Ni0.167Co0.167 Mn0.501O2 | ~10−9 | Ultrasonic-assisted co-precipitation method | CV | [113] | |
| Carbon-coated Li1.2Co0.4Mn0.4O2 | 10−12~10−9 | Self-combustion reaction (SCR) | GITT | [114] | |
| Spinel | LiNi0.5Mn1.5O4(ordered) | ~5 × 10−10 | Acquired from corporation | GITT | [106] |
| LiNi0.5Mn1.5O4 (ordered) | 10−9~10−8 | - | Theoretical studies | [115] | |
| thin film LiNi0.5Mn1.5O4 (ordered) | 10−12~10−10 | Electrostatic spray deposition technique | EIS | [116] | |
| LiNi0.5Mn1.5O4 (ordered) | 1.252 × 10−14 | Co-precipitation method | EIS | [107] | |
| thin film LiNi0.5Mn1.5O4 (ordered) | 10−12~10−10 | PLD | PITT | [117] | |
| LiNi0.5Mn1.5O4 (disordered) | ~10−9 | Acquired from corporation | GITT | [106] | |
| thin film LiNi0.5Mn1.5O4-δ (disordered) | 10−12~10−10 | PLD | PITT | [118] | |
| LiNi0.25Cu0.25Mn1.5O4 | 10−14~10−13 | Sol-gel method | PITT | [94] | |
| LiNi0.45Cu0.05Mn1.5O4 | ~10−9 | Sol–gel method | GITT | [119] | |
| LiNi0.45Co0.1Mn1.45O4 | 8 × 10−12~7 × 10−10 | PVP-combustion method | PITT | [120] | |
| LiNi0.5Mn1.5O4/Li7La3Zr2O12 composite cathode | 1.83 × 10−10 | Spray drying method | CV | [121] | |
| SiO2-Coated LiNi0.5Mn1.5O4 (ordered) | 1.437 × 10−14 | Co-precipitation method | EIS | [107] | |
| Polyimide-Coated LiNi0.5Mn1.5O4 (ordered) | 1.154 × 10−14 | Co-precipitation method | EIS | [107] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Jin, Y.; Xiang, L. Understanding Mn-Based Intercalation Cathodes from Thermodynamics and Kinetics. Crystals 2017, 7, 221. https://doi.org/10.3390/cryst7070221
Xie Y, Jin Y, Xiang L. Understanding Mn-Based Intercalation Cathodes from Thermodynamics and Kinetics. Crystals. 2017; 7(7):221. https://doi.org/10.3390/cryst7070221
Chicago/Turabian StyleXie, Yin, Yongcheng Jin, and Lan Xiang. 2017. "Understanding Mn-Based Intercalation Cathodes from Thermodynamics and Kinetics" Crystals 7, no. 7: 221. https://doi.org/10.3390/cryst7070221





