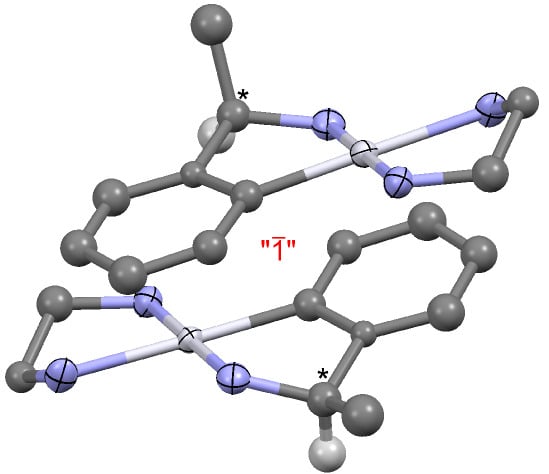
Cleavage of the Pt-I bond in a Primary Cycloplatinated Amine by Chelation
Abstract
:1. Introduction
2. Results and Discussion
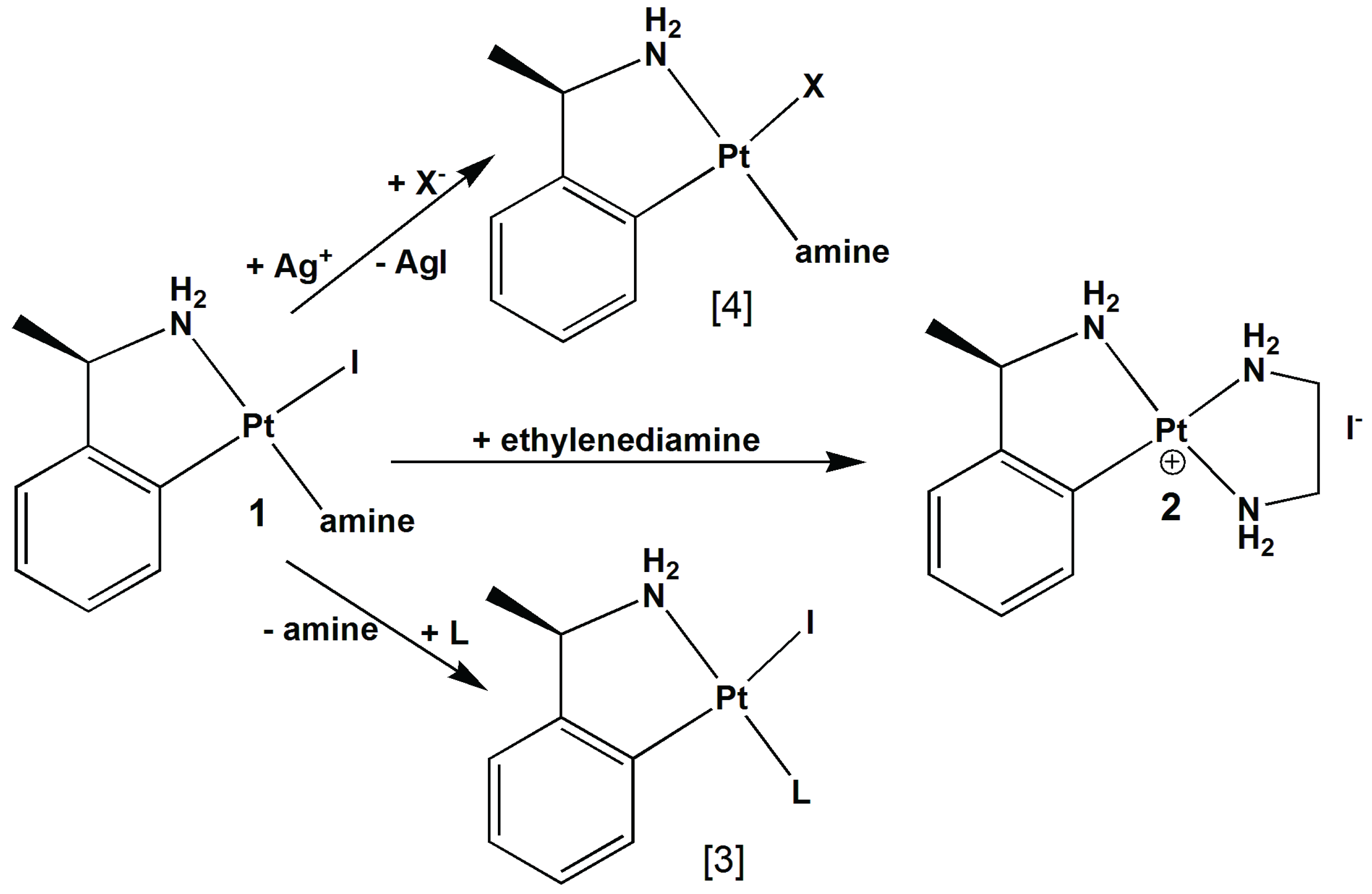
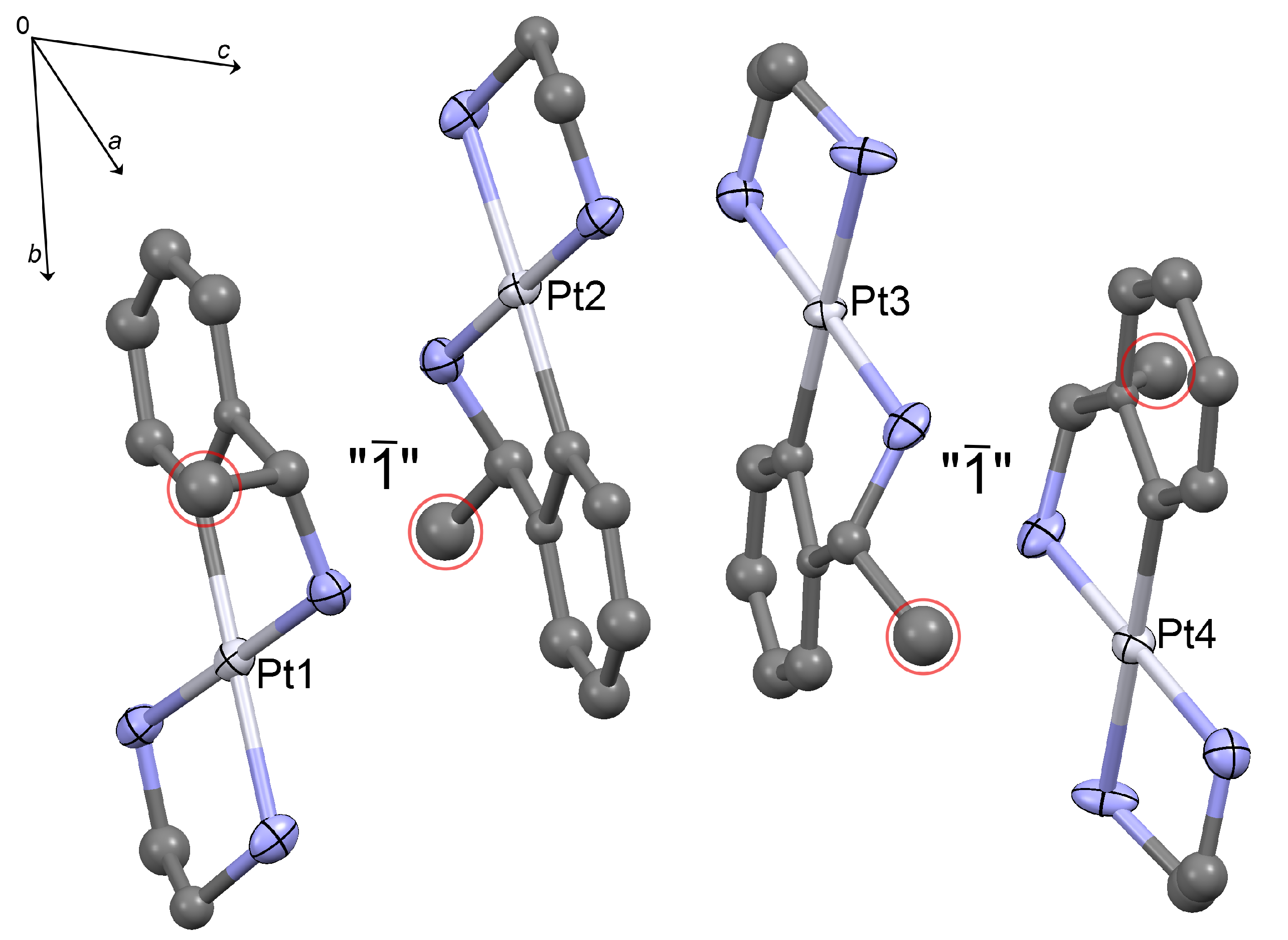
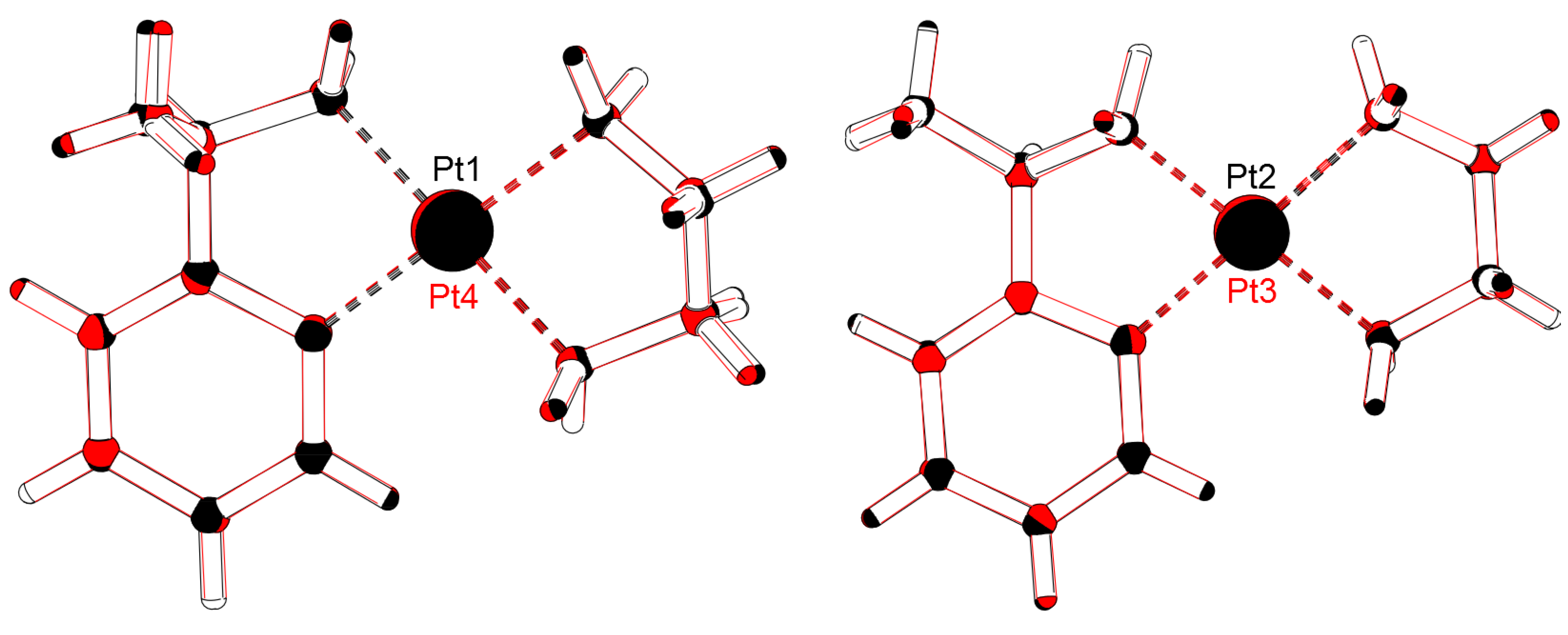
2.1. Crystal Structure Determination
3. Experimental Section
3.1. Instrumentation
3.2. Synthesis
3.3. Characterization
3.4. X-ray Data Collection and Structure Refinement
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cope, A.C.; Friedrich, E.C. Electrophilic aromatic substitution reactions by platinum(II) and palladium(II) chlorides on N,N-dimethylbenzylamines. J. Am. Chem. Soc. 1968, 90, 909–913. [Google Scholar] [CrossRef]
- Capapé, A.C.; Crespo, M.; Granell, J.; Font-Bardia, M.; Solans, X. A comparative study of the structures and reactivity of cyclometallated platinum compounds of N-benzylidenebenzylamines and cycloplatination of a primary amine. Dalton Trans. 2007, 28, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Calmuschi-Cula, B.; Englert, U. Orthoplatination of primary amines. Organometallics 2008, 27, 3124–3130. [Google Scholar] [CrossRef]
