Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure
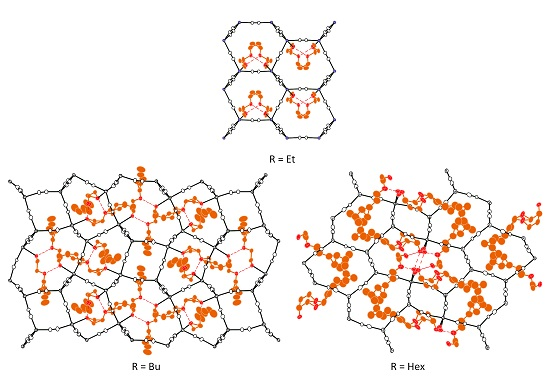
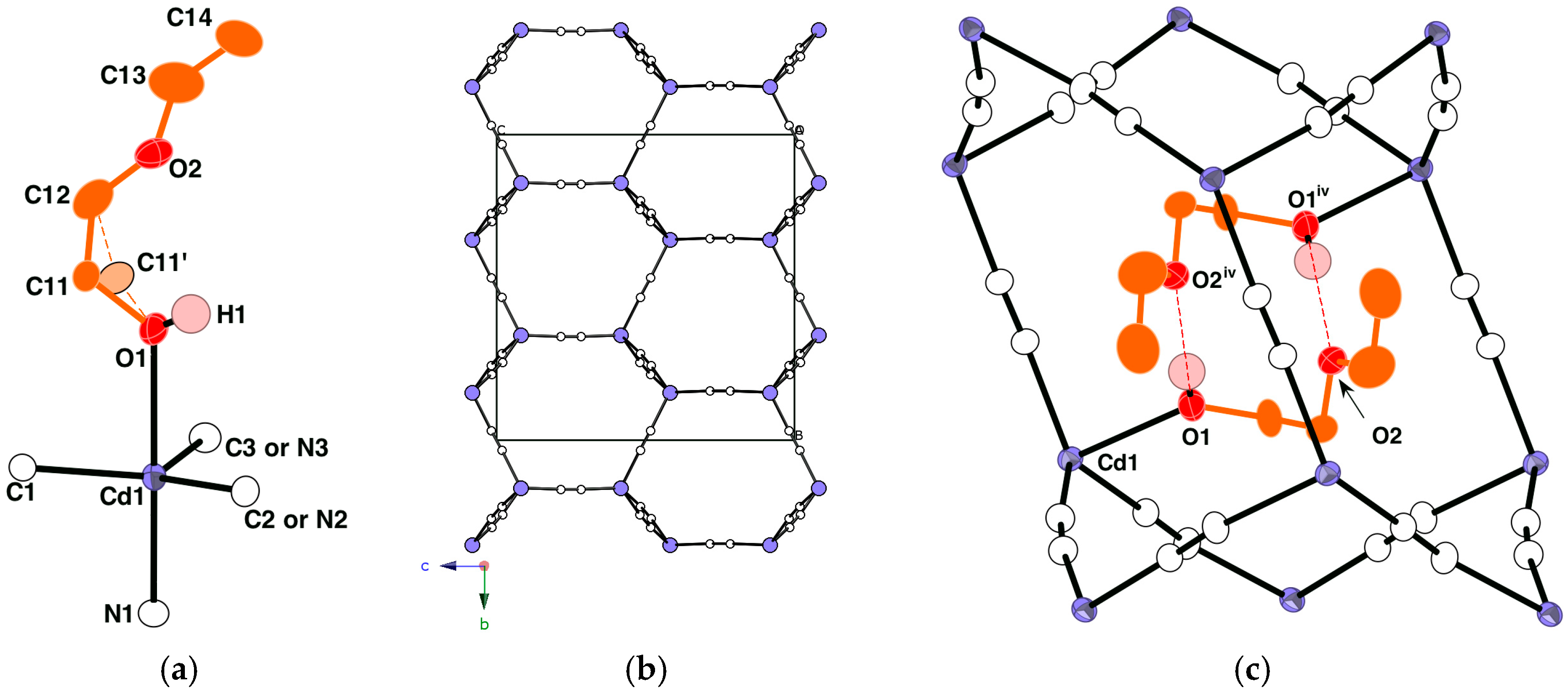
2.1.1. Crystal Structure of [Cd(CN)2(Etcel)]n I
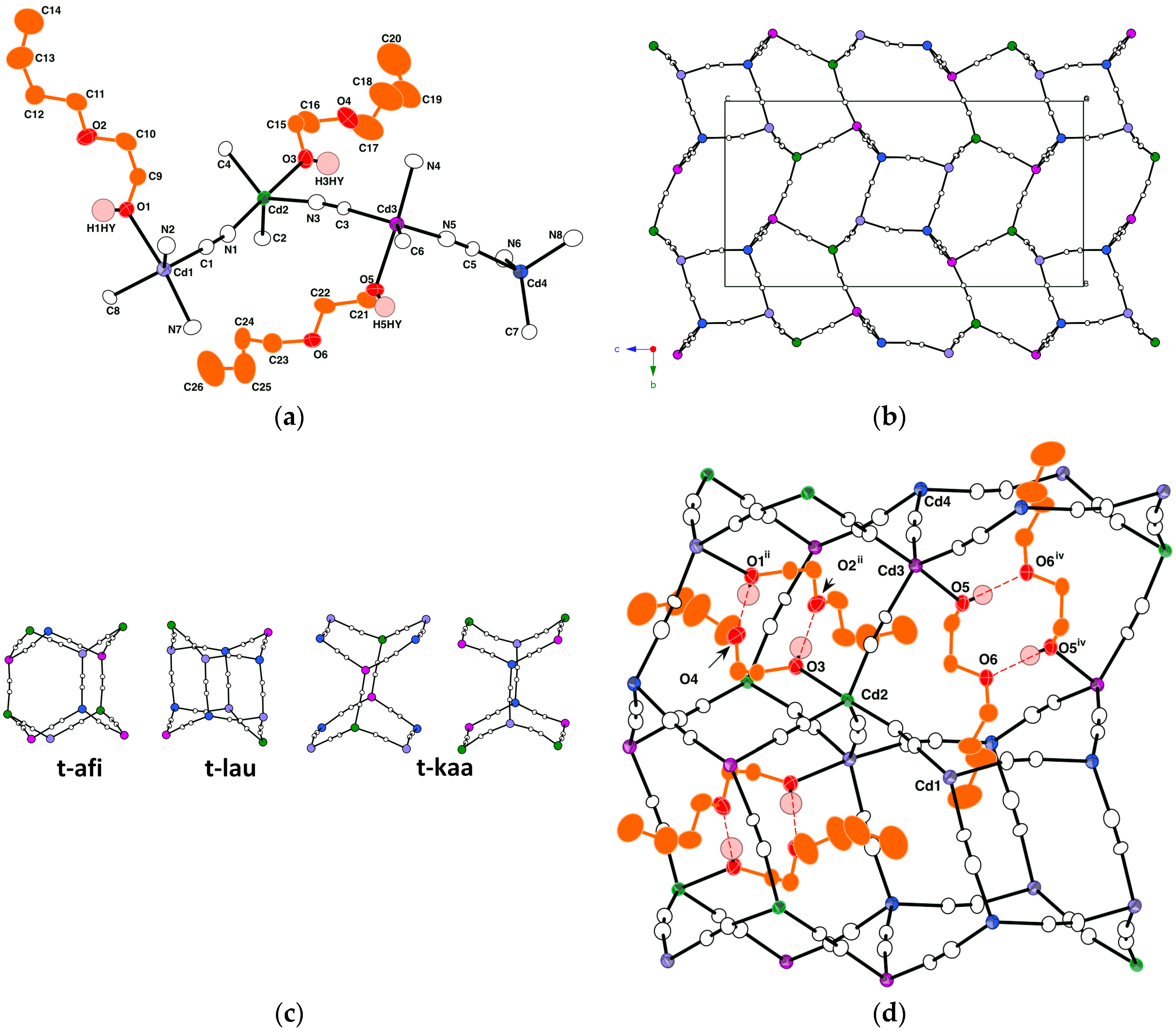
2.1.2. Crystal Structure of [{Cd(CN)2(Bucel)}3{Cd(CN)2}]n II
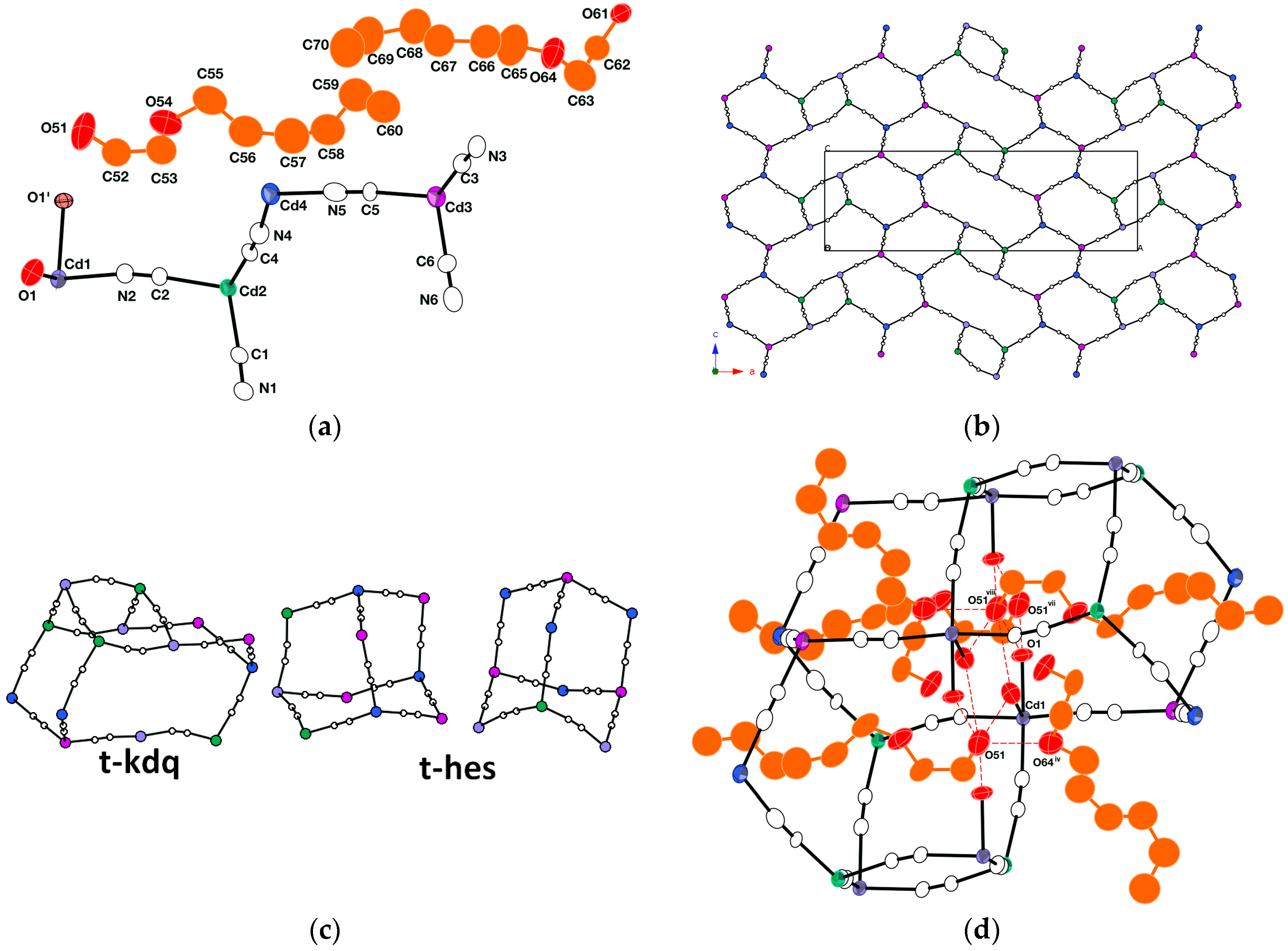
2.1.3. Crystal Structure of [{Cd(CN)2(H2O)2}{Cd(CN)2}3·2(Hexcel)]n III
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis for [Cd(CN)2(Etcel)]n I
3.1.2. Synthesis for [{Cd(CN)2(Bucel)}3{Cd(CN)2}]n II
3.1.3. Synthesis for [{Cd(CN)2(H2O)2}{Cd(CN)2}3·2(Hexcel)]n III
3.2. Single Crystal X-ray Diffraction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitazawa, T.; Nishikiori, S.; Kuroda, K.; Iwamoto, T. Novel Clathrate Compound of Cadmium Cyanide Host with an Adamantane-like Caity. Cadmium Cyanide-Carbon Tetrachloride(1/1). Chem. Lett. 1988, 17, 1729–1732. [Google Scholar] [CrossRef]
- Phillips, A.E.; Goodwin, A.L.; Halder, G.J.; Southon, P.D.; Kepert, C.J. Nanoporosity and Exceptional Negative Thermal Expansion in Single-Network Cadmium Cyanide. Angew. Chem. Int. Ed. 2008, 47, 1396–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazawa, T. A new mineralomimetic Cd(CN)2 host framework which is intermediate between H- and L-cristobalite-like frameworks. Chem. Commun. 1999, 10, 891–892. [Google Scholar] [CrossRef]
- Kitazawa, T. A new type of mineralomimetic cadmium cyanide host framework containing methyl acetate. J. Mater. Chem. 1998, 8, 671–674. [Google Scholar] [CrossRef]
