Preparation, Crystal and Properties of Nitrogen-Rich Energetic Salt of Bis(semicarbazide) 5,5′-Bitetrazole-1,1′-diolate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Energetic Salt 2(SCZ)·BTO
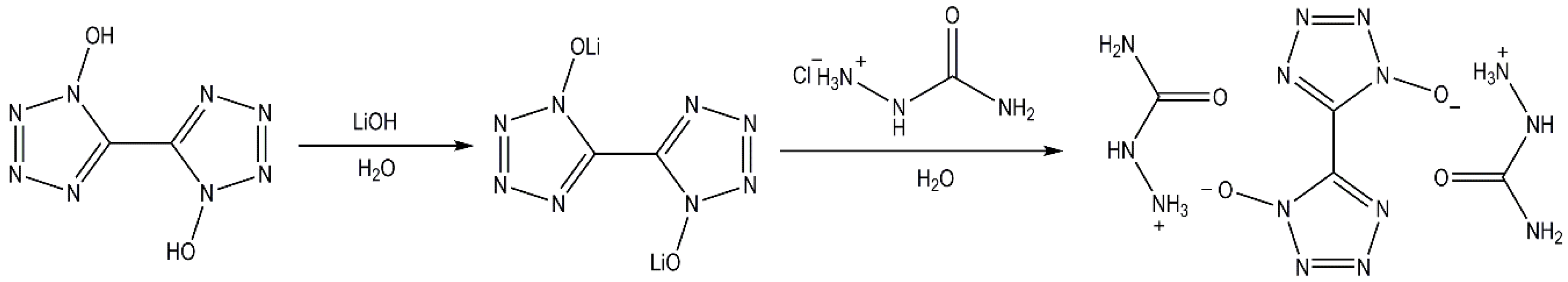
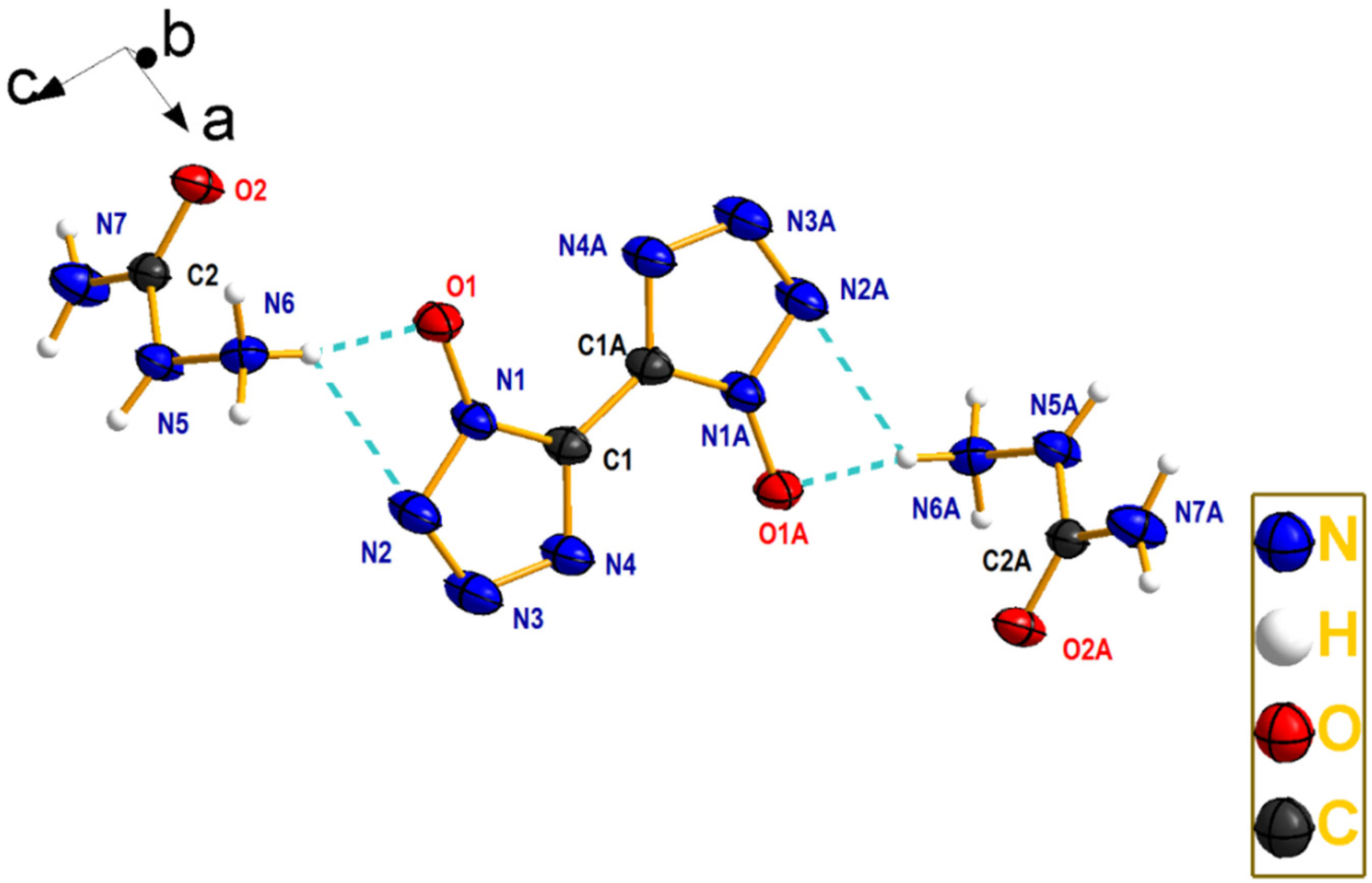
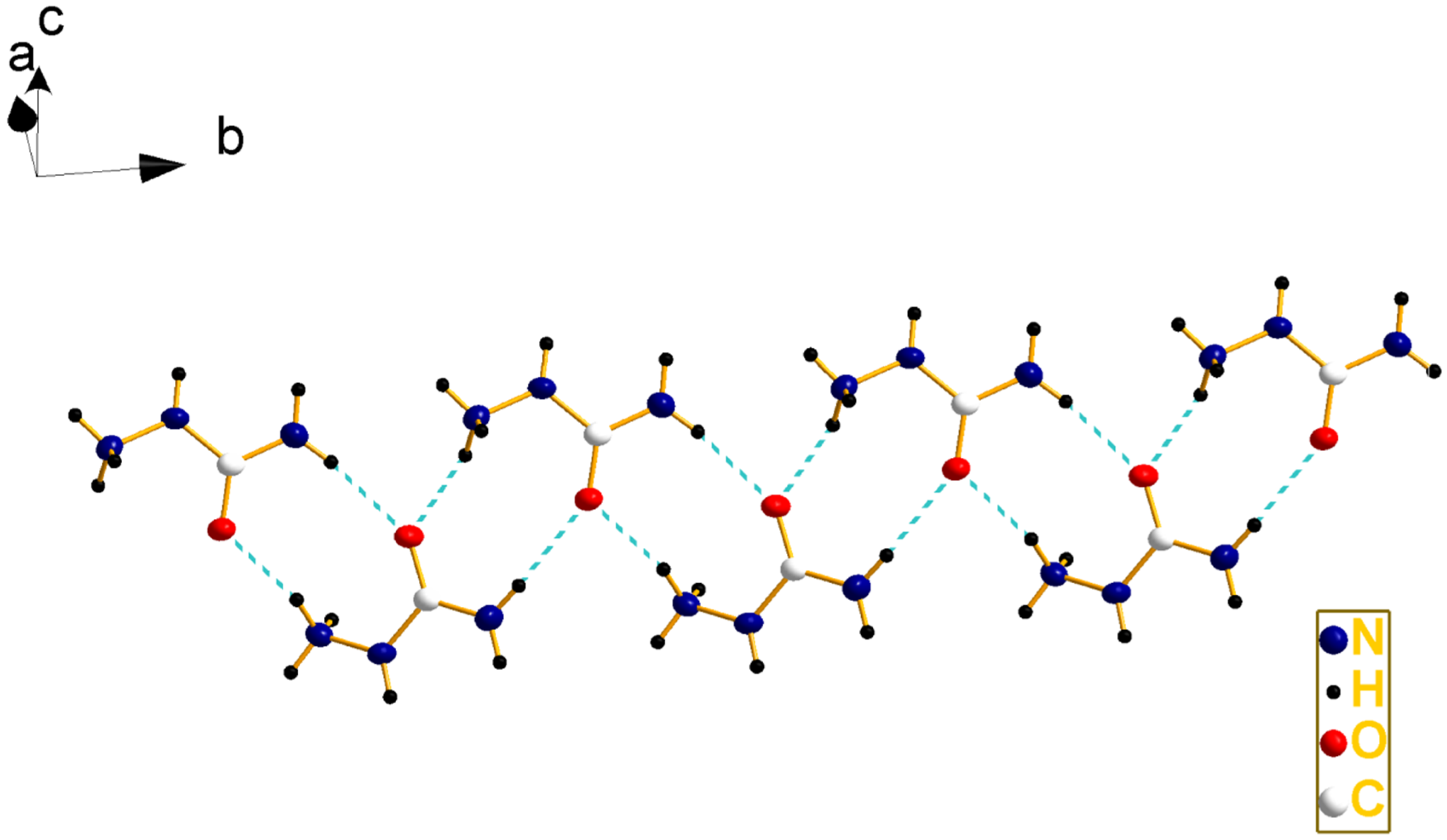
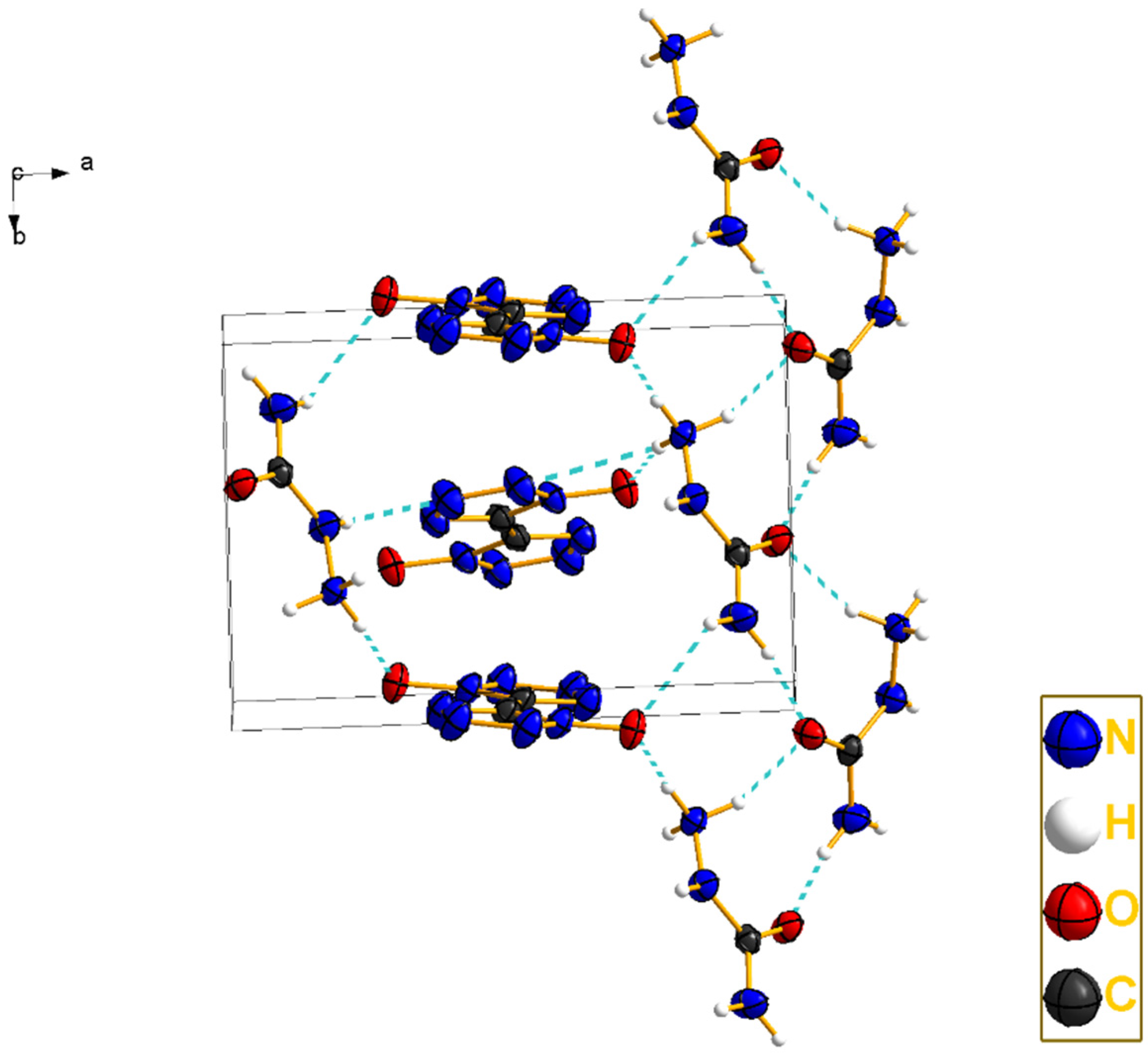
2.2. Crystal Structure
| Parameter | 2(SCZ)·BTO |
|---|---|
| empirical formula | C4H12N14O4 |
| formula mass | 320.28 |
| temperature/K | 298(2) |
| crystal system | monoclinic |
| space group | P21/c |
| Z | 2 |
| a/Å | 10.2389(9) |
| b/Å | 6.9576(6) |
| c/Å | 8.9756(7) |
| β/° | 99.099(2) |
| cell volume/Å3 | 631.36(9) |
| Dc/g·cm−3 | 1.685 |
| μ(Mo Kα)/mm−1 | 0.145 |
| F(000) | 332 |
| θ/° | 3.55–25.02 |
| h, k and l range | −10 to 12, −8 to 8, −10 to 10 |
| reflections collected | 3023 |
| reflections unique [Rint] | 1109[Rint = 0.0284] |
| data/restraint/parameter | 1109/0/102 |
| goodness-of-fit on F2 | 1.049 |
| R1, [I > 2σ(I)] | 0.0381 |
| wR2, [I > 2σ(I)] | wR2 = 0.1074 a |
| R1, (all data) | 0.0448 |
| wR2, (all data) | wR2 = 0.1147 a |
| Δρmax, Δρmin (e·Å−3) | 0.453, −0.469 |
| CCDC | 1437024 |
