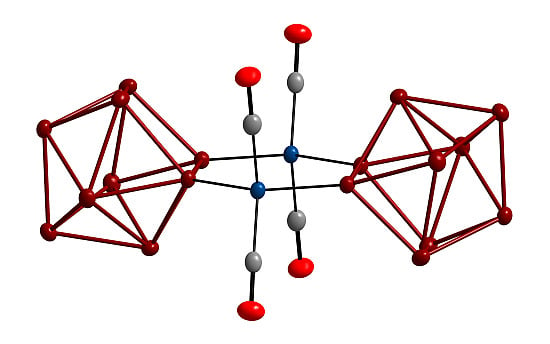
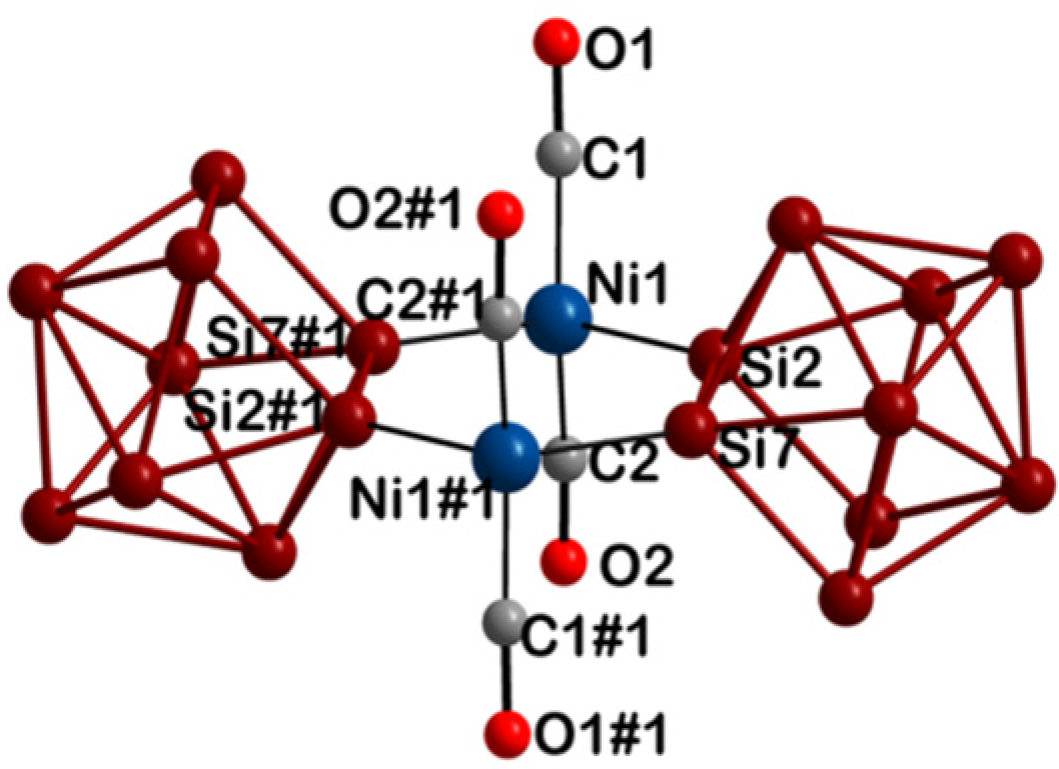
The First Chelate-Free Crystal Structure of a Silicide Transition Metal Complex [K0.28Rb7.72Si9Ni(CO)2]2·16NH3
Abstract
:1. Introduction
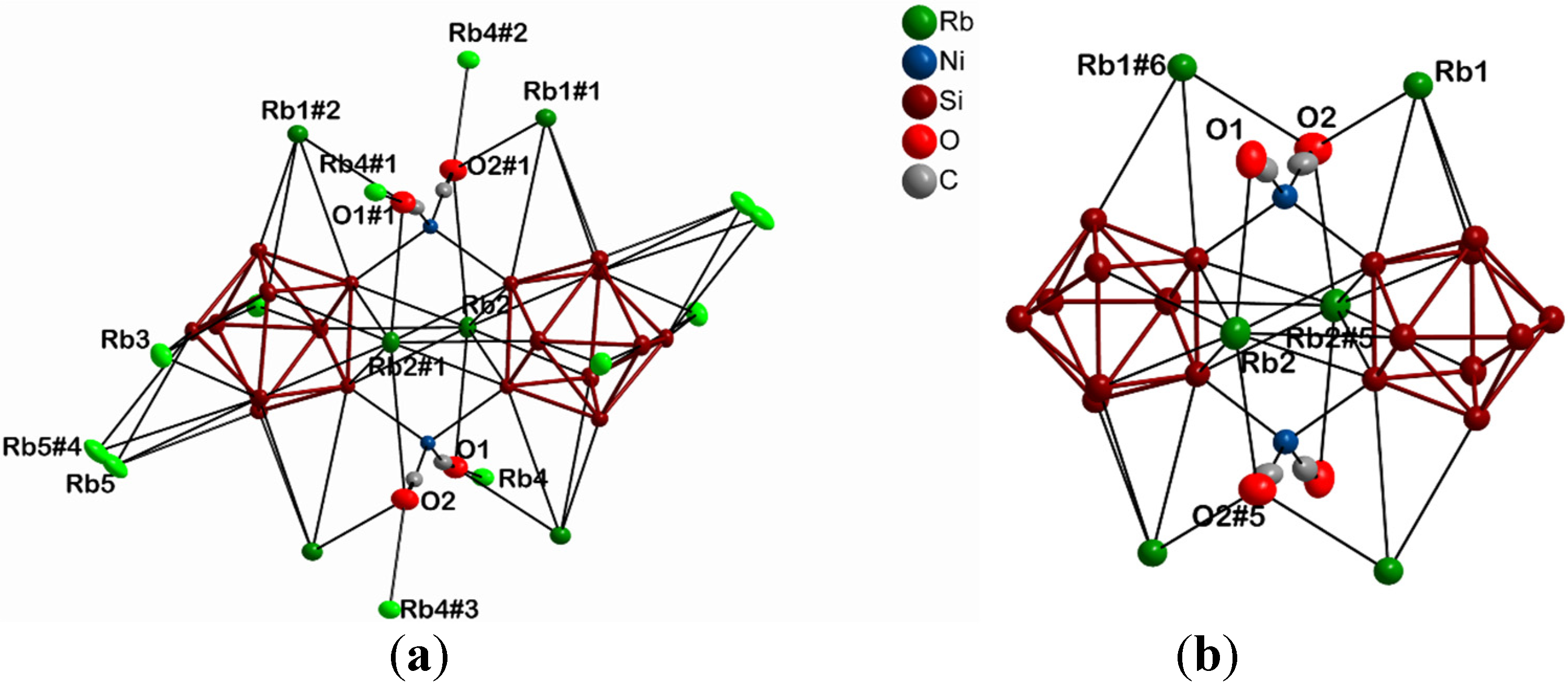
2. Results and Discussion
| Item | Numerical Value |
|---|---|
| CCDC No. | 1043014 |
| Chemical formula | [K0.28Rb7.72Si9Ni(CO)2]2·16NH3 |
| Mr (g·mol−1) | 1678.63 |
| Temperature (K) | 123(2) |
| Crystal system | monoclinic |
| Space group | C2/c |
| a (Å) | 30.669(6) |
| b (Å) | 9.919(2) |
| c (Å) | 19.894(4) |
| α (°) | 90 |
| β (°) | 110.18(3) |
| γ (°) | 90 |
| V (Å3) | 5680(2) |
| Z | 4 |
| ρcalc (g/cm3) | 1.963 |
| μ (mm−1) | 7.676 |
| F(000) (e) | 3260.0 |
| Crystal size (mm3) | 0.18 × 0.12 × 0.1 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection (°) | 4.636 to 55.28 |
| Index ranges | −39 ≤ h ≤ 39, −12 ≤ k ≤ 12, −25 ≤ l ≤ 25 |
| Reflections collected | 46,537 |
| Independent reflections | 6534 [Rint = 0.0729, Rsigma = 0.0523] |
| Data/restraints/parameters | 6534/6/253 |
| Goodness-of-fit on F2 | 0.954 |
| R1, wR2 (I ≥ 2σ (I)) | R1 = 0.0349, wR2 = 0.0667 |
| R1, wR2 (all data) | R1 = 0.0568, wR2 = 0.0711 |
| Δρmax, Δρmin (e Å−3) | 0.90/−0.46 |
| 1 | 2 | ||||
|---|---|---|---|---|---|
| Atom 1 | Atom 2 | Distance (Å) | Atom 1 | Atom 2 | Distance (Å) |
| Ni1 | Si2 | 2.3052(12) | Ni1 | Si2 | 2.3033(17) |
| Ni1 | Si7 | 2.3044(13) | Ni1 | Si7 | 2.3001(18) |
| Ni1 | C1 | 1.738(4) | Ni1 | C1 | 1.743(8) |
| Ni1 | C2 | 1.746(4) | Ni1 | C2 | 1.729(6) |
| C1 | O1 | 1.167(5) | C1 | O1 | 1.169(8) |
| C2 | O2 | 1.159(5) | C2 | O2 | 1.183(8) |

| Atom 1 | Atom 2 | Distance (Å) | Atom 1 | Atom 2 | Distance (Å) |
|---|---|---|---|---|---|
| Rb1 | Si7 | 3.9366(14) | Rb4 | N2 | 3.300(4) |
| Si9#7 | 3.7493(15) | N3 | 3.007(4) | ||
| Si2#7 | 3.8338(14) | O1 | 2.832(3) | ||
| Si4 | 3.6312(14) | O2#3 | 2.855(4) | ||
| Si7 | 3.9366(14) | K4 | O1 | 2.70(3) | |