- Kitazawa, T.; Nishikiori, S.; Kuroda, K.; Iwamoto, T. Two novel metal-complex host structures consisting of cyanocadmate coordination polyhedra. Clay-like and zeolite-like structures. Chem. Lett. 1988, 17, 459–462. [Google Scholar] [CrossRef]
- Nishikiori, S.; Ratcliffe, C.I.; Ripmeester, J.A. Crystal Structure of Cd5(CN)10(H2O)4·4C6H11OH Studied by X-ray Diffraction and Solid-State 113Cd NMR. A New Type of Cristobalite-like Framework Host with a Site Interacting with Cyclohexanol by Hydrogen Bonding. J. Am. Chem. Soc. 1992, 114, 8590–8595. [Google Scholar] [CrossRef]
- Kim, J.; Whang, D.; Lee, J.I.; Kim, K. Guest-dependent [Cd(CN)2]n Host Structures of Cadmium Cyanide-Alcohol Clathrates: Two New [Cd(CN)2]n Frameworks formed with PrnOH and PriOH Guests. J. Chem. Soc. Chem. Commun. 1993, 18, 1400–1402. [Google Scholar] [CrossRef]
- Kim, J.; Whang, D.; Koh, Y.-S.; Kim, K. Two New [Cd(CN)2]n Frameworks with Linear Channels of Large, Elongated Hexagonal Cross-section: Structures of Cadmium Cyanide-Guest (Guest = dmf and Me2SO) Clathrates. J. Chem. Soc. Chem. Commun. 1994, 5, 637–638. [Google Scholar] [CrossRef]
- Kitazawa, T.; Kukuyama, T.; Takahashi, M.; Takeda, M. Cadmium Cyanide-Ether Clathrates: Crystal Structures of Cd8(CN)16(H2O)6·6G (G = Et2O or Pri2O) and Cd3(CN)6(H2O)2·2Prn2O. J. Chem. Soc. Dalton Trans. 1994, 20, 2933–2937. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Hoskinis, B.F.; Lam, Y.-H.; Robson, R.; Separovic, F.; Woodberry, P. A Reexamination of the Structure of “Honeycomb Cadmium Cyanide”. J. Solid State Chem. 2001, 156, 51–56. [Google Scholar] [CrossRef]