| Bond | Length | Bond | Angle |
|---|---|---|---|
| N1–O1 | 1.325(19) | N1–N2–O1 | 121.11(15) |
| N1–N2 | 1.336(2) | N1–C1–O1 | 129.89(15) |
| N1–C1 | 1.342(2) | N2–N1–C1 | 109.00(15) |
| N2–N3 | 1.315(2) | N3–N2–N1 | 105.53(15) |
| N3–N4 | 1.337(2) | N2–N3–N4 | 111.55(15) |
| N4–C1 | 1.329(2) | C1–N4–N3 | 105.70(16) |
| N5–C2 | 1.360(2) | C2–N5–N6 | 115.98(14) |
| N5–N6 | 1.421(2) | N4–C1–N1 | 108.21(17) |
| N7–C2 | 1.334(2) | N4–C1–C1 #1 | 127.30(2) |
| C2–O2 | 1.238(2) | N1–C1–C1 #1 | 124.50(2) |
| C1–C1#1 | 1.446(4) | N7–C2–O2 | 123.09(16) |
| N6–O1 | 2.780(21) | N5–C2–O2 | 120.70(16) |
| N6–N2 | 3.261(24) | N7–C2–N5 | 116.11(16) |
| D–H···A | Length(D–H) | Length(H···A) | Length(D···A) | Angle(D–H···A) |
|---|---|---|---|---|
| N5–H5···N3 i | 0.860 | 2.136 | 2.882 | 144.92 |
| N6–H6A···O2 ii | 0.890 | 1.958 | 2.801 | 157.36 |
| N6–H6B···O1 iii | 0.890 | 1.936 | 2.786 | 159.14 |
| N6–H6B···N4 iv | 0.890 | 2.481 | 2.997 | 117.39 |
| N7–H7A···O2 v | 0.860 | 2.101 | 2.906 | 155.60 |
| N7–H7B···O1 vi | 0.860 | 2.466 | 3.108 | 131.95 |
| N6–H6C···O1 | 0.890 | 1.906 | 2.780 | 166.85 |
| N6–H6C···N1 | 0.890 | 2.594 | 3.438 | 158.53 |
| N6–H6C···N2 | 0.890 | 2.621 | 3.261 | 129.52 |