- Raven, W.; Hermes, P.; Kalf, I.; Schmaljohann, J.; Englert, U. A cationic aqua complex as versatile intermediate for the synthesis of neutral and cationic orthoplatinated primary amines. J. Organomet. Chem. 2014, 766, 34–39. [Google Scholar] [CrossRef]
- Kvam, P.; Engebretsen, T.; Maartmann-Moe, K.; Songstad, J. Crystal structures of Bu4N[Pt(ppy)Cl2], Et4N[Pt(tpy)Cl2] and [Pt(ppy)en]Cl (ppy = N,C'-chelated 2-phenylpyridinate, tpy = N,C'-chelated 2-(2'-thienyl)pyridinate, en = N,N'-chelated 1,2-diaminoethane). Acta Chem. Scand. 1996, 50, 107–113. [Google Scholar] [CrossRef]
- Failes, T.W.; Hall, M.D.; Hambley, T.W. The first examples of platinum amine hydroxamate complexes: Structures and biological activity. Dalton Trans. 2003, 8, 1596–1600. [Google Scholar] [CrossRef]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Raven, W.; Kalf, I.; Englert, U. Platinum(IV) complexes of primary Amines via oxidative addition. J. Organomet. Chem. 2015. submitted. [Google Scholar]
- Calmuschi, B.; Englert, U. (R)-Di-μ-acetato-κ2O:O'-bis[2-(1-aminoethyl)phenyl -κ2C1,N] palladium(II), (R)-di-μ-chloro-bis[2-(1-aminoethyl)phenyl-κ2C1,N]palladium(II) and [SP-4-4]-(R)-[2-(1-aminoethyl)phenyl-κ2C1,N]chloro(pyridine-κ N)palladium(II). Acta Crystallogr. 2002, C58, m545–m548. [Google Scholar]