- Nishikiori, S. Reversible reconstructive transition of the [CuZn(CN)4]- framework host induced by guest exchange. CrystEngComm 2014, 16, 10173–10176. [Google Scholar] [CrossRef]
- Nishikiori, S.; Ratcliffe, C.I.; Ripmeester, J.A. 113Cd NMR studies of Hofmann-type clathrates and related compounds: Evidence form two room temperature orientational glasses. Can. J. Chem. 1990, 68, 2270–2273. [Google Scholar] [CrossRef]
- Nishikiori, S.; Ratcliffe, C.I.; Ripmeester, J.A. Framework ordering in solid cadmium cyanides from cadmium-113 NMR spectroscopy. J. Chem. Soc. Chem. Commun. 1991, 10, 735–736. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. Sect. A 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijin, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Siewe, A.D.; Kim, J.-Y.; Kim, S.; Park, I.-H.; Lee, S.S. Regioisomer-Dependent Endo- and Exocyclic Coordination of Bis-Dithiamacrocycles. Inorg. Chem. 2014, 53, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Delgado-Friedrichs, O.; O’Keeffe, M.; Proserpio, D.M. Three-periodic nets and tilings: Natural tilings for nets. Acta Crystallogr. Sect. A 2007, 63, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Proserpio, D.M. Natural Tilings for Zeolite-Type Frameworks. J. Phys. Chem. C 2010, 114, 10160–10170. [Google Scholar] [CrossRef]
- Bryant, M.R.; Burrows, A.D.; Fitchett, C.M.; Hawes, C.S.; Hunter, S.O.; Keenan, L.L.; Kelly, D.J.; Kruger, P.E.; Mahon, M.F.; Richardson, C. The synthesis and characterisation of coordination and hydrogen-bonded networks based on 4-(3,5-dimethyl-1H-pyrazol-4-yl)benzoic acid. Dalton Trans. 