Spectroscopy, Crystal and Molecular Structures of New 4-Acylpyrazolone Dinitrophenylhydrazones
Abstract
:1. Introduction
2. Results
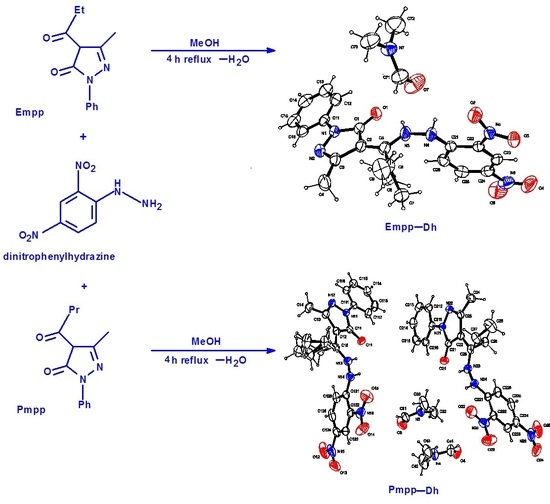
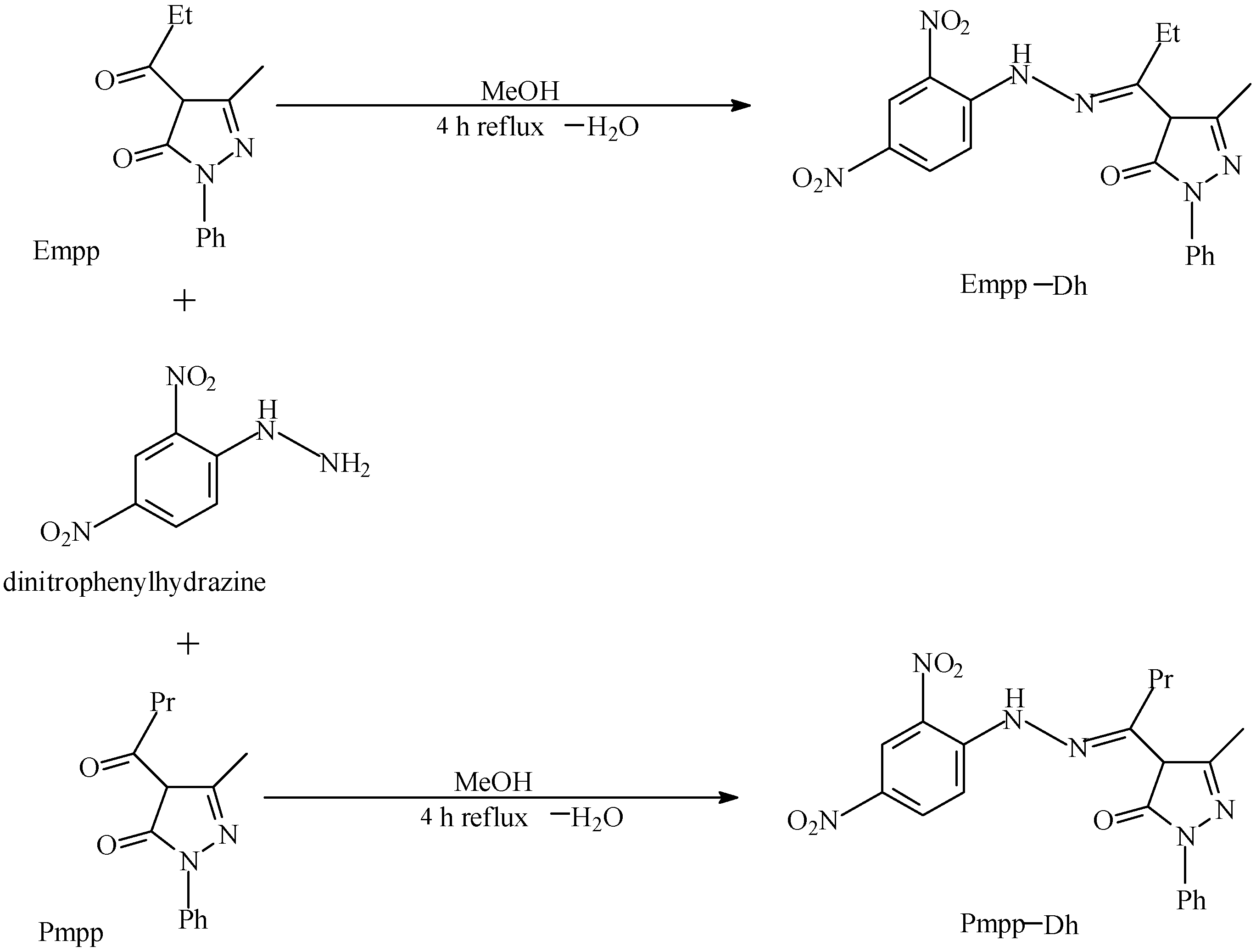
2.1. Synthesis and Elemental Analysis
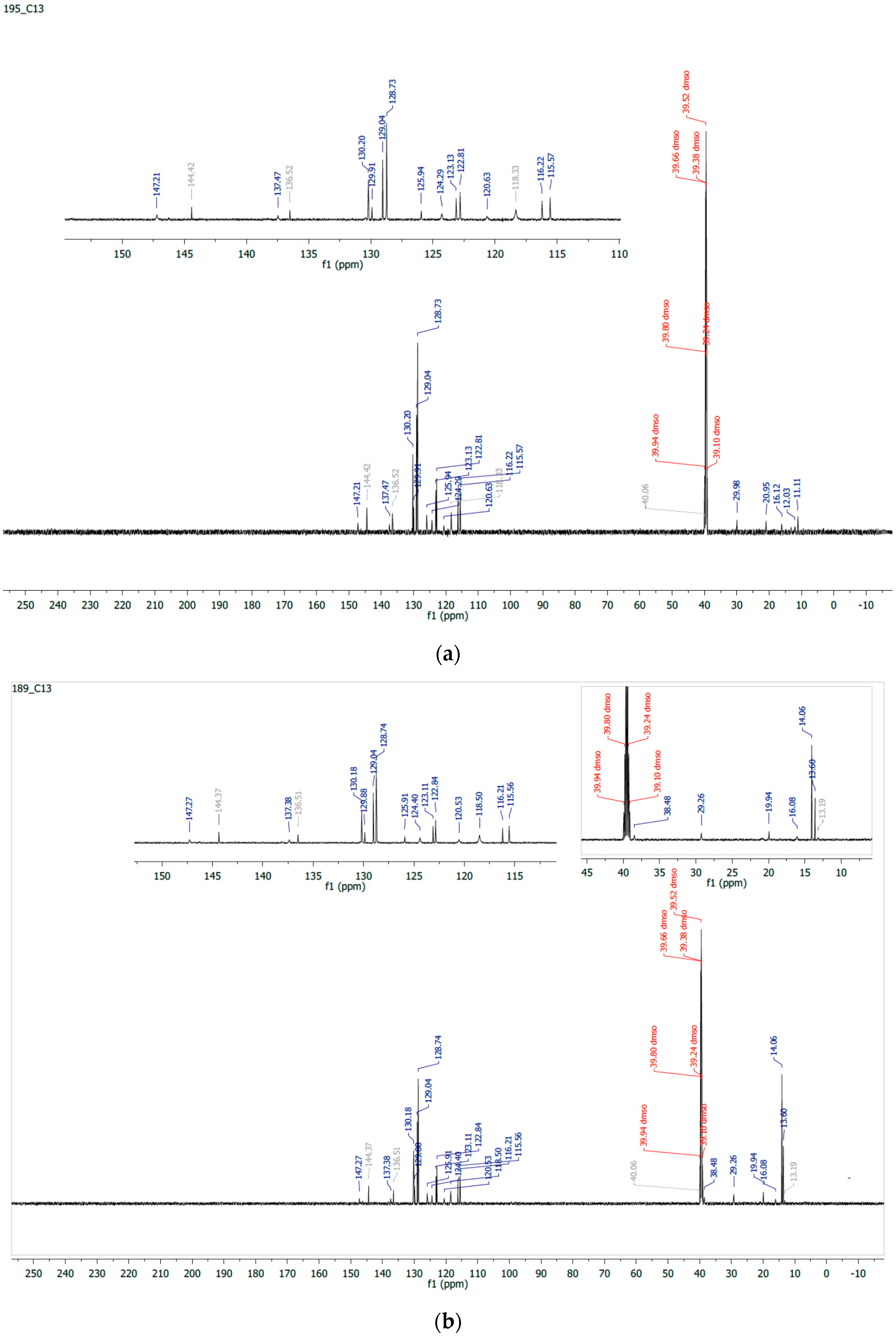
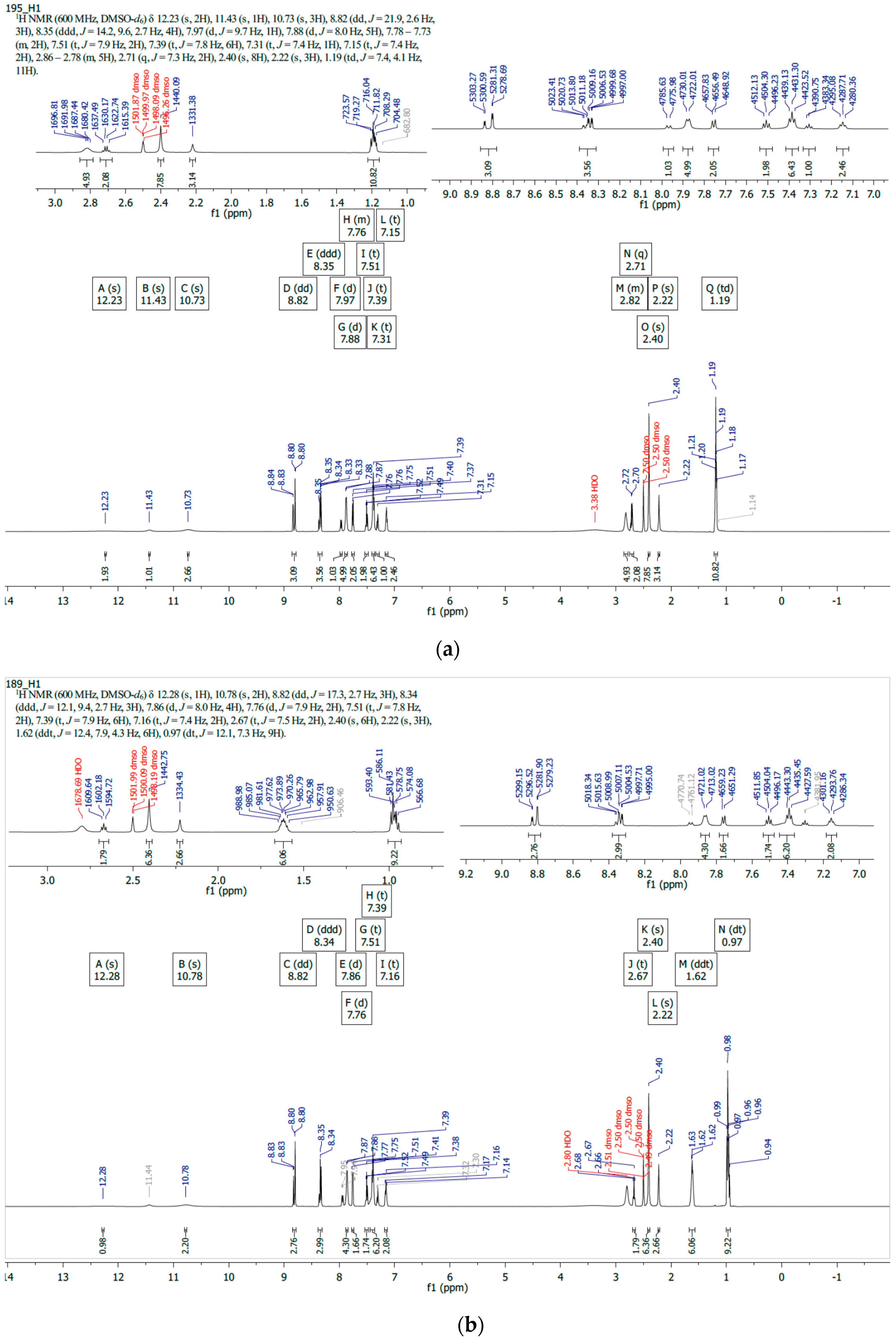
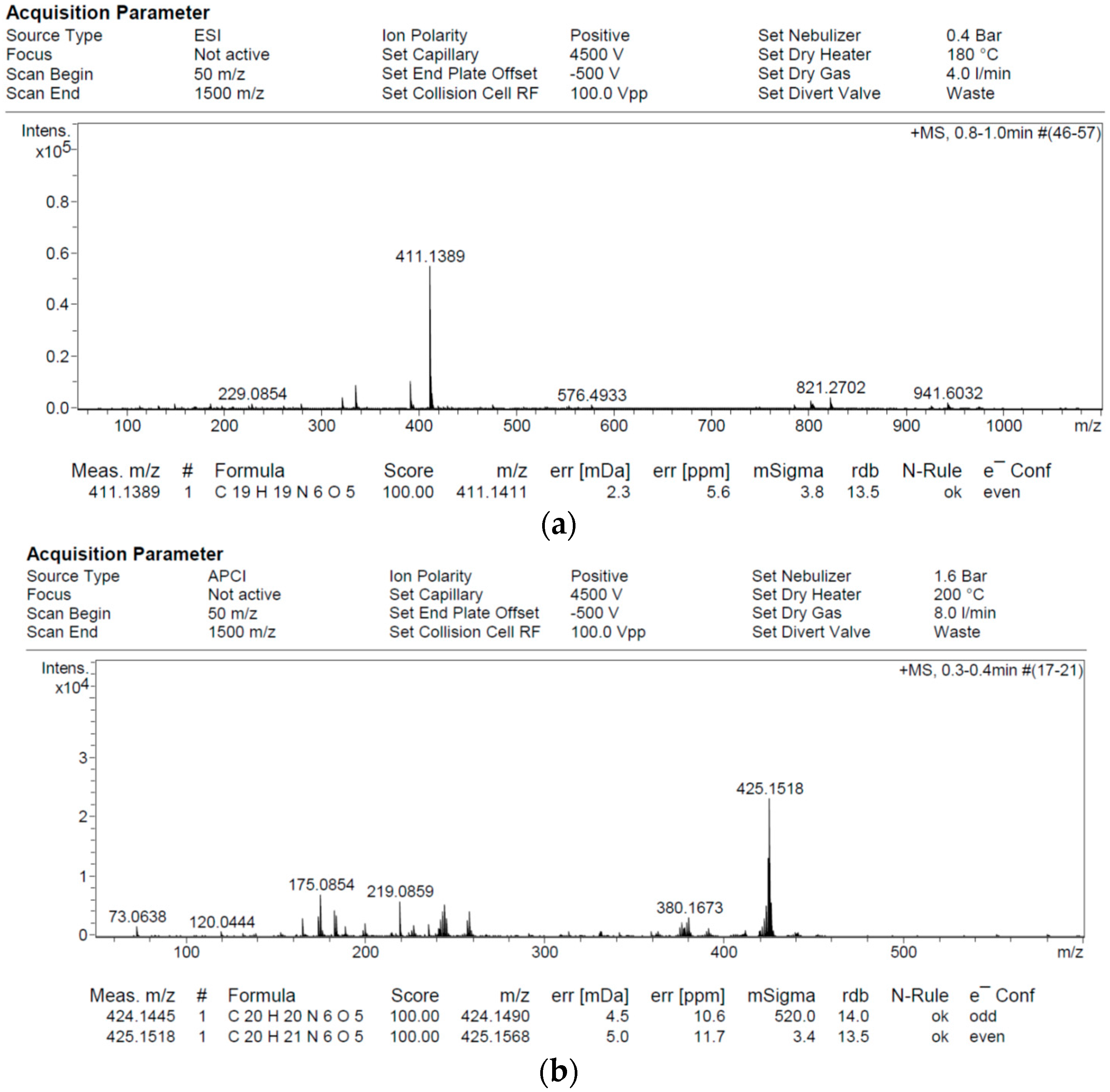
2.2. 1H/13C NMR and Mass Spectroscopy
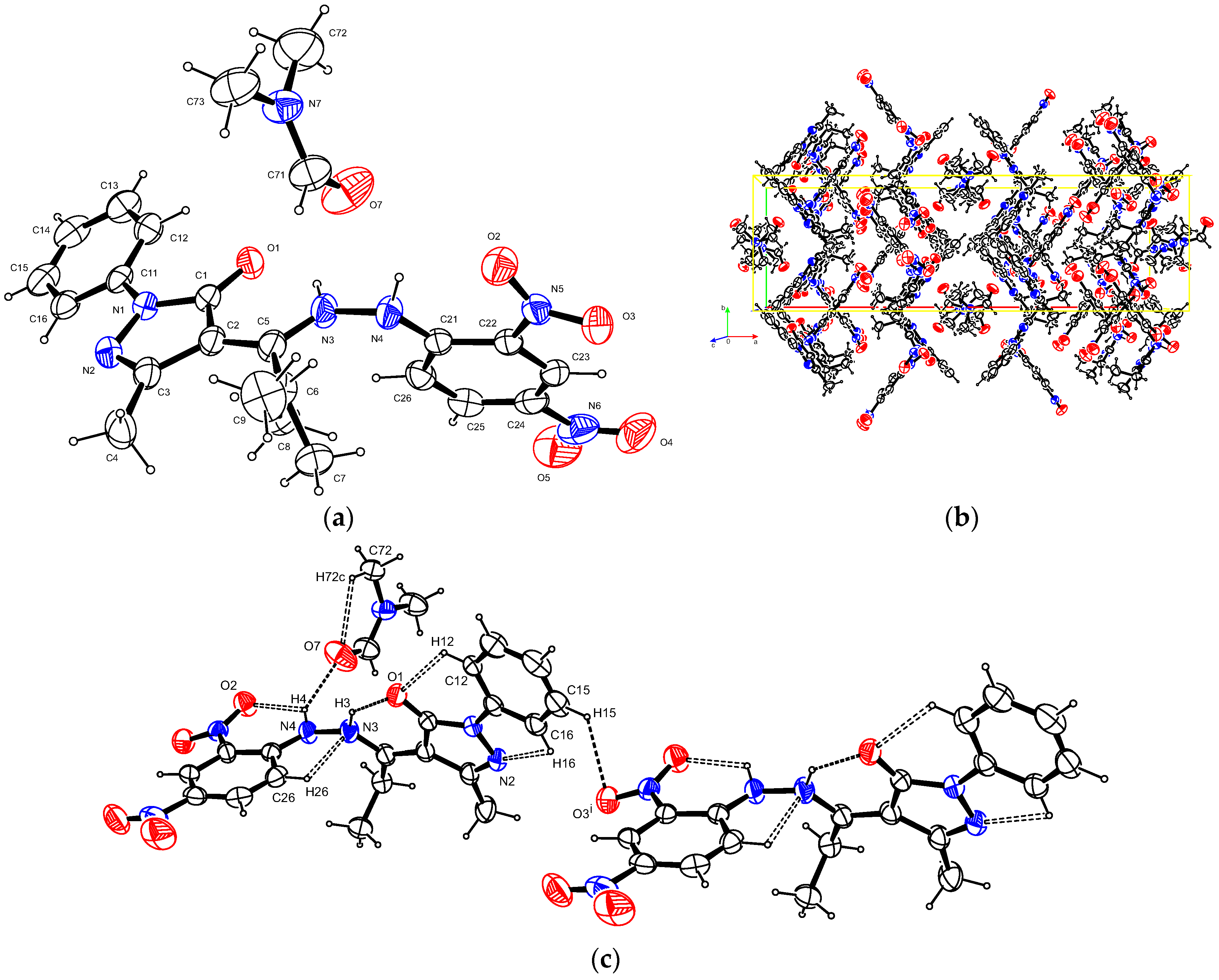
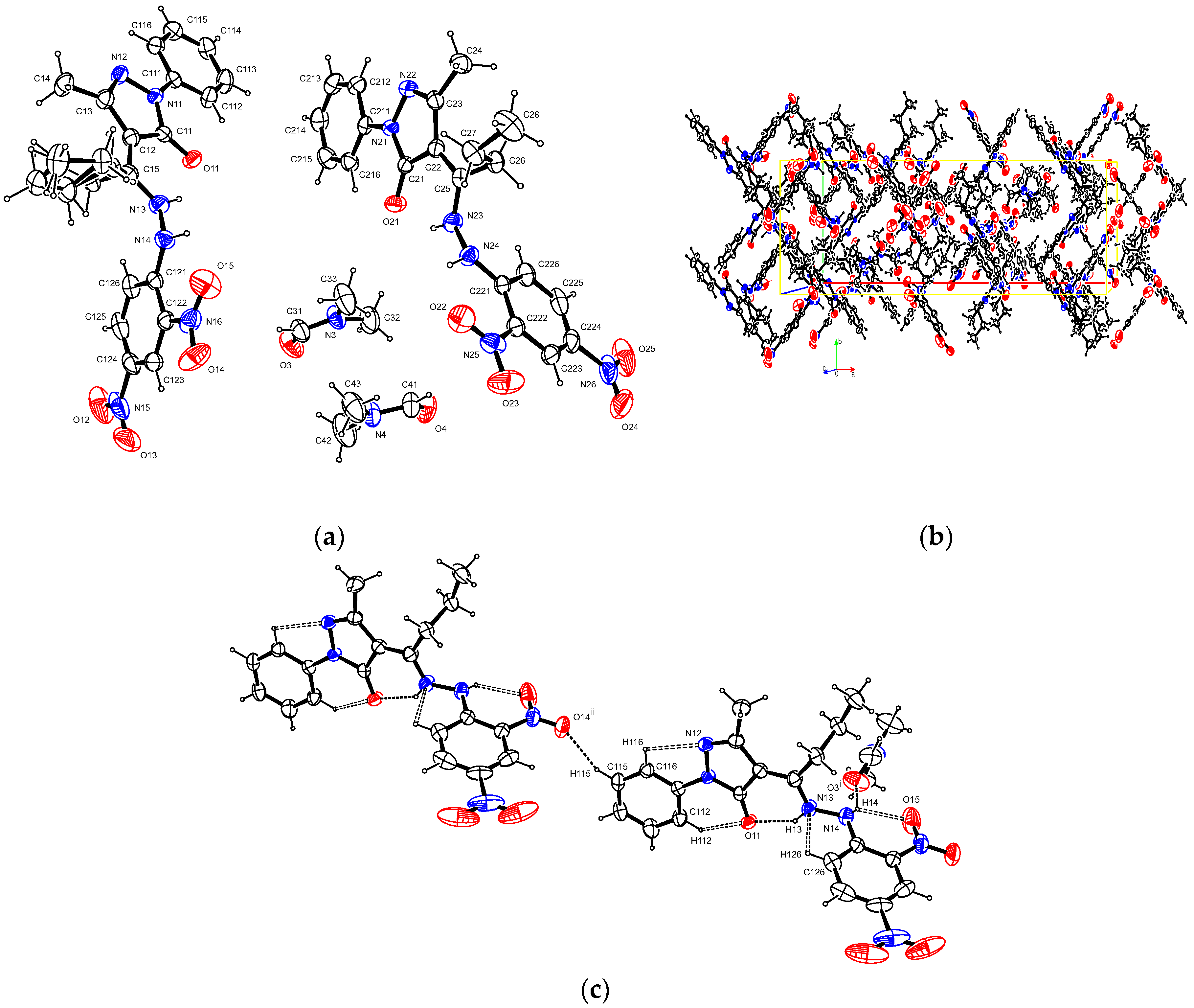
2.3. X-Ray Crystallography
3. Experimental
3.1. General Methods and Synthesis
3.2. 4-Ethyl-5-Methyl-2-Phenyl-Pyrazol-3-one Dinitrophenylhydrazone, C19H18N6O5 Empp-Dh
3.3. 4-Propyl-5-Methyl-2-Phenyl-Pyrazol-3-One Dinitrophenylhydrazone, C20H20N6O5 Pmpp-Dh
