A New 1D Chained Coordination Polymer: Synthesis, Crystal Structure, Antitumor Activity and Luminescent Property
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Description of {[Zn(L)2(4,4′-bipy)]·(H2O)}n (1)

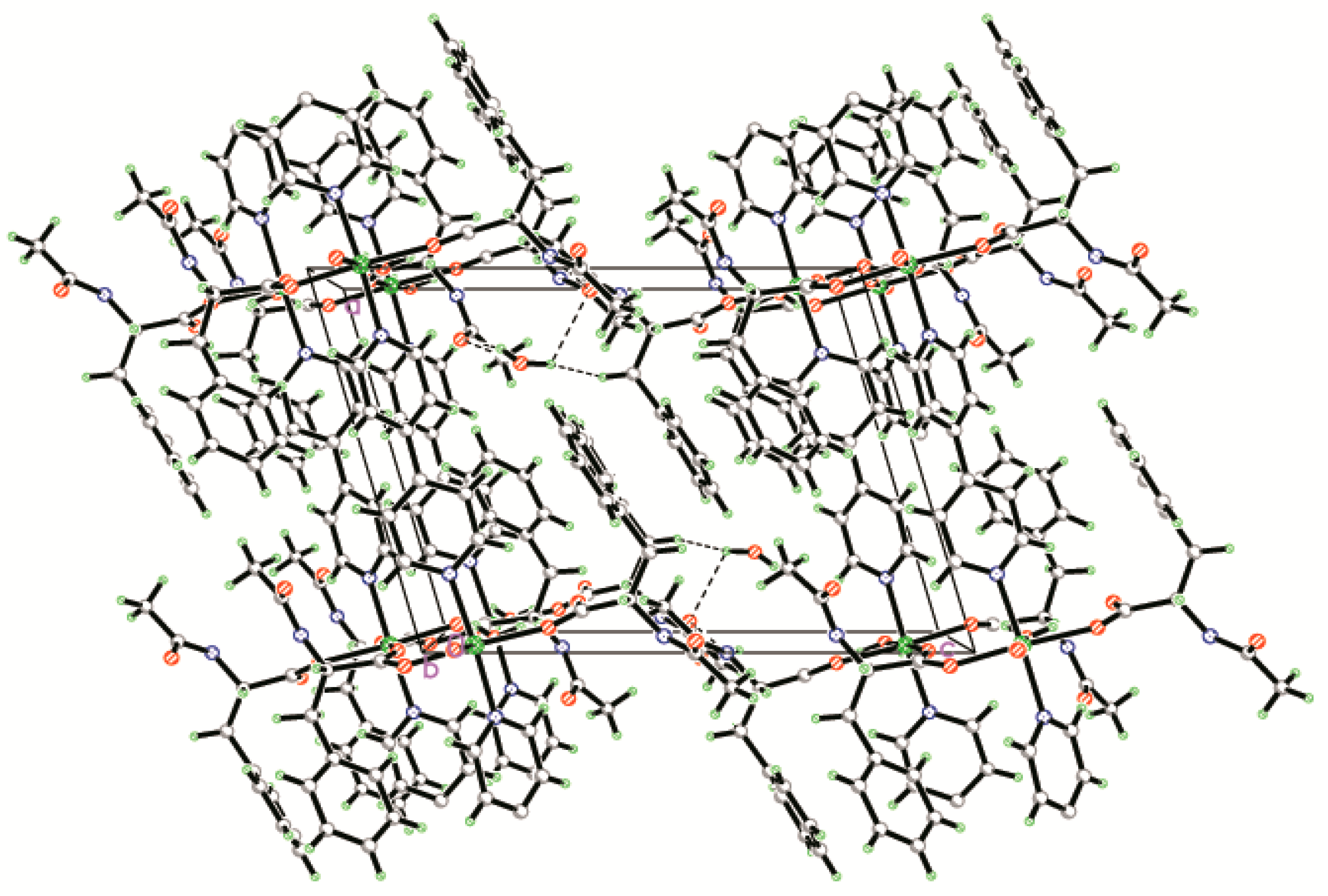
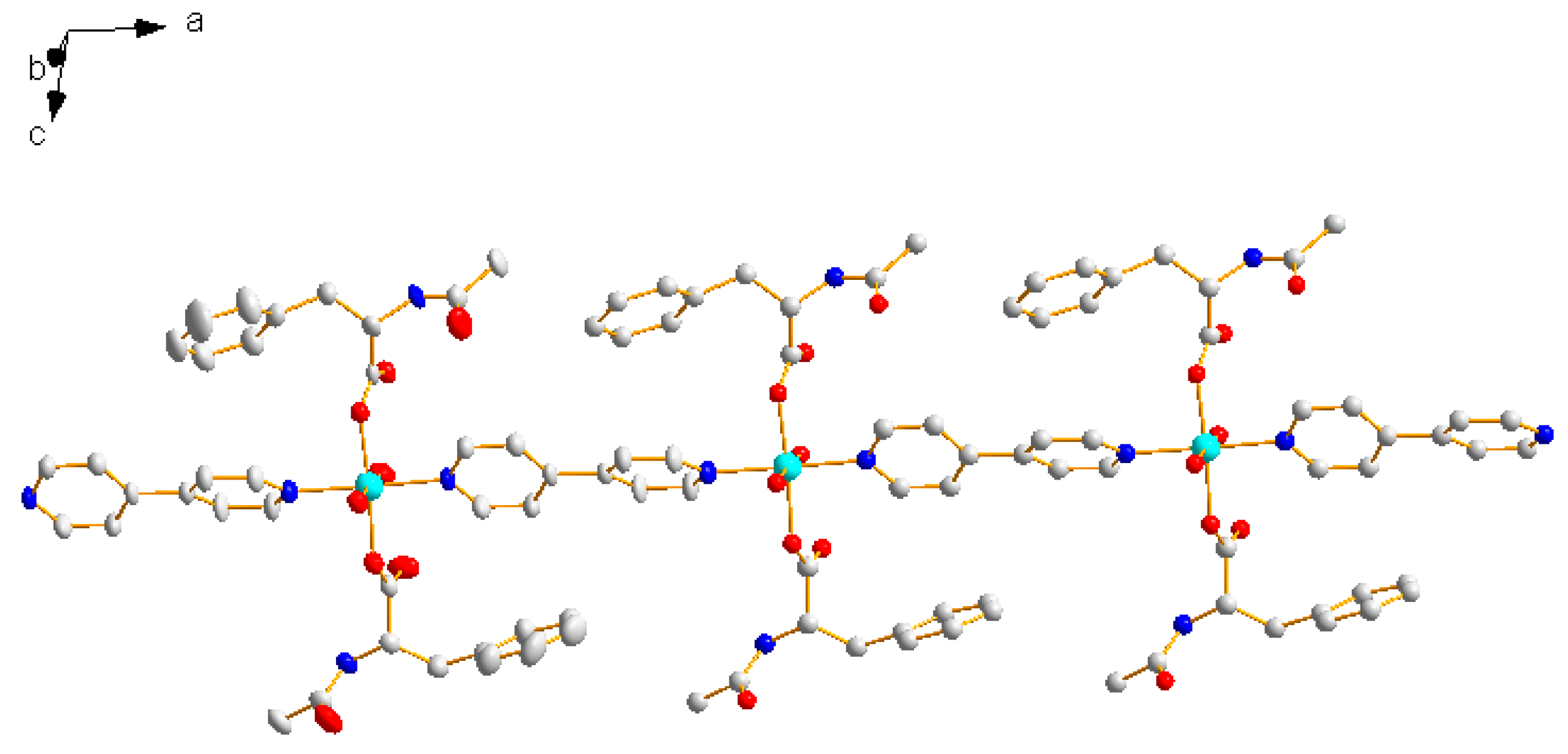
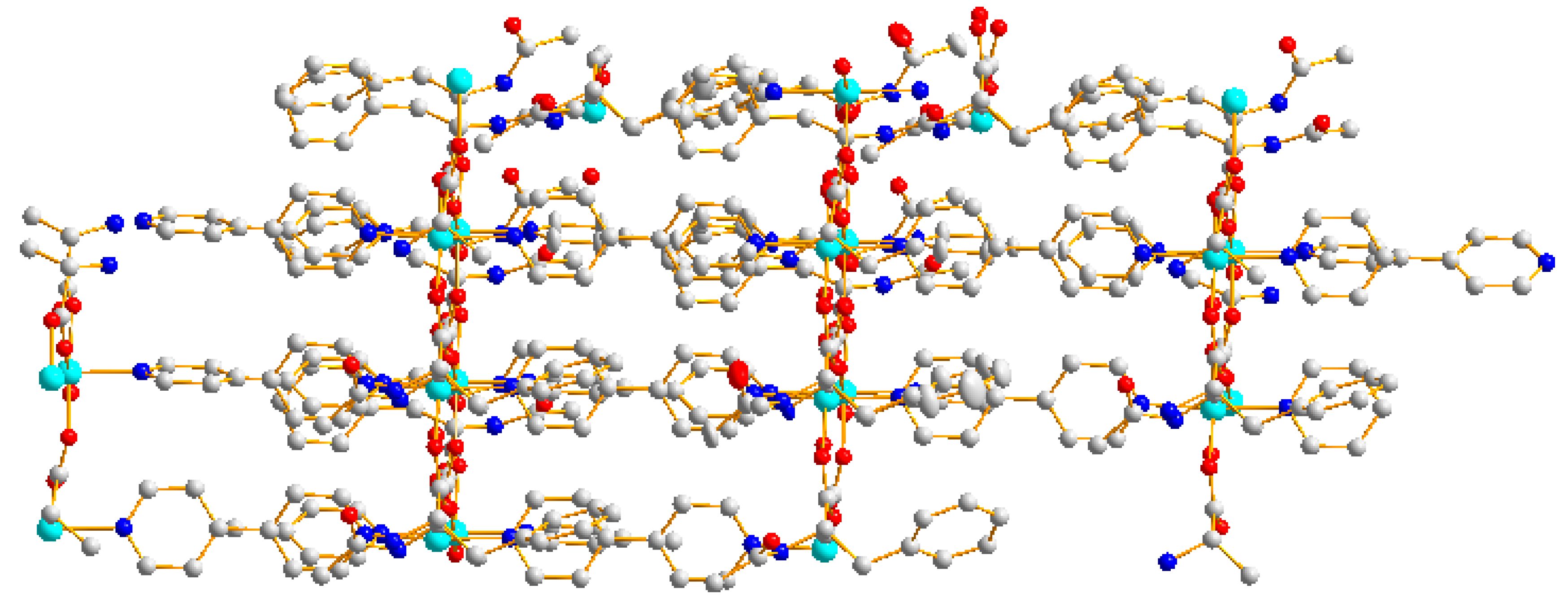
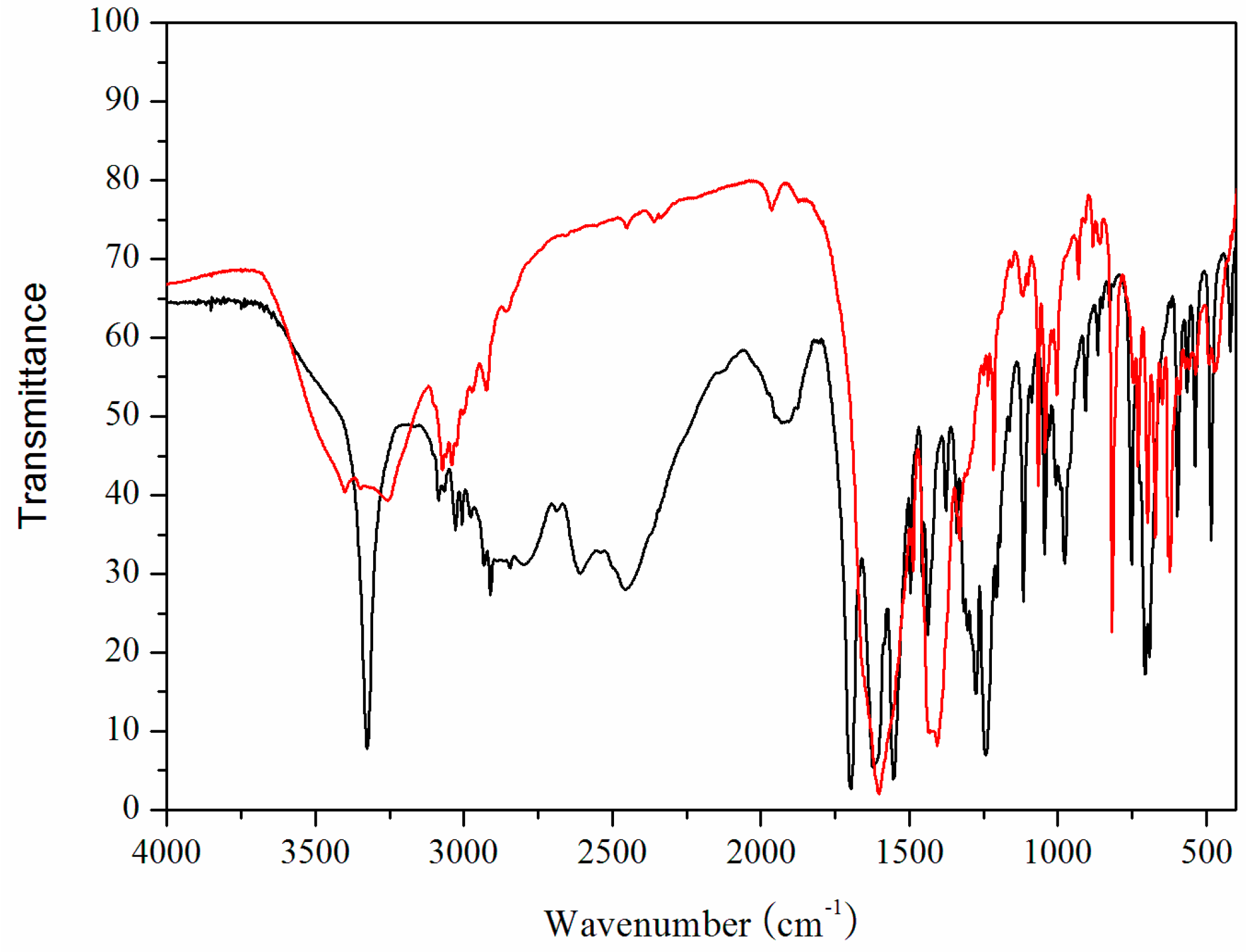
2.2. IR Spectra
2.3. AntitumorActivity
| Compound | IC50 (μg/mL) | ||
|---|---|---|---|
| SMMC-7721 | WiDr | A549 | |
| N-acetyl-l-phenylalanine | -- | 18 ± 0.3 | 28 ± 0.1 |
| Complex 1 | 12 ± 0.2 | 25 ± 0.9 | -- |
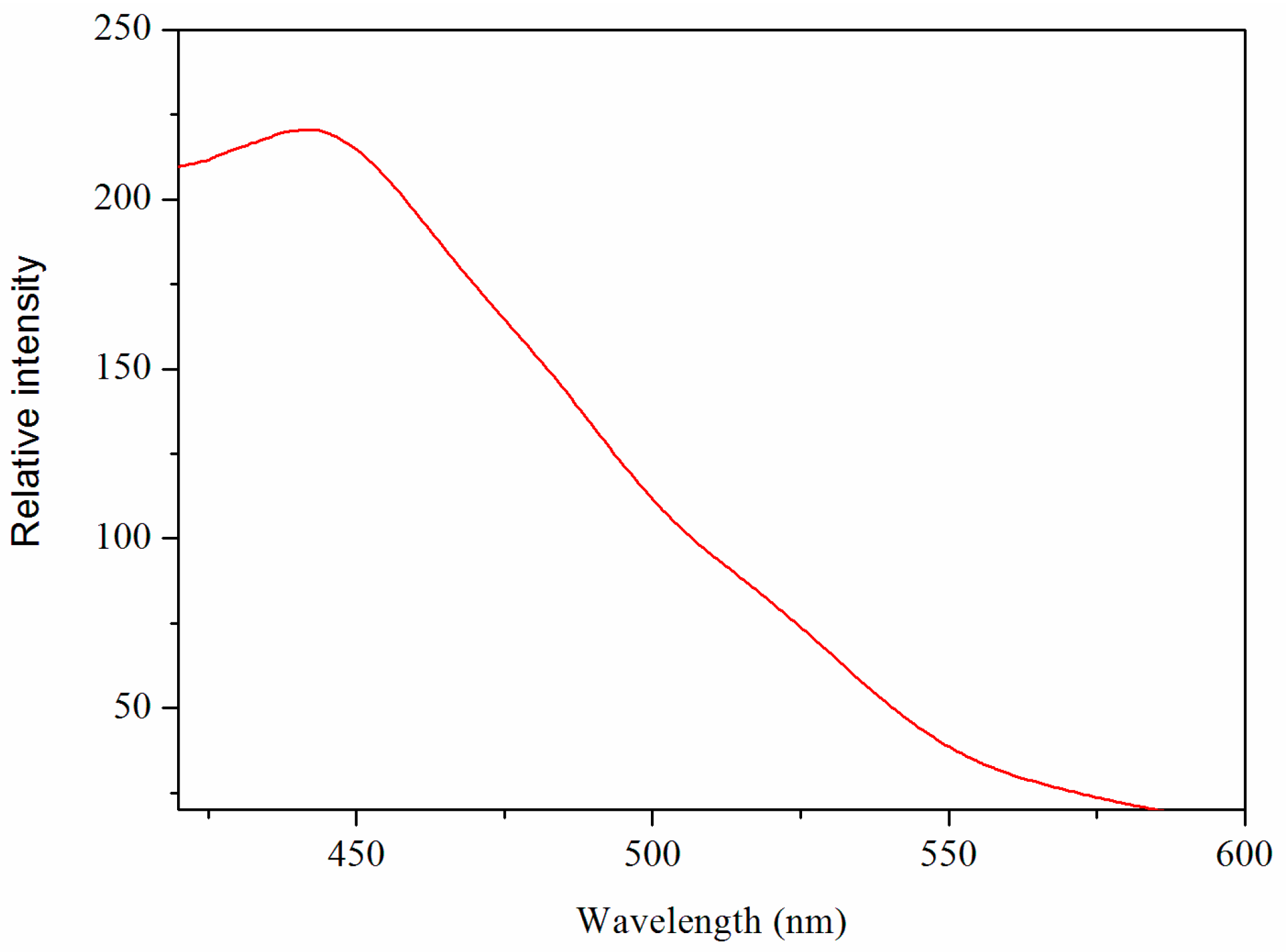
2.4. Luminescent Property
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis of {[Zn(L)2(4,4′-bipy)]·(H2O)}n(1)
3.3. Data Collection, Structural Determination, and Refinement
| Empirical Formula | C32H34N4O8Zn |
|---|---|
| Formula weight | 668.00 |
| Temperature/K | 293(2) |
| Crystal system | Monoclinic |
| Space group | P21 |
| a/Å | 11.421(2) |
| b/Å | 9.2213(17) |
| c/Å | 15.188(3) |
| β/° | 106.112(3) |
| Volume/Å3 | 1536.7(5) |
| Z | 2 |
| ρcalcmg/mm3 | 1.444 |
| μ/mm‑1 | 0.857 |
| S | 1.005 |
| F(000) | 696 |
| Index ranges | −15 ≤ h ≤ 13 |
| −9 ≤ k ≤ 12 | |
| −18 ≤ l ≤ 20 | |
| Reflections collected | 8997 |