| Si8 | 4.0366(15) | O1#8 | 2.99(3) | ||
| N2 | 3.116(4) | O2#3 | 2.79(2) | ||
| N1 | 3.322(4) | O2#11 | 2.98(2) | ||
| O2 | 3.853(4) | N2 | 3.04(3) | ||
| O1#3 | 3.241(4) | N2#8 | 3.58(2) | ||
| Rb2 | Si7#1 | 3.5962(15) | N3 | 2.776(17) | |
| Si6#1 | 3.5971(15) | N3#8 | 3.246(19) | ||
| Si6 | 3.8961(13) | Rb5 * | Si5 | 3.619(4) | |
| Si3 | 3.4674(13) | Si5#4 | 3.639(4) | ||
| Si9#1 | 3.5273(17) | Si4 | 4.049(3) | ||
| Si8#1 | 3.8988(17) | Si8#4 | 3.744(4) | ||
| Si7#1 | 3.5961(15) | Si8 | 3.988(3) | ||
| N3 | 3.406(4) | N7 | 3.234(6) | ||
| N3#8 | 3.367(4) | N7#4 | 3.222(6) | ||
| N4#8 | 3.157(4) | N1 | 3.258(5) | ||
| N4 | 3.090(4) | ||||
| O1 | 3.263(4) | ||||
| O2#1 | 3.702(4) | ||||
| Rb3 | Si5 | 3.7988(13) | |||
| Si1#10 | 3.7609(14) | ||||
| Si8 | 3.6490(13) | ||||
| Si3#10 | 4.0429(18) | ||||
| Si9 | 3.7252(14) | ||||
| N5 | 2.935(5) | ||||
| N7#9 | 3.227(5) | ||||
| N6 | 3.000(4) |
3. Experimental Section
4. Conclusions
Supplementary Information
Author Contributions
Conflicts of Interest
References
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl ions, cage compounds, and intermetalloid clusters of group 14 and group 15 elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670. [Google Scholar] [CrossRef] [PubMed]
- Scharfe, S.; Fässler, T.F. Polyhedral nine-atom clusters of tetrel elements and intermetalloid derivatives. Phil. Trans. R. Soc. A 2010, 368, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, S.; Korber, N. 1.09-Zintl anions. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 251–267. [Google Scholar]
- Gärtner, S.; Korber, N. Polyanions of group 14 and group 15 elements in alkali and alkaline earth metal solid state compounds and solvate structures. In Zintl Ions Principles and Recent Developments; Fässler, T.F., Ed.; Springer-Verlag: Berlin, Germany; Heidelberg, Germany, 2011; Volume 140, pp. 25–56. [Google Scholar]