2015, 44, 9269–9280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruker. APEX2, version 2.0-2 ed; Bruker AXS Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Bruker. SAINT, version 7.23A ed; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Bruker. SMART, version 5.625 ed; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Bruker. SAINT, version 6.22 ed; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
| Complex | I | II | III |
|---|---|---|---|
| Empirical formula | C6H10CdN2O2 | C26H42Cd4N8O6 | C24H40Cd4N8O6 |
| Formula weight | 254.56 | 1012.28 | 986.24 |
| Temperature (K) | 253 | 273 | 90 |
| Crystal system | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | C2221 | P21/c | Pnma |
| a (Å) | 8.1727(3) | 8.9441(4) | 38.0252(19) |
| b (Å) | 15.7149(6) | 15.2192(7) | 8.7786(4) |
| c (Å) | 15.3108(6) | 29.4582(13) | 12.0948(6) |
| α (°) | 90 | 90 | 90 |
| β (°) | 90 | 91.6930(10) | 90 |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 1966.41(13) | 4008.2(3) | 4037.3(3) |
| Z | 8 | 4 | 4 |
| dcalc (g cm−3) | 1.720 | 1.677 | 1.623 |
| μ (mm−1) | 2.180 | 2.135 | 2.118 |
| F(000) | 992 | 1976 | 1920 |
| Reflections collected | 7375 | 29345 | 20635 |
| Rint | 0.0142 | 0.0228 | 0.0320 |
| Data/restraints/parameters | 2909/0/115 | 9947/63/438 | 3823/343/320 |
| GOF | 1.030 | 1.010 | 1.181 |
| R1, wR2 [I > 2σ(I)] | 0.0164, 0.0369 | 0.0326, 0.0749 | 0.0633, 0.1312 |
| Flack parameter [14] | 0.00(3) | - | - |
| Δρmax, Δρmin (e Å−3) | 0.327, −0.333 | 0.501, 0.688 | 1.089, −1.559 |
| CdTB–O/Å | |
| Cd1–O1 | 2.5207 (19) |
| CdTB–(CN)ax/Å | |
| Cd1–N1 | 2.3449 (19) |
| CdTB–(CN)eq/Å | |
| Cd1–C1 | 2.197 (2) |
| Cd1–N2 | 2.2060 (18) |
| Cd1–N3 | 2.210 (2) |