| Reflections with I> 2σ(I) | 4217 |
| Absolute structure parameter | 0.026(16) |
| Independent reflections | 5465 [R(int) = 0.0362] |
| Data/restraints/parameters | 5465/1/411 |
| Goodness-of-fit on F2 | 1.005 |
| Final R indexes [≥2σ (I)] | R1 = 0.0439, wR2 = 0.1013 |
| Final R indexes [all data] | R1 = 0.0711, wR2 = 0.1156 |
| Largest diff. peak/hole/e Å−3 | 0.87/−0.88 |
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Yanai, N.; Kitayama, K.; Hijikata, Y.; Sato, H.; Matsuda, R.; Kubota, Y.; Takata, M.; Mizuno, M.; Uemura, T.; Kitagawa, S. Gas detection by structural variations of fluorescent guest molecules in a flexible porous coordination polymer. Nat. Mater. 2011, 10, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Su, Y.C.; Wang, J.J. Synthesis and characterization of a new 3D pillared bilayer Cd(II) coordination polymer based on 6,6′-dinitro-2,2′,4,4′-biphenyltetracarboxylic acid. Chin. J. Struct. Chem. 2015, 34, 1385–1390. [Google Scholar]
- Lee, G.M.; Lee, S.W. Silver-tetrapyridyl coordination polymers: [Ag2(L)](NO3)2(H2O)2, [Ag(L)](PF6), and [Ag2I2L](CH2Cl2) {L = 1,1,2,2-tetrakis(4-(pyridin-3-yl)phenyl)ethene}. Polyhedron 2015, 87, 338–348. [Google Scholar] [CrossRef]