- Ugrinov, A.; Sevov, S.C. Ge9=Ge9=Ge9=Ge98−: A linear tetramer of nine-atom germanium clusters, a nanorod. Inorg. Chem. 2003, 42, 5789–5791. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.W.; Sevov, S.C. Functionalization of nine-atom deltahedral zintl ions with organic substituents: Detailed studies of the reactions. J. Am. Chem. Soc. 2009, 131, 9026–9037. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.J.; Sevov, S.C. Tin-based organo-zintl ions: Alkylation and alkenylation of Sn94−. Inorg. Chem. 2008, 47, 6009–6013. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.M.; Sevov, S.C. Deltahedral germanium clusters: Insertion of transition-metal atoms and addition of organometallic fragments. J. Am. Chem. Soc. 2006, 128, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.; Denning, M.S.; Kays, D.L.; Goicoechea, J.M. Synthesis and isolation of [Fe@Ge10]3−: A pentagonal prismatic zintl ion cage encapsulating an interstitial iron atom. J. Am. Chem. Soc. 2009, 131, 2802–2803. [Google Scholar] [CrossRef] [PubMed]
- Scharfe, S.; Fässler, T.F.; Stegmaier, S.; Hoffmann, S.D.; Ruhland, K. [Cu@Sn9]3− and [Cu@Pb9]3−: Intermetalloid clusters with endohedral Cu atoms in spherical environments. Chem. Eur. J. 2008, 14, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.M.; Sevov, S.C. Ligand-free deltahedral clusters of silicon in solution: Synthesis, structure, and electrochemistry of Si92−. Inorg. Chem. 2005, 44, 2654–2658. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, J.M.; Sevov, S.C. Naked deltahedral silicon clusters in solution: Synthesis and characterization of Si93− and Si52−. J. Am. Chem. Soc. 2004, 126, 6860–6861. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Suchentrunk, C.; Korber, N. Dissolving silicides: Syntheses and crystal structures of new ammoniates containing Si52− and Si94− polyanions and the role of ammonia of crystallisation. Z. Naturforsch. B 2010, 65, 1059–1065. [Google Scholar] [CrossRef]
