Flux Growth and Crystal Structure Refinement of Calcite Type Borate GaBO3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Growth
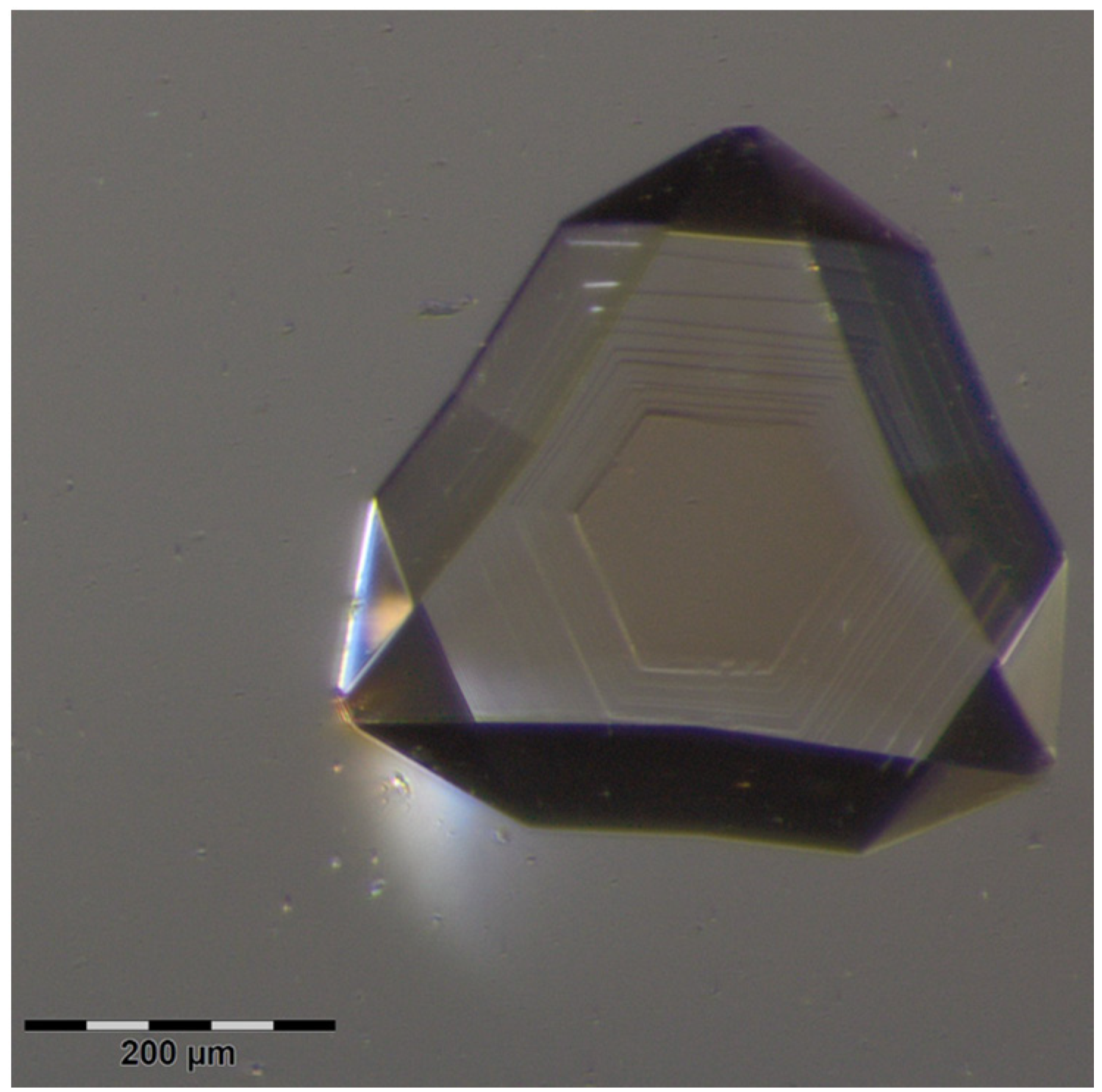
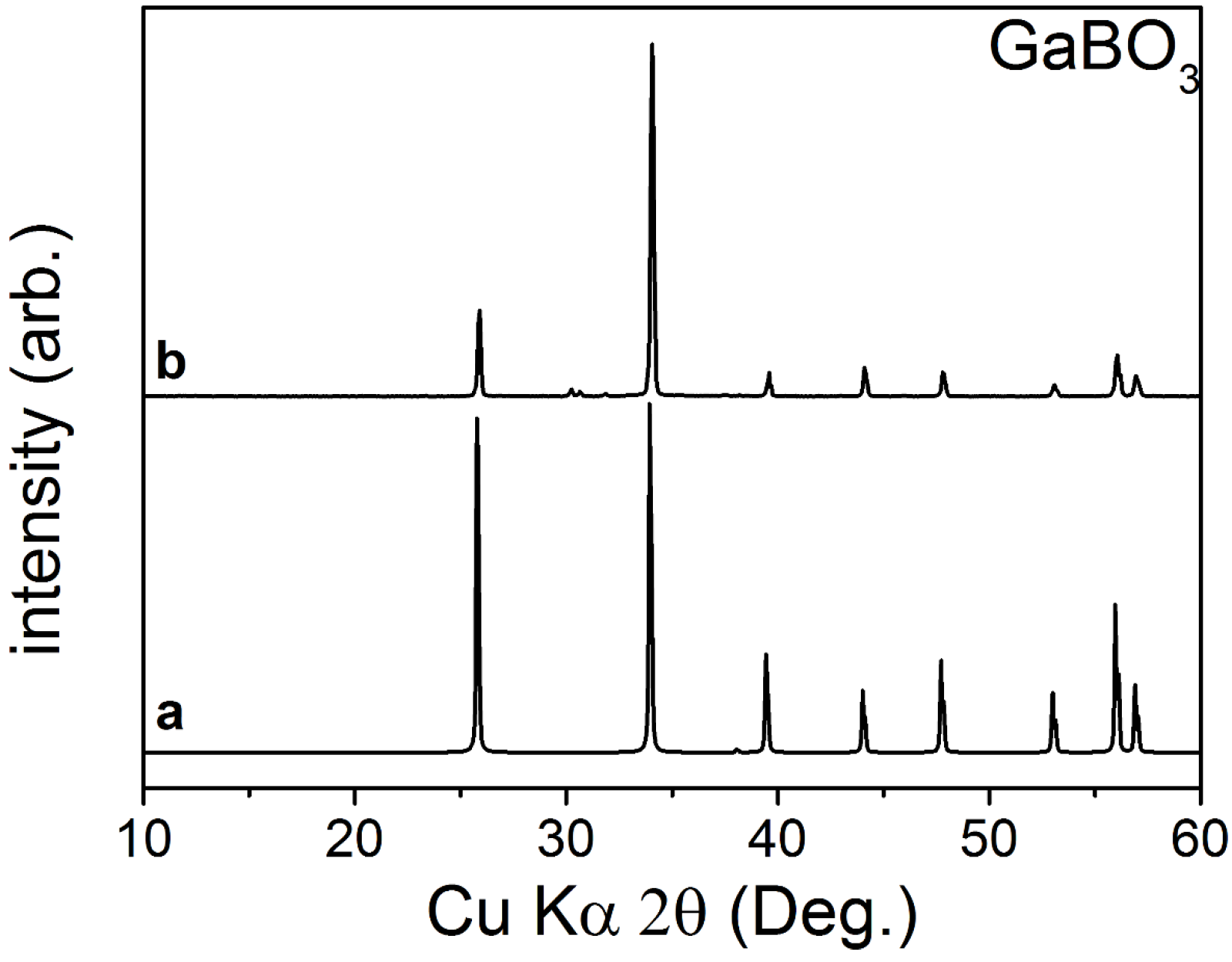
2.2. Structural Analysis

| Bond lengths (Å) | Bond angles (°) | ||
|---|---|---|---|
| Ga–O | 1.9875(2) | O–Ga–O | 180.00(3) |
| O–Ga–O | 91.718(7) | ||
| O–Ga–O | 91.719(8) | ||
| B–O–Ga | 117.055(14) | ||
| O–B–O | 120 | ||
| B–O | 1.3789(5) | O–Ga–O | 180 |
| O–Ga–O | 88.282(7) | ||
| O–Ga–O | 88.281(8) | ||
| Ga–O–Ga | 125.89(3) | ||

2.3. Optical Properties

3. Experimental Section
3.1. Growth
3.2. Elemental Analysis
3.3. X-ray Measurements
| Parameter | Data |
|---|---|
| Formula mass (amu) | 128.53 |
| Crystal system | Trigonal |
| Space group | R-3c |
| a (Å) | 4.56590(10) |
| c (Å) | 14.1764(4) |
| V(Å3) | 255.946(11) |
| Z | 6 |
| Crystal size (mm) | 0.15 × 0.11 × 0.07 |
| ρ(calcd) (g/cm3) | 5.003 |
| F(000) | 360 |
| μ (mm−1) | 15.716 |
| Absorption correction | Multi-scan |
| Temperature (K) | 100(2) |
| Wavelength (Å) | 0.71073 |
| θ (deg) | 5.91–45.16 |
| Index range | −8 ≤ h ≤ 9 |
| −8 ≤ k ≤ 9 | |
| −26 ≤ l ≤ 28 | |
| Rint | 0.0353 |
| Reflections collected | 6025 |
| Independent reflections | 242 |
| Reflections (I > 2σ(I)) | 237 |
| Completeness | 100% |
| Data/Restraints/Parameters | 242/0/11 |
| R/wR (I > 2σ (I) ) | 0.0153/0.0432 |
| R/wR (all data) | 0.0155/0.0433 |