- Wen, Y.H.; Dou, L.T.; Yao, K.; Xu, G.F. Syntheses, structures, and fluorescent properties of four Co(II) coordination polymers based on 5-hydroxyisophthalic acid and structurally related bis(benzimidazole) ligands. J. Coord. Chem. 2015, 68, 38–54. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, structural characterization, and antitumor activity of a Ca(II) coordination polymer based on 1,6-naphthalenedisulfonate and 4,4′-bipyridyl. Materials 2013, 6, 3547–3555. [Google Scholar] [CrossRef]
- Yue, Y.M.; Sun, J.W.; Yan, P.F.; Li, G.M. Single molecule magnet of flexible Salen-type dysprosium coordination polymer with 1D ionic chain structure. Inorg. Chem. Commun. 2015, 51, 42–45. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, W.J.; Hua, Z.J.; Wang, G.N.; Uvdala, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Samsonenko, D.G.; Vostrikova, K.E. Heterobimetallic coordination polymers involving 3d metal complexes and heavier transition metals cyanometallates. J. Solid State Chem. 2015, 224, 107–114. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhang, X.; Shen, Y.C.; Yao, Y.G. A novel luminescent zinc(II) coordination polymer with 2D→3D polythreaded motif. Chin. J. Struct. Chem. 2015, 34, 1399–1404. [Google Scholar]
- Lu, W.G.; Jiang, L.; Feng, X.L.; Lu, T.B. Three 3D coordination polymers constructed by Cd(II) and Zn(II) with imidazole-4,5-dicarboxylate and 4,4′-bipyridyl building blocks. Cryst. Growth Des. 2006, 6, 564–571. [Google Scholar] [CrossRef]
