A Family of Nitrogen-Enriched Metal Organic Frameworks with CCS Potential
Abstract
:1. Introduction
2. Experimental
2.1. Materials Used
2.2. Methods
2.2.1. Single Crystal Synthesis
2.2.2. Single Crystal X-ray Diffraction (SCXRD)
2.2.3. CO2 Adsorption
3. Results and Discussion
3.1. 1D Chain Coordination Polymer: [Zn(pytz(hydrolyzed))2(OH2)2(OCOCH3)2]·H2O
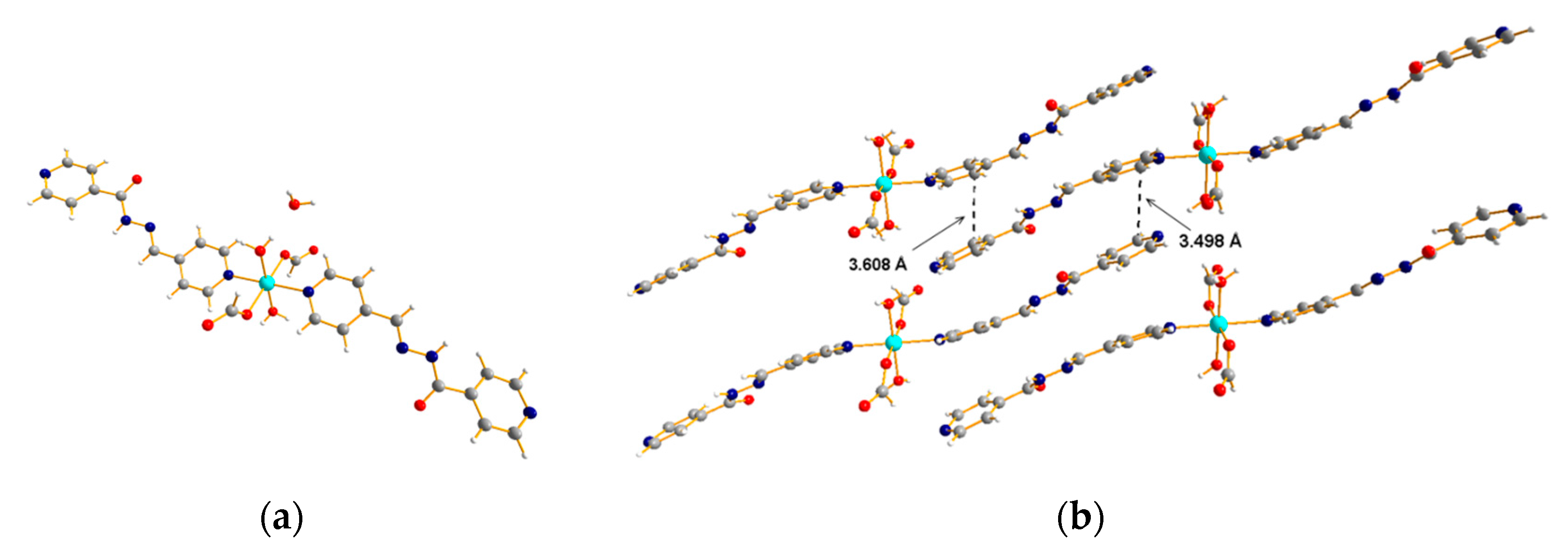
3.2. 1D Ladder Coordination Polymer: [Ni(pytz)1.5(NO3)]·nDCM (n = 2–3)
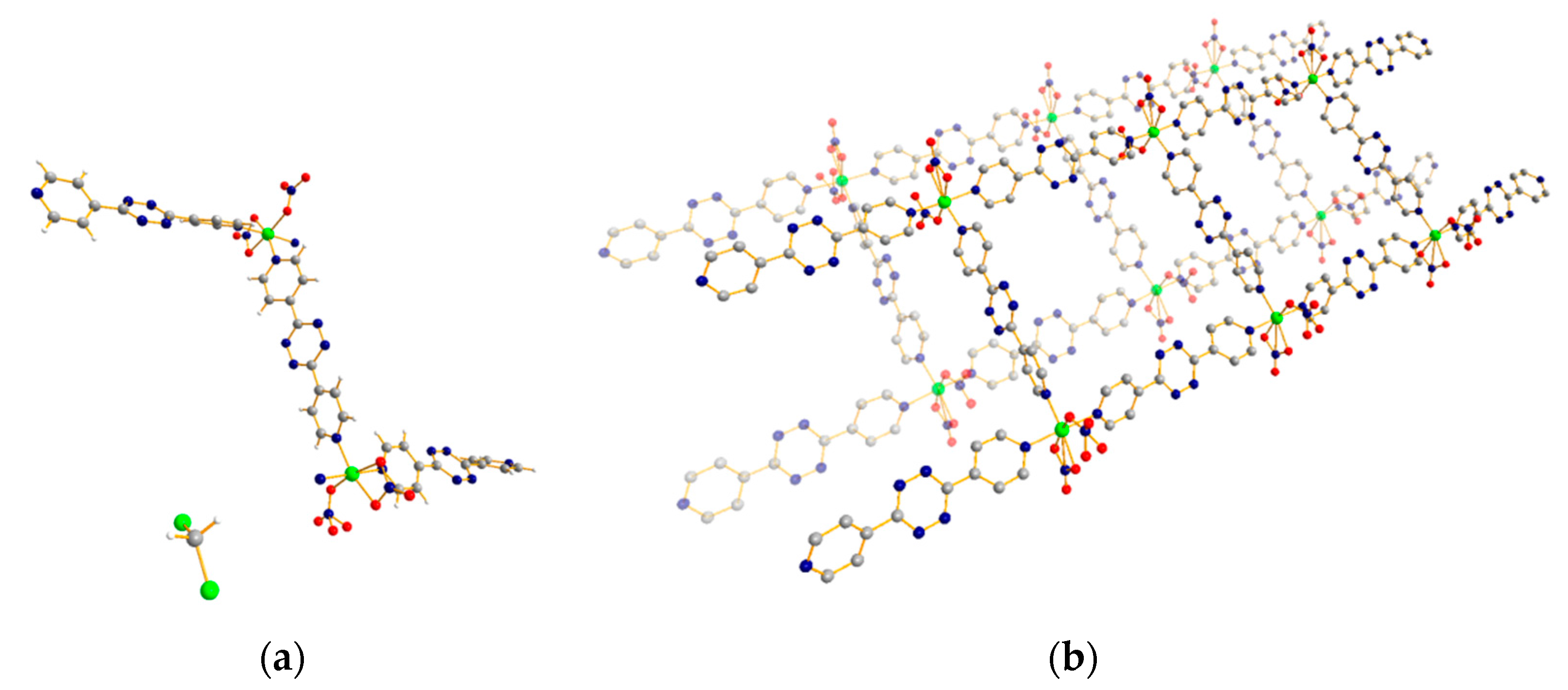
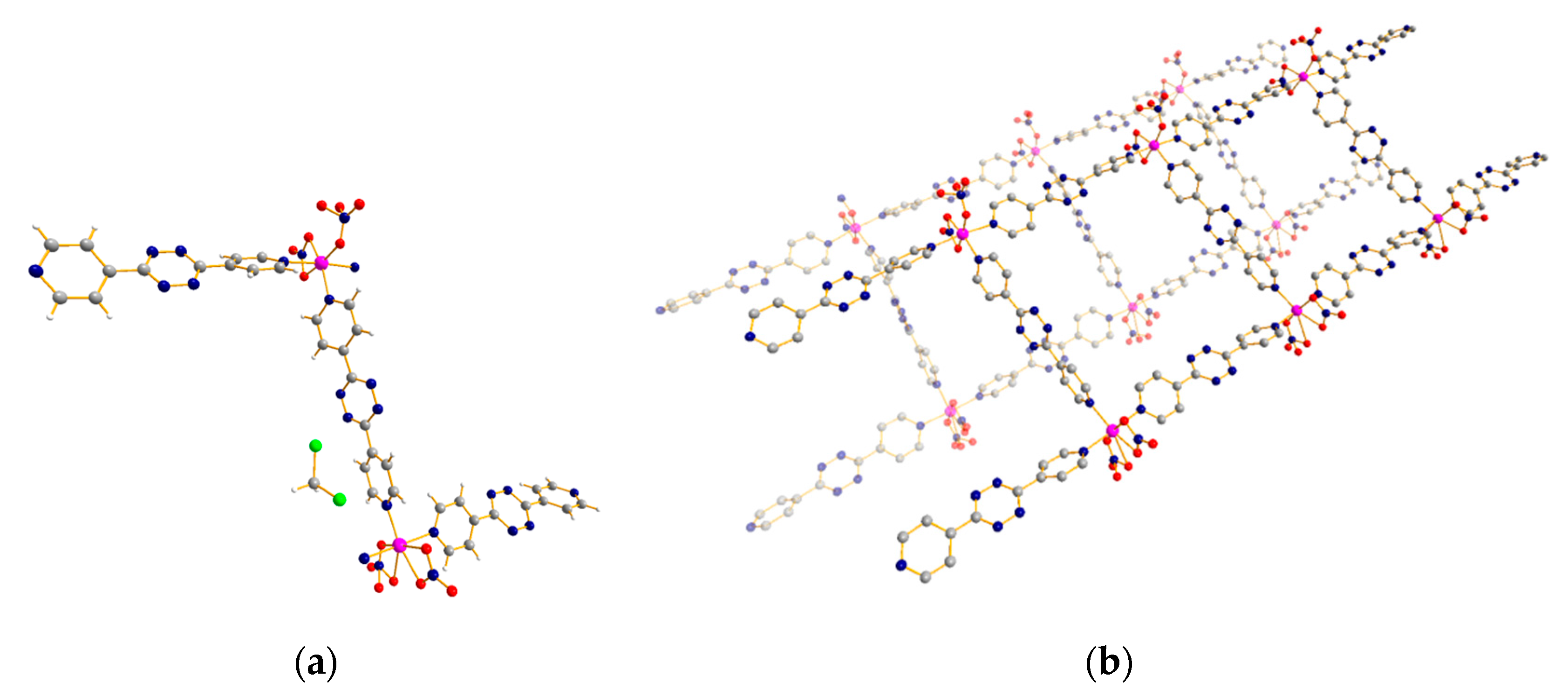
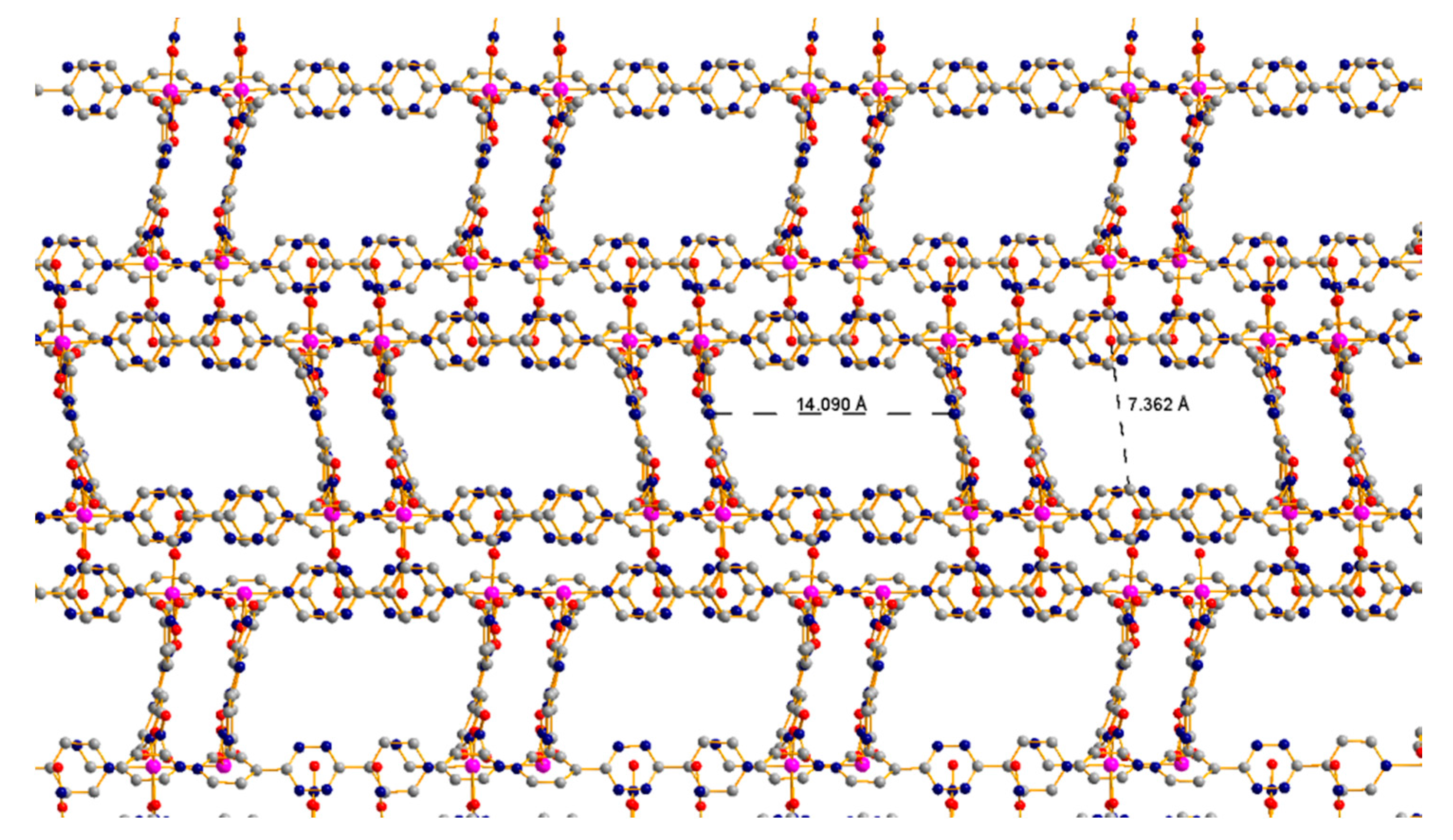
3.3. 1D Ladder Coordination Polymer: [Co2(pytz)3(NO3)4]·nDCM (n = 4–6)

3.4. CO2 Adsorption of Co-pytz
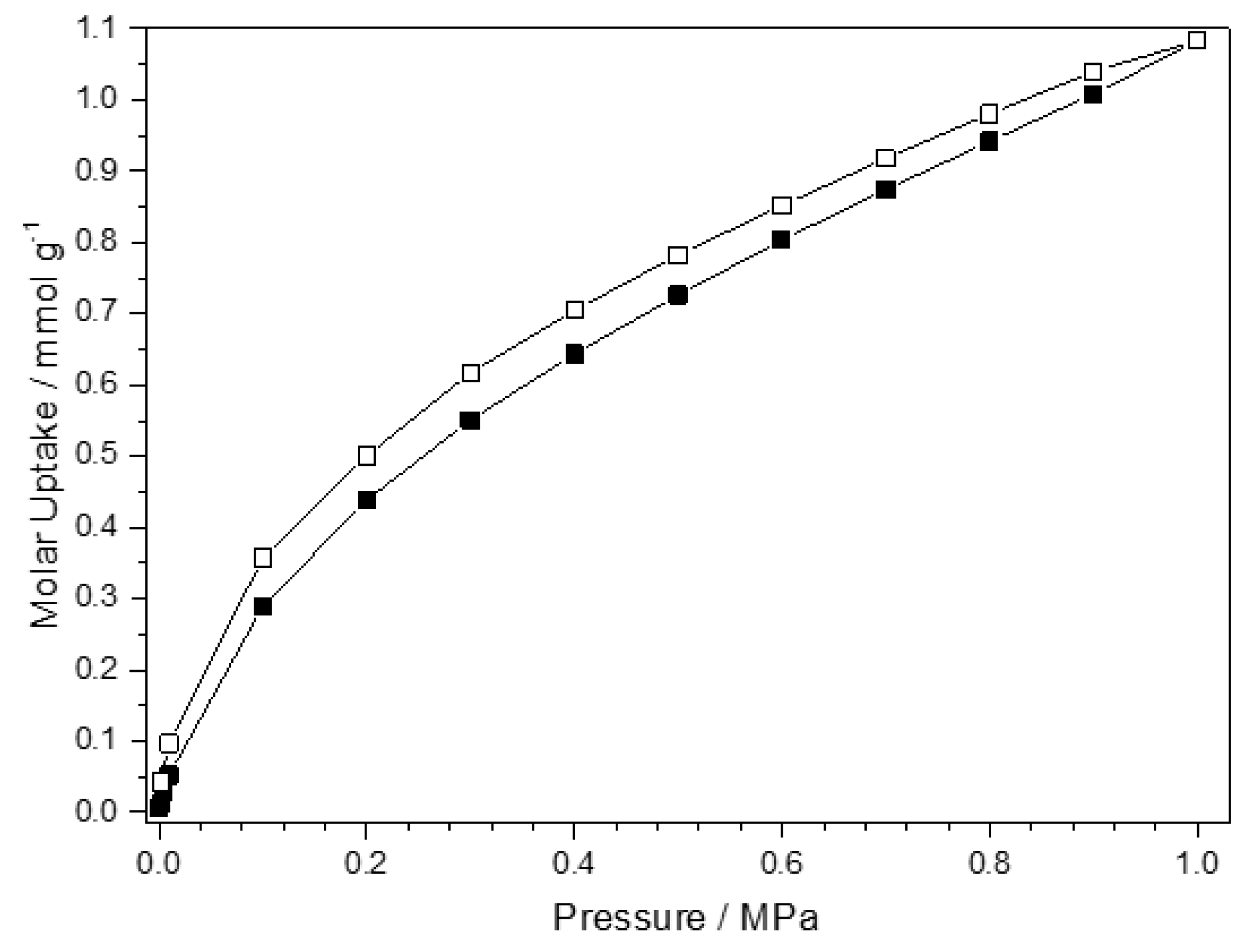
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2011, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273, 76–86. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2011, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 2011, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Intergovenmental Panel on Climate Change. Intergovernmental Panel on Climate Change (IPCC) Fifth Assessment Report: Climate Change; Intergovenmental Panel on Climate Change: Genève, Switzerland, 2014. [Google Scholar]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; McCarthy, M.C.; Sachdeva, S.; Lee, A.K.; Jeong, H.-K. Current status of metal-organic framework membranes for gas separations: Promises and challenges. Ind. Eng. Chem. Res. 2012, 51, 2179–2199. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Li, H.L.; Yaghi, O.M. Highly porous and stable metal-organic frameworks: Structure design and sorption properties. J. Am. Chem. Soc. 2000, 122, 1391–1397. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xiang, S.; Qian, G. Metal-organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Couck, S.; Denayer, J.F.; Baron, G.V.; Rémy, T.; Gascon, J.; Kapteijn, F. An amine-functionalized MIL-53 metal-organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar] [CrossRef] [PubMed]
- Vaidhyanathan, R.; Iremonger, S.S.; Shimizu, G.K.; Boyd, P.G.; Alavi, S.; Woo, T.K. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 2010, 330, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; Li, Y.; Yao, K.; Zhu, Y.; Deng, Z.; Yang, F.; Zhou, X.; Li, G.; Wu, H. Enhanced binding affinity, remarkable selectivity, and high capacity of CO2 by dual functionalization of a rht-type metal-organic framework. Angew. Chem. Int. Ed. 2012, 51, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.K.; Suresh, E. Spontaneous resolution to absolute chiral induction: Pseudo-kagomé type homochiral Zn(II)/Co(II) coordination polymers with achiral precursors. J. Am. Chem. Soc. 2013, 135, 15690–15693. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, Y.; Horike, S.; Sugimoto, M.; Inukai, M.; Fukushima, T.; Kitagawa, S. Pore design of two-dimensional coordination polymers toward selective adsorption. Inorg. Chem. 2013, 52, 3634–3642. [Google Scholar] [CrossRef] [PubMed]
- Hulvey, Z.; Furman, J.D.; Turner, S.A.; Tang, M.; Cheetham, A.K. Dimensionality trends in metal-organic frameworks containing perfluorinated or nonfluorinated benzenedicarboxylates. Cryst. Growth Des. 2010, 10, 2041–2043. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth Heinemann: Oxford, UK, 1998. [Google Scholar]
- Hunter, C.A.; Sanders, J.K. The nature of π-π. Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Felloni, M.; Blake, A.J.; Hubberstey, P.; Wilson, C.; Schroder, M. Hydrogen-bonding interactions between linear bipyridinium cations and nitrate anions. CrystEngComm 2002, 4, 483–495. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dooris, E.; McAnally, C.A.; Cussen, E.J.; Kennedy, A.R.; Fletcher, A.J. A Family of Nitrogen-Enriched Metal Organic Frameworks with CCS Potential. Crystals 2016, 6, 14. https://doi.org/10.3390/cryst6010014
Dooris E, McAnally CA, Cussen EJ, Kennedy AR, Fletcher AJ. A Family of Nitrogen-Enriched Metal Organic Frameworks with CCS Potential. Crystals. 2016; 6(1):14. https://doi.org/10.3390/cryst6010014
Chicago/Turabian StyleDooris, Emma, Craig A. McAnally, Edmund J. Cussen, Alan R. Kennedy, and Ashleigh J. Fletcher. 2016. "A Family of Nitrogen-Enriched Metal Organic Frameworks with CCS Potential" Crystals 6, no. 1: 14. https://doi.org/10.3390/cryst6010014
APA StyleDooris, E., McAnally, C. A., Cussen, E. J., Kennedy, A. R., & Fletcher, A. J. (2016). A Family of Nitrogen-Enriched Metal Organic Frameworks with CCS Potential. Crystals, 6(1), 14. https://doi.org/10.3390/cryst6010014