- Calmuschi, B.; U. Englert, U. (S)-(Acetyl-acetonato-κ2O,O') [2-(1-aminoethyl) -phenyl-κ2C1,N]-palladium(II). Acta Crystallogr. 2005, E61, m164–m165. [Google Scholar]
- Calmuschi, B.; Englert, U. (S)-[2-(1-Amino-ethyl)-phenyl-κ2C1,N]4 (hexafluoro-acetyl-acetonato-κ2O,O') palladium(II). Acta Crystallogr. 2005, E61, m166–m167. [Google Scholar]
- Calmuschi, B.; Englert, U. rac-[2-(1-Amino-ethyl)phenyl-κ2C1,N] (hexafluoro- acetyl-acetonato-κ2O,O') palladium(II). Acta Crystallogr. 2005, E61, m168–m170. [Google Scholar]
- Kalf, I.; Wang, R.; Englert, U. Unexpected solids from enantiopure cationic palladium complexes and racemic anions: A structural study of chiral non-discrimination. J. Organomet. Chem. 2006, 691, 2277–2285. [Google Scholar] [CrossRef]
- Kalf, I.; Wang, R.; Englert, U. A robust conglomerate structure type in salts of cationic organopalladium complexes and non-coordinating anions. CrystEngComm 2008, 10, 39–47. [Google Scholar] [CrossRef]
- Şerb, M.D.; Kalf, I.; Englert, U. [rac-2-(1-Aminoethyl)phenyl -κ2C1,N] (ethylendiamine-κ2N,N')palladium(II)3-methyl-benzoate monohydrate. Acta Crystallogr. 2010, E66, m976. [Google Scholar]