- Li, F.A.; Zhao, X.J.; Yang, W.C.; Li, S.Q. A Co(II) coordinated polymer with2-foldinterpenetration 3D architecture based on 5-(imidazol-1-ylmethyl)isophthalate: Hydrothermal synthesis, crystal structure and properties. Chin. J. Struct. Chem. 2015, 34, 1281–1287. [Google Scholar]
- Wang, R.M.; Zhang, M.H.; Liu, X.Q.; Liu, X.B.; Wang, W.; Sun, D.F. Synthesis, crystal structure and luminescence of a trinuclear magnesium coordination polymer based on a triangle flexible carboxylic ligand. Chin. J. Struct. Chem. 2015, 34, 1288–1294. [Google Scholar]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of Ca(II) coordination polymer based on 1,5-naphthalenedisulfonate. J. Inorg. Organomet. Polym. 2013, 23, 1354–1357. [Google Scholar] [CrossRef]
- Asif, S.K.; Brahma, S.; Dhamija, A.; Rath, S.P. Building-up novel coordination polymer with Zn(II) porphyrin dimer: Synthesis, structures, surface morphology and effect of axial ligands. J. Chem. Sci. 2014, 126, 1451–1461. [Google Scholar]
- Tian, F.; Wang, H.D.; He, M.Y.; Chen, Q.; Chen, S.C. Anion effect on the structural diversity of 1-D and 2-D zinc(II) coordination polymers with aflexible fluorinated bis(imidazole) ligand. Z. Naturforsch. 2014, 69, 878–884. [Google Scholar] [CrossRef]