| (CN)–(CN)/Å | |
| N1–C1i | 1.135 (3) |
| N2–C2ii | 1.141 (4) |
| N3–C3iii | 1.135 (4) |
| (CN)eq–CdTB–(CN)eq/° | |
| C1–Cd1–N2 | 128.65 (8) |
| C1–Cd1–N3 | 116.08 (8) |
| N2–Cd1–N3 | 113.44 (8) |
| (CN)ax–CdTB–(CN)eq/° | |
| N1–Cd1–C1 | 95.06 (7) |
| N1–Cd1–N2 | 94.67 (7) |
| N1–Cd1–N3 | 93.53 (7) |
| (CN)ax–CdTB–O/° | |
| N1–Cd1–O1 | 177.94 (7) |
| (CN)eq–CdTB–O/° | |
| C1–Cd1–O1 | 84.83 (7) |
| N2–Cd1–O1 | 86.99 (7) |
| N3–Cd1–O1 | 84.67 (7) |
| Hydrogen bond/Å | |
| O1···O2iv | 2.774 (2) |
| torsion angle/° | |
| O1–C11–C12–O2 | −68.6 (4) |
| O1–C11’–C12–O2 | 54.1 (9) |
| CdTB–O/Å | |||||
| Cd1–O1 | 2.530(3) | Cd2–O3 | 2.547(3) | Cd3–O5 | 2.480(2) |
| CdTB–(CN)ax/Å | |||||
| Cd1–N7 | 2.322(3) | Cd2–N1 | 2.323(3) | Cd3–N4 | 2.303(3) |
| CdTB–(CN)eq/Å | |||||
| Cd1–C1 | 2.200(3) | Cd2–C2 | 2.182(3) | Cd3–C3 | 2.190(3) |
| Cd1–C8 | 2.185(3) | Cd2–C4 | 2.194(3) | Cd3–C6 | 2.180(3) |
| Cd1–N2 | 2.218(3) | Cd2–N3 | 2.212(3) | Cd3–N5 | 2.223(3) |
| CdT–(CN)/Å | |||||
| Cd4–C5 | 2.203(3) | Cd4–N6 | 2.229(3) | ||
| Cd4–C7 | 2.176(3) | Cd4–N8 | 2.215(4) | ||
| (CN)–(CN)/Å | |||||
| N1–C1 | 1.123(4) | N2–C2i | 1.136(4) | N3–C3 | 1.140(4) |
| N4–C4ii | 1.130(4) | N5–C5 | 1.140(4) | N6–C6iii | 1.137(4) |
| N7–C7iv | 1.125(4) | N8–C8v | 1.137(5) | ||
| (CN)ax–CdTB–O/° | |||||
| N7–Cd1–O1 | 173.88(10) | N1–Cd2–O3 | 178.06(11) | N4–Cd3–O5 | 176.27(10) |
| (CN)eq–CdTB–O/° | |||||
| C1–Cd1–O1 | 85.07(10) | C2–Cd2–O3 | 83.78(12) | C3–Cd3–O5 | 83.28(10) |
| C8–Cd1–O1 | 82.64(11) | C4–Cd2–O3 | 84.57(11) | C6–Cd3–O5 | 87.31(10) |
| N2–Cd1–O1 | 85.01(11) | N3–Cd2–O3 | 84.01(10) | N5–Cd3–O5 | 85.06(10) |