- Şerb, M.D.; Kalf, I.; Englert, U. [rac-2-(1-Aminoethyl)phenyl-κ2C1,N] (ethylendiamine-κ2N,N')palladium(II) 3,5-dimethyl-benzoate. Acta Crystallogr. 2010, E66, m977. [Google Scholar]
- Le Page, Y. MISSYM1.1—A flexible new release. J. Appl. Cryst. 1988, 21, 983–984. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- SAINT+, Version 7.68, Bruker AXS Inc.: Madison, WI, USA, 2009.
- SMART, Version 5.624, Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Parsons, S.; Flack, H.D. Precise absolute-structure determination in light-atom crystals. Acta Crystallogr. 2004, A60, S61. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raven, W.; Kalf, I.; Englert, U. Cleavage of the Pt-I bond in a Primary Cycloplatinated Amine by Chelation. Crystals 2015, 5, 244-251. https://doi.org/10.3390/cryst5020244
Raven W, Kalf I, Englert U. Cleavage of the Pt-I bond in a Primary Cycloplatinated Amine by Chelation. Crystals. 2015; 5(2):244-251. https://doi.org/10.3390/cryst5020244
Chicago/Turabian StyleRaven, William, Irmgard Kalf, and Ulli Englert. 2015. "Cleavage of the Pt-I bond in a Primary Cycloplatinated Amine by Chelation" Crystals 5, no. 2: 244-251. https://doi.org/10.3390/cryst5020244