- Smith, T.M.; Symester, D.; Perrin, K.A.; Hudson, B.S.; Zubieta, J. Metal-organodiphosphonate chemistry: Hydrothermal syntheses and structures of Zn(II) and Cd(II) coordination polymers with xylyldiphosphonate ligands. Inorg. Chim. Acta 2014, 411, 172–187. [Google Scholar] [CrossRef]
- Wang, J.J.; Hou, X.Y.; Gao, L.J.; Zhang, M.L.; Ren, Y.X.; Fu, F. Hydrothermal synthesis, crystal structure and luminescence of a2D bilayer Zn(II) coordination polymer based on terphenyl-2,2′,4,4′-tetracarboxylic acid. Chin. J. Inorg. Chem. 2014, 30, 379–383. [Google Scholar]
- Mishra, A.; Kim, H.; Lee, H.C.; Min, J.W.; Lee, M.H.; Chi, K.W. Cadmium(II) and zinc(II) coordination polymers built with an ethynyl backbone containing an unsymmterical amide ligand: Syntheses, crystal structures, and photoluminescent properties. Inorg. Chim. Acta 2013, 405, 77–82. [Google Scholar] [CrossRef]
- Larionov, S.V.; Kokina, T.E.; Agafontsev, A.M.; Marenin, K.S.; Glinskaya, L.A.; Korol’kov, I.V.; Rakhmanova, M.I.; Uskov, E.M.; Plyusnin, P.E.; Tkachev, A.V. Synthesis and properties of ZnII and CdII complexes with chiral N-derivatives of aminoacetic acid based on natural monoterpenes (+)-3-carene and (−)-α-pinene. Crystal structure of coordination polymer [Zn(HL)Cl·2H2O]n. Russ. Chem. Bull. 2011, 60, 2555–2563. [Google Scholar] [CrossRef]