| GOF on F2 | 1.172 |
| Largest diff. peak and hole (e/Å−3) | 1.504 and −1.156 |
| Atom | x | y | z | Wyckoff | Ueq(Å2) | Occupancy |
|---|---|---|---|---|---|---|
| Ga | 0 | 0 | 0 | 6b | 0.00262(8) | 1 |
| B | 0 | 0 | 1/4 | 6a | 0.0043(3) | 1 |
| O | 0.30201(12) | 0 | 1/4 | 18e | 0.00391(11) | 1 |
3.4. Optical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levin, E.M.; Roth, R.S.; Martin, J.B. Polymorphism of ABO3 type rare earth borates. Am. Mineral. 1961, 46, 1030–1055. [Google Scholar]
- Bernal, I.; Struck, C.W.; White, J.G. New transition metal borates with the calcite structure. Acta Crystallogr. 1963, 16, 849–850. [Google Scholar] [CrossRef]
- Diehl, R. Crystal structure refinement of ferric borate, FeBO3. Solid State Commun. 1975, 17, 743–745. [Google Scholar] [CrossRef]
- Vegas, A.; Cano, F.H.; Garciablanco, S. Refinement of aluminium orthoborate Sample: 30–50 degree reflections, Al3+, O- Note: Calcite structure type. Acta Crystallogr. Sect. B Struct. Sci. 1977, 33, 3607–3609. [Google Scholar] [CrossRef]
- Keszler, D.A.; Sun, H.X. Structure of ScBO3. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 1505–1057. [Google Scholar] [CrossRef]
- Cox, J.R.; Keszler, D.A. InBO3. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 1857–1859. [Google Scholar] [CrossRef]
- Ding, X.X.; Huang, Z.X.; Huang, X.T.; Gan, Z.W.; Cheng, C.; Tang, C.; Qi, S.R. Synthesis of gallium borate nanowires. J. Cryst. Growth 2004, 263, 504–509. [Google Scholar] [CrossRef]