2.3. Thermal Decomposition and Non-Isothermal Kinetic Analysis
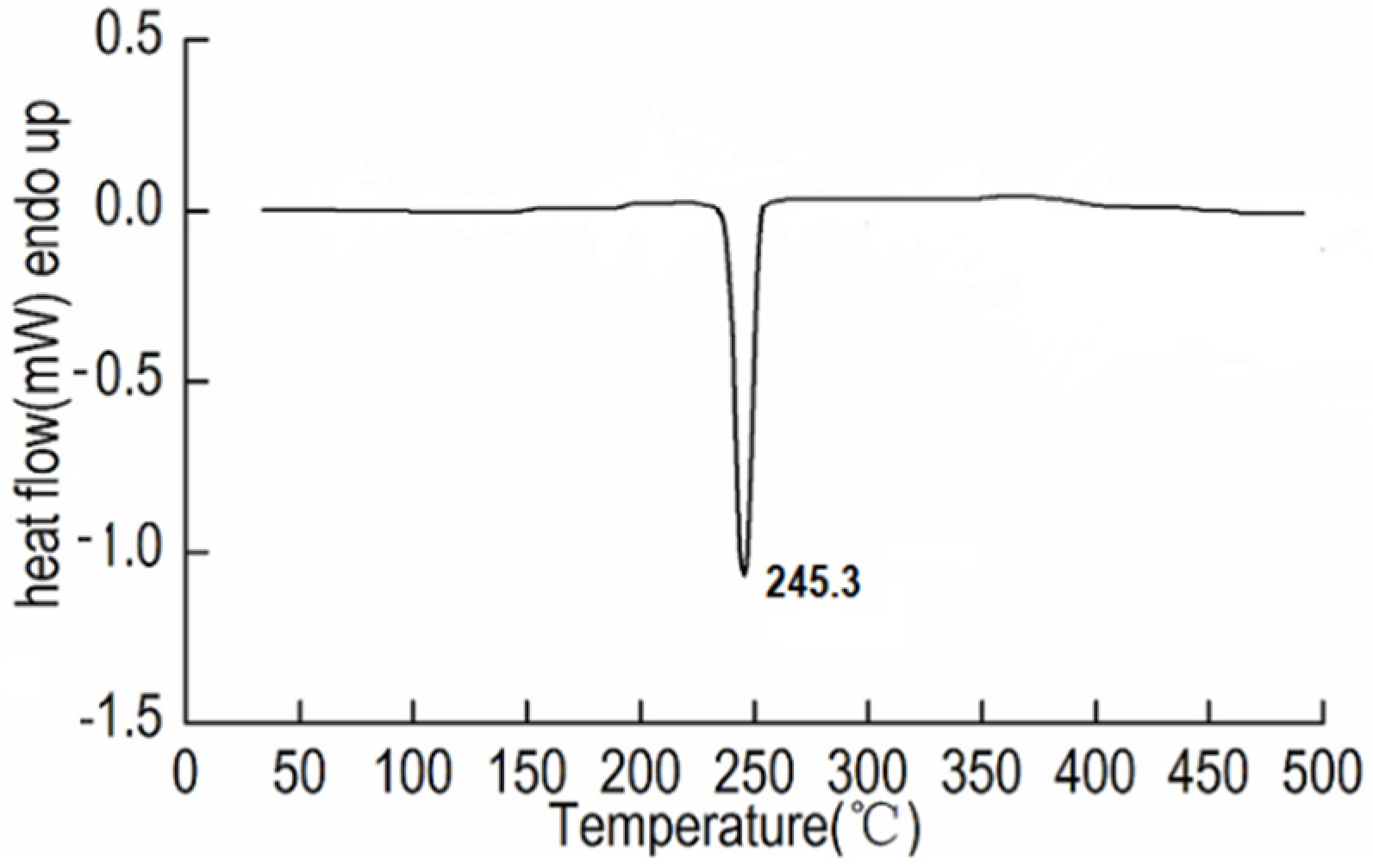
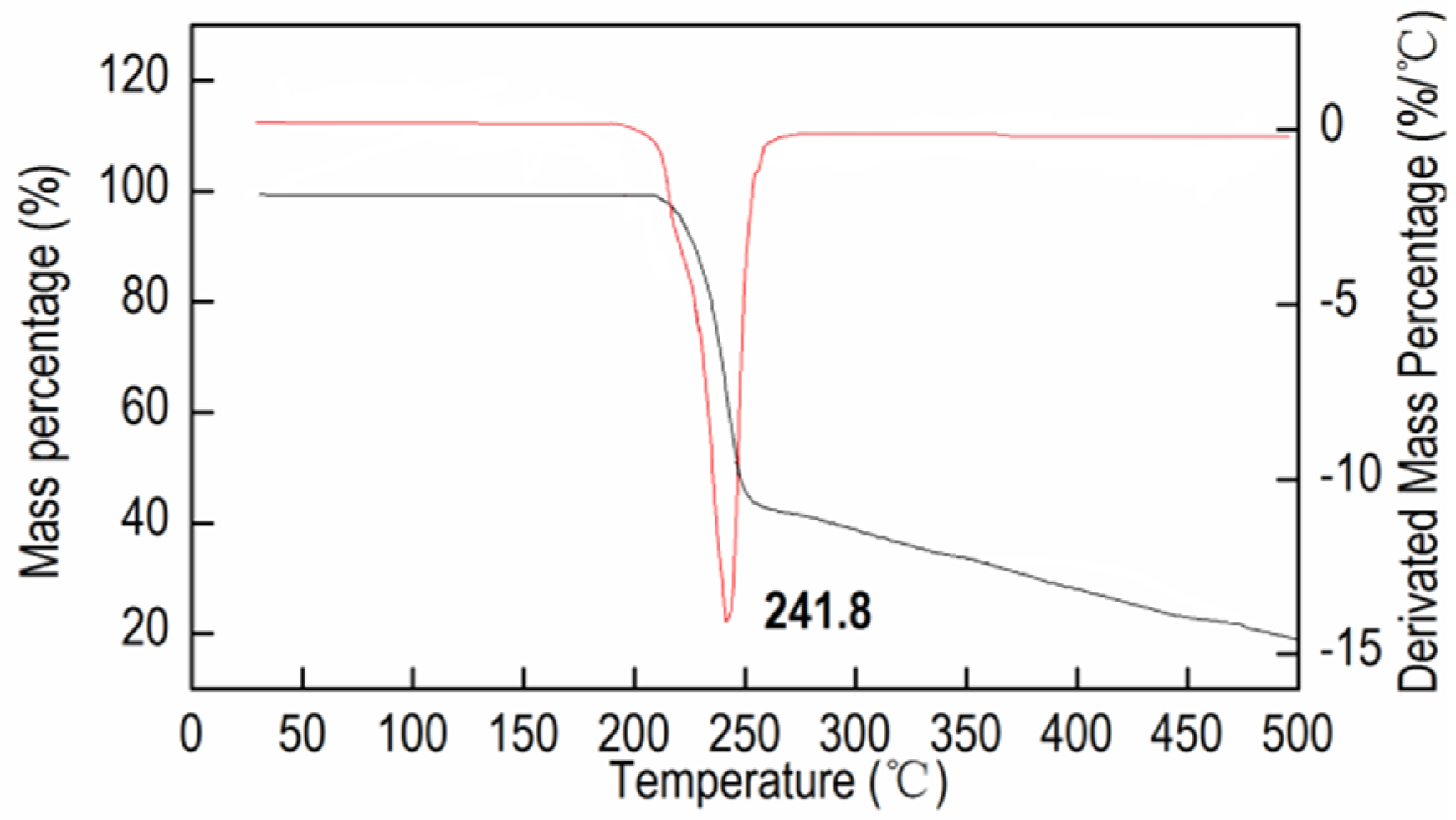
| Heating rates (°C·min−1) | 5 | 10 | 15 | 20 |
| Peak temperatures Tp (℃) | 245.3 | 252.7 | 256.1 | 258.4 |
| Kissinger’s Method | Ozawa’s Method | |||
| Ek(kJ·mol−1) | lg[Ak(s−1)] | Rk | Eo(kJ·mol−1) | Ro |
| 231.2 | 21.21 | −0.9968 | 228.1 | −0.9970 |
2.4. Calculation of the Thermal Explosion Properties and Enthalpy of Formation
2.5. Detonation Parameters
3. Materials and Methods
3.1. Materials and Physical Techniques
3.2. Synthesis of the Energetic Salt 2(SCZ)·BTO
3.3. X-ray Single-Crystal Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Abbreviations | Definition |
| SCZ | semicarbazide |
| BTO | 1H,1’H-5,5’-bitetrazole-1,1’-diol |
| FT-IR | Fourier Transform infrared spectroscopy |
| DSC | Differential Scanning Calorimetry |
| TG-DTG | Thermogravimetric differential heat |
| ΔS≠ | The entropy of activation |
| ΔH≠ | The enthalpy of activation |
| ΔG≠ | The free energy of activation |
| P | detonation pressure |
| D | detonation velocities |
Appendix B
References