- Lin, J.G.; Wang, F.M.; Xu, Y.Y.; Lu, C.S.; Meng, Q.J.; Wu, P.H. Organic-inorganic hybrid coordination polymers based on tetratopic pyridyl-bridging ligand: Syntheses, structures, and luminescent properties. Inorg. Chim. Acta 2009, 362, 5219–5223. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, S.J.; Kim, C.; Kim, S.J.; Kim, Y.M. Novel infinite hexanuclear zinc coordination polymer with a flexible bipyridyl ligand and its catalytic activity. Inorg. Chem. Commun. 2005, 8, 692–696. [Google Scholar] [CrossRef]
- Liu, G.X.; Huang, Y.Q.; Chu, Q.; Okamura, T.; Sun, W.Y.; Liang, H.; Ueyama, N. Effect of N-donor ancillary ligands on supramolecular architectures of a series of zinc(II) and cadmium(II) complexes with flexible tricarboxylate. Cryst. Growth Des. 2008, 8, 3233–3245. [Google Scholar] [CrossRef]
- Gong, Y.Q.; Mi, T.Q.; Jiang, F.L. Synthese, crystal structure and photoluminescence of a Zn(II) coordination polymers derived from 1, 1′-biphenyl-2, 2′, 6, 6′-tetracarboxylic acid. Chin. J. Struct. Chem. 2015, 34, 1087–1091. [Google Scholar]