| (CN)eq–CdTB–(CN)ax/° | |||||
| C1–Cd1–N7 | 99.90(11) | C2–Cd2–N1 | 97.20(12) | C3–Cd3–N4 | 93.05(11) |
| C8–Cd1–N7 | 91.44(12) | C4–Cd2–N1 | 96.41(11) | C6–Cd3–N4 | 95.48(11) |
| N2–Cd1–N7 | 95.90(12) | N3–Cd2–N1 | 94.06(11) | N5–Cd3–N4 | 96.03(11) |
| (CN)eq–CdTB–(CN)eq/° | |||||
| C8–Cd1–C1 | 128.24(13) | C2–Cd2–C4 | 117.75(13) | C6–Cd3–C3 | 126.97(12) |
| C1–Cd1–N2 | 116.09(13) | C2–Cd2–N3 | 113.13(12) | C3–Cd3–N5 | 117.07(12) |
| C8–Cd1–N2 | 112.61(12) | C4–Cd2–N3 | 126.01(12) | C6–Cd3–N5 | 113.87(12) |
| (CN)–CdT–(CN)/° | |||||
| C7–Cd4–C5 | 110.76(12) | C7–Cd4–N6 | 115.46(13) | C7–Cd4–N8 | 111.38(13) |
| C5–Cd4–N6 | 103.02(12) | C5–Cd4–N8 | 110.93(12) | N8–Cd4–N6 | 104.89(12) |
| Hydrogen bond/Å | |||||
| O1···O4vi | 2.763(4) | O3···O2ii | 2.777(4) | O5···O6iv | 2.708(3) |
| torsion angle/° | |||||
| O1–C9–C10–O2 | −69.5(7) | O1–C9’–C10–O2 | 44.6(19) | ||
| O3–C15–C16–O4 | −71.5(6) | O3–C15’–C16–O4 | 42(2) | ||
| O5–C21–C22–O6 | −74.2(4) |
| CdOC–O/Å | |||||
| Cd1–O1 | 2.274(12) | Cd1–O1’ | 2.49(2) | ||
| CdOC–(CN)/Å | |||||
| Cd1–N1i | 2.267(8) | Cd1–N2 | 2.306(10) | ||
| Cd1–N1ii | 2.267(8) | Cd1–N3iv | 2.285(11) | ||
| CdT–(CN)/Å | |||||
| Cd2–C1 | 2.191(9) | Cd3–C3 | 2.157(13) | Cd4–N4 | 2.221(15) |
| Cd2–C1iii | 2.191(9) | Cd3–C5 | 2.157(11) | Cd4–N5 | 2.179(13) |
| Cd2–C2 | 2.221(12) | Cd3–C6 | 2.151(10) | Cd4–N6v | 2.230(11) |
| Cd2–C4 | 2.184(12) | Cd3–C6iii | 2.151(10) | Cd4–N6vi | 2.230(11) |
| (CN)–(CN)/Å | |||||
| N1–C1 | 1.148(11) | N3–C3 | 1.128(15) | N5–C5 | 1.162(15) |
| N2–C2 | 1.124(14) | N4–C4 | 1.146(16) | N6–C6 | 1.152(12) |
| O–CdOC–O/° | |||||