- Santamaria-Perez, D.; Gomis, O.; Sans, J.A.; Ortiz, H.M.; Vegas, A.; Errandonea, D.; Ruiz-Fuertes, J.; Martinez-Garcia, D.; Garcia-Domene, B.; Pereira, A.L.J.; et al. Compressibility systematics of calcite-type borates: An experimental and theoretical structural study on ABO3 (A = Al, Sc, Fe, and In). J. Phys. Chem. C 2014, 118, 4354–4361. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Crystal structure and fluorescence of some lanthanide gallium borates. J. Inorg. Nucl. Chem. 1967, 29, 266–267. [Google Scholar] [CrossRef]
- Dirksen, G.J.; Hoffman, A.; Vandebout, T.P.; Laudy, M.P.G.; Blasse, G. Luminescence spectra of pure and doped GaBO3 and LiGaO2. J. Mater. Chem. 1991, 1, 1001–1005. [Google Scholar] [CrossRef]
- Dotsenko, V.P.; Efryushina, N.P.; Berezovskaya, I.V. Luminescence properties of GaBO3:Bi3+. Mater. Lett. 1996, 28, 517–520. [Google Scholar]
- Sajuti, D.; Yano, M.; Narushima, T.; Iguchi, Y. Phase diagrams of the Ga2O3-B2O3 and In2O3-B2O3 binary systems. Mater. Trans. JIM 1993, 34, 1195–1199. [Google Scholar] [CrossRef]
- Tajima, K.; Hino, Y.; Narushima, T.; Iguchi, Y. Activity of Ga2O3 in B2O3 flux and free energies of formation of GaBO3 and InBO3. Mater. Trans. JIM 2000, 41, 714–718. [Google Scholar] [CrossRef]
- Hoch, M. Thermodynamic properties and phase diagrams of the binary systems B2O3-Ga2O3, B2O3-Al2O3 and B2O3-In2O3. J. Alloys Compd. 2001, 320, 267–275. [Google Scholar] [CrossRef]