3.4. X-Ray Diffraction Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Subbaiyan, S.; Chinnasamy, J. Synthesis, characterization, DNA binding and cleavage activity of Ruthenium(II) complexes with heterocyclic substituted thiosemicarbazones. J. Chil. Chem. Soc. 2013, 58, 1637–1642. [Google Scholar]
- Mohamed, G.G.; Zayed, M.A.; Abdallah, S.M. Metal Complexes of a Novel Schiff Base Derived from Sulphametrole and Varelaldehyde. Synthesis, Spectral, Thermal Characterization and Biological Activity. J. Mol. Struct. 2010, 979, 62–71. [Google Scholar] [CrossRef]
- Gharamaleki, J.A.; Akbari, F.; Karbalaei, A.; Ghiassi, K.B.; Olmstead, M.M. Synthesis, Characterization and Crystal Structure of a New Schiff Base Ligand from a Bis(Thiazoline) Template and Hydrolytic Cleavage of the Imine Bond Induced by a Co(II) Cation. Open J. Inorg. Chem. 2016, 6, 76–88. [Google Scholar] [CrossRef]
- Castagnolo, D.; De Logu, A.; Radi, M.; Bechi, B.; Manetti, F.; Magnani, M.; Supino, S.; Meleddu, R.; Chisu, L.; Botta, M. Synthesis, biological evaluation and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis. Bioorg. Med. Chem. 2008, 16, 8587–8591. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone ligands: Synthesis, structures, metal coordination chemistry and applications. Coord. Chem. Rev. 2005, 249, 2909–2945. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, R.; Pettinari, C. Recent advances in acylpyrazolone metal complexes and their potential applications. Coord. Chem. Rev. 2015, 303, 1–31. [Google Scholar] [CrossRef]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. Crystal structure of bis(4-benzoyl-3-methyl-1-phenyl pyrazol-5-one)-bis(N,N-dimethylformamide)cobalt(II), C40H40CoN6O6. Z. Kristallogr. New. Cryst. Struct. 2015, 230, 83–84. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Parihar, S.; Vyas, K.; Gupta, V.K. Synthesis and crystal structure of a series of pyrazolone based Schiff base ligands and DNA binding studies of their copper complexes. J. Mol. Struct. 2013, 1013, 86–94. [Google Scholar] [CrossRef]
- Idemudia, O.G.; Sadimenko, A.P.; Afolayan, A.J. Potential Therapeutic Mn2+ and Ni2+ Complexes of New 4-Acetyl-3-Methyl-1-Phenyl-2-Pyrazoline-5-One Phenylhydrazone Ampp-Ph. Macromol. Symp. 2015, 351, 61–68. [Google Scholar] [CrossRef]
- Brady, O.L.; Elsmie, G.V. The use of 2,4-dinitrophenylhydrazine as a reagent for aldehydes and ketones. Analyst 1926, 51, 77–78. [Google Scholar] [CrossRef]
- Jesmin, M.; Ali, M.M.; Khanam, J.A. Antitumour activities of some Schiff bases derived from benzoin, salicylaldehyde, amino phenol and 2,4 dinitrophenyl hydrazine. Thai J. Pharm. Sci. 2010, 34, 20–31. [Google Scholar]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. 4-{[2-(2,4-Dinitrophenyl) hydrazinylidene] (phenyl)methyl}-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one ethanol monosolvate. Acta Cryst. 2012, E68, o3380. [Google Scholar] [CrossRef] [PubMed]
- Idemudia, O.G.; Sadimenko, A.P.; Afolayan, A.J.; Hosten, E.C. Synthesis and Characterization of Bioactive Acylpyrazolone Sulfanilamides and Their Transition Metal Complexes: Single Crystal Structure of 4-Benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one Sulfanilamide. Bioinorg. Chem. Appl. 2015, 2015, 717089. [Google Scholar] [CrossRef] [PubMed]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. Crystal structure of 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one sulfadiazine dimethylformamide monosolvate, C30H29N7O4S. Z. Kristallogr. New. Cryst. Struct. 