- Steinhauser, G.; Klapötke, T.M. “Green” pyrotechnics: A chemists’challenge. Angew. Chem. Int. Ed. 2008, 47, 3330–3347. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Sabaté, C.M. Bistetrazoles: Nitrogen-rich, high-performing, insensitive energetic compounds. Chem. Mater. 2008, 20, 3629–3637. [Google Scholar] [CrossRef]
- Göbel, M.; Karaghiosoff, K.; Klapötke, T.M. Nitrotetrazolate-2N-oxides and the strategy of N-oxide introduction. J. Am. Chem. Soc. 2010, 132, 17216–17226. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Xu, H.Y.; Guo, Y.; Sa, R.J.; Shreeve, J.M. Bis[3-(5-nitroimino-1,2,4-triazolate)]-Based Energetic Salts: Synthesis and Promising Properties of a New Family of High-Density Insensitive Materials. J. Am. Chem. Soc. 2010, 132, 11904–11905. [Google Scholar] [CrossRef] [PubMed]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Sabaté, C.M.; Welch, J.M. Alkaline earth metal salts of 5-nitro-2H-tetrazole: Prospective candidates for environmentally friendly energetic applications. Eur. J. Inorg. Chem. 2009, 2009, 769–776. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M.; Stierstorfer, J. Neutral 5-nitrotetrazoles: Easy initiation with low pollution. New J. Chem. 2009, 33, 136–147. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Karaghiosoff, K.; Mayer, P. Synthesis and characterization of 1,4-dimethyl-5-aminotetrazolium 5-Nitrotetrazolate. Propellants Explos. Pyrotech. 2006, 31, 188–195. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M.; Winter, N. Insensitive nitrogen-rich energetic compounds based on the 5,5’-Dinitro-3,3’-bi-1,2,4-triazol-2-ide anion. Eur. J. Inorg. Chem. 2012, 2012, 3474–3484. [Google Scholar] [CrossRef]
- Wu, J.T.; Zhang, J.G.; Yin, X. Synthesis, characterization, and thermal analysis of two energetic ionic salts based on 3,4-diamino-1,2,4-triazole (DATr). Z. Anorg. Allg. Chem. 2013, 639, 2354–2358. [Google Scholar] [CrossRef]
- Fendt, T.; Fischer, N.; Klapötke, T.M. N-rich Salts of 2-methyl-5-nitraminotetrazole: Secondary explosives with low sensitivities. Inorg. Chem. 2011, 50, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M. Nitrogen-rich tetrazolium azotetrazolate salts: A new family of insensitive energetic materials. Chem. Mater. 2008, 20, 1750–1763. [Google Scholar] [CrossRef]
- Fischer, N.; Klapötke, T.M.; Stierstorfer, J. Hydrazinium nitriminotetrazolates. Z Anorg. Allg. Chem. 2011, 637, 1273–1276. [Google Scholar] [CrossRef]