- Pelzer, H.; Muller, F. Thermochemistry of GaBO3 and phase equilibria in the Ga2O3-B2O3 system. J. Alloys Compd. 2001, 320, 262–266. [Google Scholar] [CrossRef]
- Bither, T.A.; Young, H.S. MBO3 Calcite-type borates of Al, Ga, Tl, and Rh. J. Solid State Chem. 1973, 6, 502–508. [Google Scholar] [CrossRef]
- Rudenko, V.V. High-temperature solution growth of GaBO3 crystals. Kristallografiya 1995, 40, 382–384. [Google Scholar]
- Vitzthum, D.; Hering, S.A.; Perfler, L.; Huppertz, H. High-pressure syntheses and crystal structures of orthorhombic DyGaO3 and trigonal GaBO3. Z. Naturforschung Sect. B J. Chem. Sci. 2015, 70, 207–214. [Google Scholar]
- Levin, E.M.; Mc Daniel, C.L. The system Bi2O3-B2O3. J. Am. Ceram. Soc. 1962, 45, 355–360. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ye, N.; Poeppelmeier, K.R. Flux Growth and Crystal Structure Refinement of Calcite Type Borate GaBO3. Crystals 2015, 5, 252-260. https://doi.org/10.3390/cryst5020252
Wang S, Ye N, Poeppelmeier KR. Flux Growth and Crystal Structure Refinement of Calcite Type Borate GaBO3. Crystals. 2015; 5(2):252-260. https://doi.org/10.3390/cryst5020252
Chicago/Turabian StyleWang, Shichao, Ning Ye, and Kenneth R. Poeppelmeier. 2015. "Flux Growth and Crystal Structure Refinement of Calcite Type Borate GaBO3" Crystals 5, no. 2: 252-260. https://doi.org/10.3390/cryst5020252