- Hao, J.M.; Zhang, H.; Li, G.Y.; Cui, G.H. Two 1-D zinc(II) coordination polymers based on flexible bis(2-methylbenzimidazole) and rigid dicarboxylate co-ligands. J. Coord. Chem. 2014, 67, 1992–2003. [Google Scholar] [CrossRef]
- Li, D.X.; Ren, Z.G.; Young, D.J.; Lang, J.P. Synthesis of two coordination polymer photocatalysts and significant enhancement of their catalytic photodegradation activity by doping with Co2+ ions. Eur. J. Inorg. Chem. 2015, 2015, 1981–1988. [Google Scholar] [CrossRef]
- Liu, S.J.; Xie, C.C.; Jia, J.M.; Zhao, J.P.; Han, S.D.; Cui, Y.; Li, Y.; Bu, X.H. Low-dimensional carboxylate-bridged GdIII complexes for magnetic refrigeration. Chem. Asian J. 2014, 9, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, C.; Kinnibrugh, T.L.; Xu, H.; Lindline, J.; Timofeeva, T.; Wei, Q. Synthesis offramework isomer MOFs containing zinc and 4-tetrazolyl benzenecarboxylic acid via a structure directing solvothermal approach. Crystals 2015, 5, 193–205. [Google Scholar] [CrossRef]
- Tai, X.S.; Liu, L.L.; Yin, J. Synthesis, crystal structure of tetra-nuclear macrocyclic Cu(II) complex material and its application as catalysts for A3 coupling reaction. J. Inorg. Organomet. Polym. 2014, 24, 1014–1020. [Google Scholar] [CrossRef]