| O1–Cd1–O1’ | 87(2) | ||||
| (CN)–CdOC–O/° | |||||
| N1i–Cd1–O1 | 164.9(11) | N3iv–Cd1–O1 | 85.4(5) | N2–Cd1–O1’ | 82.4(6) |
| N1ii–Cd1–O1 | 100.0(11) | N1i–Cd1–O1’ | 78.3(12) | N3iv–Cd1–O1’ | 93.0(7) |
| N2–Cd1–O1 | 88.0(5) | N1ii–Cd1–O1’ | 171.5(12) | ||
| (CN)–CdOC–(CN)/° | |||||
| N1i–Cd1–N1ii | 95.2(4) | N1i–Cd1–N3iv | 92.9(3) | ||
| N1i–Cd1–N2 | 92.5(2) | N3iv–Cd1–N2 | 172.1(4) | ||
| (CN)–CdT–(CN)/° | |||||
| C1–Cd2–C1iii | 111.2(5) | C3–Cd3–C5 | 119.5(4) | N5–Cd4–N4 | 115.8(4) |
| C1–Cd2–C2 | 109.4(3) | C6–Cd3–C3 | 111.5(3) | N4–Cd4–N6v | 106.5(3) |
| C4–Cd2–C1 | 110.0(3) | C6–Cd3–C5 | 104.1(3) | N5–Cd4–N6v | 110.8(3) |
| C4–Cd2–C2 | 106.7(4) | C6–Cd3–C6iii | 104.9(5) | N6v–Cd4–N6vi | 106.0(5) |
| Hydrogen bond/Å | |||||
| O1···O51vii | 3.23(4) | O1’···O511 | 2.71(5) | O511···O1’ix | 2.71(5) |
| O1···O51viii | 3.14(4) | O51···O64iv | 2.66(3) | C63···O61ix | 2.47(2) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, T.; Kitazawa, T. Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol. Crystals 2016, 6, 103. https://doi.org/10.3390/cryst6090103
Kawasaki T, Kitazawa T. Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol. Crystals. 2016; 6(9):103. https://doi.org/10.3390/cryst6090103
Chicago/Turabian StyleKawasaki, Takeshi, and Takafumi Kitazawa. 2016. "Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol" Crystals 6, no. 9: 103. https://doi.org/10.3390/cryst6090103
APA StyleKawasaki, T., & Kitazawa, T. (2016). Three-Dimensional Cadmium(II) Cyanide Coordination Polymers with Ethoxy-, Butoxy- and Hexyloxy-ethanol. Crystals, 6(9), 103. https://doi.org/10.3390/cryst6090103