- Joseph, S.; Suchentrunk, C.; Kraus, F.; Korber, N. Si94− anions in solution-structures of the solvates Rb4Si9·4.75NH3 and [Rb(18-crown-6)]Rb3Si9·4NH3, and chemical bonding in Si94−. Eur. J. Inorg. Chem. 2009, 2009, 4641–4647. [Google Scholar] [CrossRef]
- Goicoechea, J.M.; Sevov, S.C. Organozinc derivatives of deltahedral zintl ions: Synthesis and characterization of closo-[E9Zn(C6H5)]3− (E = Si, Ge, Sn, Pb). Organometallics 2006, 25, 4530–4536. [Google Scholar] [CrossRef]
- Waibel, M.; Kraus, F.; Scharfe, S.; Wahl, B.; Fässler, T.F. (MesCu)2(η3-Si4)4−: A mesitylcopper-stabilized tetrasilicide tetraanion. Angew. Chem. Int. Ed. 2010, 49, 6611–6615. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Hamberger, M.; Mutzbauer, F.; Hartl, O.; Meier, M.; Korber, N. Chemistry with bare silicon clusters in solution: A transition-metal complex of a polysilicide anion. Angew. Chem. Int. Ed. 2009, 48, 8770–8772. [Google Scholar] [CrossRef] [PubMed]
- Queneau, V.; Todorov, E.; Sevov, S.C. Synthesis and structure of isolated silicon clusters of nine atoms. J. Am. Chem. Soc. 1998, 120, 3263–3264. [Google Scholar] [CrossRef]
- Hoch, C.; Wendorff, M.; Rohr, C. Synthesis and crystal structure of the tetrelides A12M17 (A=Na, K, Rb, Cs; M=Si, Ge, Sn) and A4Pb9 (A=K, Rb). J. Alloys Compd. 2003, 361, 206–221. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gärtner, S.; Hamberger, M.; Korber, N. The First Chelate-Free Crystal Structure of a Silicide Transition Metal Complex [K0.28Rb7.72Si9Ni(CO)2]2·16NH3. Crystals 2015, 5, 275-282. https://doi.org/10.3390/cryst5030275
Gärtner S, Hamberger M, Korber N. The First Chelate-Free Crystal Structure of a Silicide Transition Metal Complex [K0.28Rb7.72Si9Ni(CO)2]2·16NH3. Crystals. 2015; 5(3):275-282. https://doi.org/10.3390/cryst5030275
Chicago/Turabian StyleGärtner, Stefanie, Markus Hamberger, and Nikolaus Korber. 2015. "The First Chelate-Free Crystal Structure of a Silicide Transition Metal Complex [K0.28Rb7.72Si9Ni(CO)2]2·16NH3" Crystals 5, no. 3: 275-282. https://doi.org/10.3390/cryst5030275