- Suresh, P.; Prabusankar, G. Cationic zinc(II) dimers and one dimensional coordination polymer from ionic carboxylic acid. J. Chem. Sci. 2014, 126, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Dolatyari, L.; Seddigi, P.; Ramazani, A.; Amiri, M.G.; Morsali, A. A new Zn(II) complex of unusual unidentate coordination of 4,4′-bipyridine, a new precursor for the preparation of zinc(II) oxide nanoparticles. J. Struct. Chem. 2013, 54, 571–576. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tai, X.S.; Zhang, Y.P.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of adinuclear calcium complex based on 1,5-naphthalenedisulfonate and 2,2′-bipyridine ligands. Res. Chem. Intermed. 2015, 41, 4339–4347. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, X.-S.; You, H.-Y. A New 1D Chained Coordination Polymer: Synthesis, Crystal Structure, Antitumor Activity and Luminescent Property. Crystals 2015, 5, 608-616. https://doi.org/10.3390/cryst5040608
Tai X-S, You H-Y. A New 1D Chained Coordination Polymer: Synthesis, Crystal Structure, Antitumor Activity and Luminescent Property. Crystals. 2015; 5(4):608-616. https://doi.org/10.3390/cryst5040608
Chicago/Turabian StyleTai, Xi-Shi, and Hai-Ying You. 2015. "A New 1D Chained Coordination Polymer: Synthesis, Crystal Structure, Antitumor Activity and Luminescent Property" Crystals 5, no. 4: 608-616. https://doi.org/10.3390/cryst5040608