2014, 229, 455–457. [Google Scholar] [CrossRef]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. Crystal structure of 4-propyl-3-methyl-1-phenyl-2-pyrazolin-5-one thiosemicarbazone, C15H19N5OS. Z. Kristallogr. New. Cryst. Struct. 2015, 230, 81–82. [Google Scholar] [CrossRef]
- Parihar, S.; Pathan, S.; Jadeja, R.N.; Patel, A.; Gupta, V.K. Synthesis and Crystal Structure of an Oxovanadium(IV) Complex with a Pyrazolone Ligand and Its Use as a Heterogeneous Catalyst for the Oxidation of Styrene under Mild Conditions. Inorg. Chem. 2012, 51, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. Metal Complexes of New Bioactive Pyrazolone Phenylhydrazones; Crystal Structure of 4-Acetyl-3-Methyl-1-Phenyl-2-Pyrazoline-5-One Phenylhydrazone Ampp-Ph. Int. J. Mol. Sci. 2016, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEXII, SAINT, SADABS, Bruker AXS Inc.: Madison, WI, USA, 2007.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. ORTEP-3 for Windows—A Version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
| Compound | Empp-Ph | Pmpp-Dh |
|---|---|---|
| Formula | C19H18N6O5, C3H7NO | C20H20N6O5, C3H7NO |
| Crystal color and form | Orange/Block | Orange/Block |
| Formula weight | 483.49 | 497.51 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/c |
| a | 31.6116(9) (Å) | 32.2379(16) (Å) |
| b | 9.8297(3) (Å) | 10.0527(5) (Å) |
| c | 14.9770(5) (Å) | 15.2972(8) (Å) |
| α | 90o | 90o |
| β | 92.114(2)o | 103.420(2)o |
| γ | 90o | 90o |
| V | 4650.7(2) (Å3) | 4822.1(4) (Å3) |
| Z | 8 | 8 |
| D(calc) | 1.381 (Mg cm−1) | 1.371 (Mg cm−1) |
| F(000) | 2032 | 2096 |
| θ range | 2.2–28.3 (°) | 0.6–28.4 (°) |
| Crystal size | 0.21 × 0.26 × 0.45 (mm) | 0.21 × 0.41 × 0.60 (mm) |
| Reflections measured | 5753 | 11985 |
| Independent/observed | 0.018/4472 | 0.032/9541 |
| Mu(MoKa) | 0.103 (/mm) | 0.102 (/mm) |
| Temperature | 200 (K) | 200 (K) |
| Parameters | 348 | 695 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idemudia, O.G.; Hosten, E.C. Spectroscopy, Crystal and Molecular Structures of New 4-Acylpyrazolone Dinitrophenylhydrazones. Crystals 2016, 6, 127. https://doi.org/10.3390/cryst6100127
Idemudia OG, Hosten EC. Spectroscopy, Crystal and Molecular Structures of New 4-Acylpyrazolone Dinitrophenylhydrazones. Crystals. 2016; 6(10):127. https://doi.org/10.3390/cryst6100127
Chicago/Turabian StyleIdemudia, Omoruyi G., and Eric C. Hosten. 2016. "Spectroscopy, Crystal and Molecular Structures of New 4-Acylpyrazolone Dinitrophenylhydrazones" Crystals 6, no. 10: 127. https://doi.org/10.3390/cryst6100127