- Joo, Y.H.; Gao, H.X.; parrish, D.A.; Cho, S.G.; Goh, E.M.; Shreeve, J.M. Energetic salts based on nitroiminotetrazole-containing acetic acid. J. Mater. Chem. 2012, 22, 6123–6130. [Google Scholar] [CrossRef]
- Wang, K.; Parrish, D.A.; Shreeve, J.M. 3-Azido-N-nitro-1H-1,2,4-triazol-5-amine-based energetic salts. Chem. Eur. J. 2011, 17, 14485–14492. [Google Scholar] [CrossRef] [PubMed]
- Tselinskii, I.V.; Mel’nikova, S.F.; Romanova, T.V. Synthesis and reactivity of carbohydroximoyl azides: I. aliphatic and aromatic carbohydroximoyl azides and 5-substituted 1-hydroxytetrazoles based thereon. Russian J. Organ. Chem. 2001, 37, 430–436. [Google Scholar] [CrossRef]
- Fischer, N.; Fischer, D.; Klapötke, T.M. Pushing the limits of energetic materials—the synthesis and characterization of dihydroxylammonium 5,5′-bitetrazole-1,1′-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Fischer, N.; Izsák, D.; Klapötke, T.M.; Rappenglück, S.; Stierstorfer, J. Nitrogen-rich 5,5′-bistetrazolates and their potential use in propellant systems: A comprehensive study. Chem. Eur. J. 2012, 18, 4051–4062. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Klapötke, T.M.; Reymann, M. Nitrogen-rich salts of 1H,1′H-5,5′-Bitetrazole-1,1′-diol: Energetic materials with high thermal stability. Eur. J. Inorg. Chem. 2013, 2013, 2167–2180. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 19, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Li, Z.M.; Xie, S.H.; Zhang, J.G.; Feng, J.L.; Wang, K. Two high nitrogen content energetic compounds: 3,6-diguanidino-1,2,4,5-tetrazine and its diperchlorate. J. Chem. Eng. Data 2012, 57, 729–736. [Google Scholar] [CrossRef]
- Zhang, T.L.; Hu, R.Z.; Xie, Y. The estimation of critical-temperatures of thermal-explosion for energetic materials using nonistheramal DSC. Thermochim. Acta 1994, 244, 171. [Google Scholar]
- Li, F.G.; Bi, Y.G.; Zhao, W.Y.; Zhang, T.L.; Zhou, Z.N.; Yang, L. Nitrogen-rich salts based on the energetic [monoaquabis (N,N-bis (1H-tetrazol-5-yl) amine)–zinc (II)] anion: A promising design in the development of new energetic materials. Inorg. Chem. 2015, 54, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Kamlet, K.B.; Jacobs, S.J. Chemistry of Detonations. I. A Simple Method for Calculating Detonation Properties of C-H-N-O Explosives. J. Chem. Phys. 1968, 48, 23–25. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Guo, Y.; Joo, Y.H.; Parrish, D.A.; Shreeve, J.M. 3,4,5-Trinitropyrazole-Based Energetic Salts. Chem. Eur. J. 2010, 16, 10778–10784. [Google Scholar] [CrossRef] [PubMed]
- Sheldick, G.M. SHELXS-97, Program for Crystal Structure Refinement; Universität of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldick, G.M. SHELXL-97, Program for Crystal Structure Solution; Universität of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-B.; Yin, L.; Yin, X.; Zhang, J.-G. Preparation, Crystal and Properties of Nitrogen-Rich Energetic Salt of Bis(semicarbazide) 5,5′-Bitetrazole-1,1′-diolate. Crystals 2016, 6, 21. https://doi.org/10.3390/cryst6020021
Zhang Z-B, Yin L, Yin X, Zhang J-G. Preparation, Crystal and Properties of Nitrogen-Rich Energetic Salt of Bis(semicarbazide) 5,5′-Bitetrazole-1,1′-diolate. Crystals. 2016; 6(2):21. https://doi.org/10.3390/cryst6020021
Chicago/Turabian StyleZhang, Zhi-Bin, Lei Yin, Xin Yin, and Jian-Guo Zhang. 2016. "Preparation, Crystal and Properties of Nitrogen-Rich Energetic Salt of Bis(semicarbazide) 5,5′-Bitetrazole-1,1′-diolate" Crystals 6, no. 2: 21. https://doi.org/10.3390/cryst6020021





