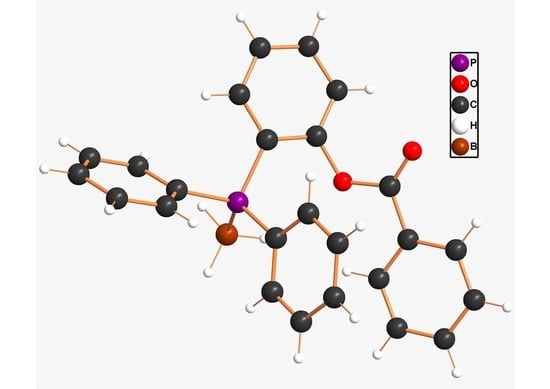
Synthesis and Molecular Structure of 2-(Diphenylphosphano)phenyl Benzoate Borane Adduct
Abstract
:1. Introduction
2. Results and Discussion
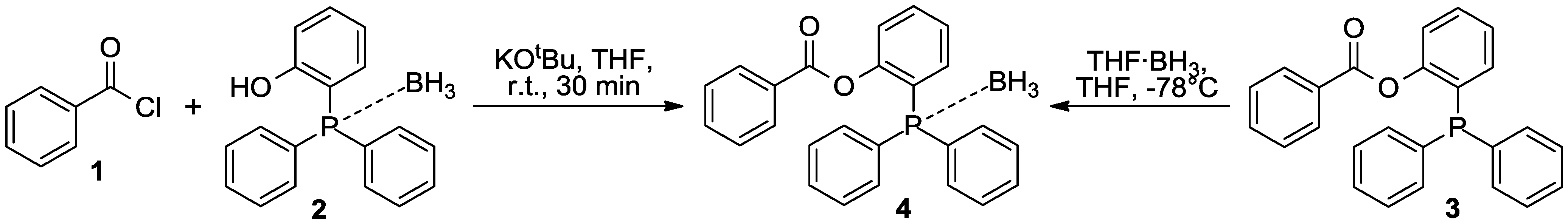
| Crystal data | Refinement | ||
|---|---|---|---|
| Formula | C25H22BO2P | F(000) | 416 |
| Formula weight | 396.20 g·mol−1 | Refinement method | Full-matrix least-squares on F2 |
| Temperature | 98 K | – | – |
| Wavelength | 0.71073 Å | Data/restraints/parameters | 5616/0/274 |
| Crystal system | Triclinic | – | – |
| Space group | P | Goodness-of-fit on F2 | 1.08 |
| Unit cell dimensions | a = 8.677(1) Å | – | – |
| b = 9.202(1) Å | Final R indices | R1 = 0.0335 | |
| c = 14.224(2) Å | [I > 2σ(I)] | wR2 = 0.0906 | |
| α = 72.600(7)° | |||
| β = 73.577(7)° | R indices (all data) | R1 = 0.0386 | |
| γ =84.399(7)° | |||
| Volume | 1039.5(2) Å3 | – | wR2 = 0.0949 |
| Z | 2 | Largest diff. peak and hole | 0.51/−0.28 e·Å−3 |
| Density (calcd.) | 1.266 g·cm−3 | – | – |
| Absorption coefficient | 0.15 mm−1 | – | – |
| Crystal size | 0.37 × 0.35 × 0.31 mm3 | – | – |
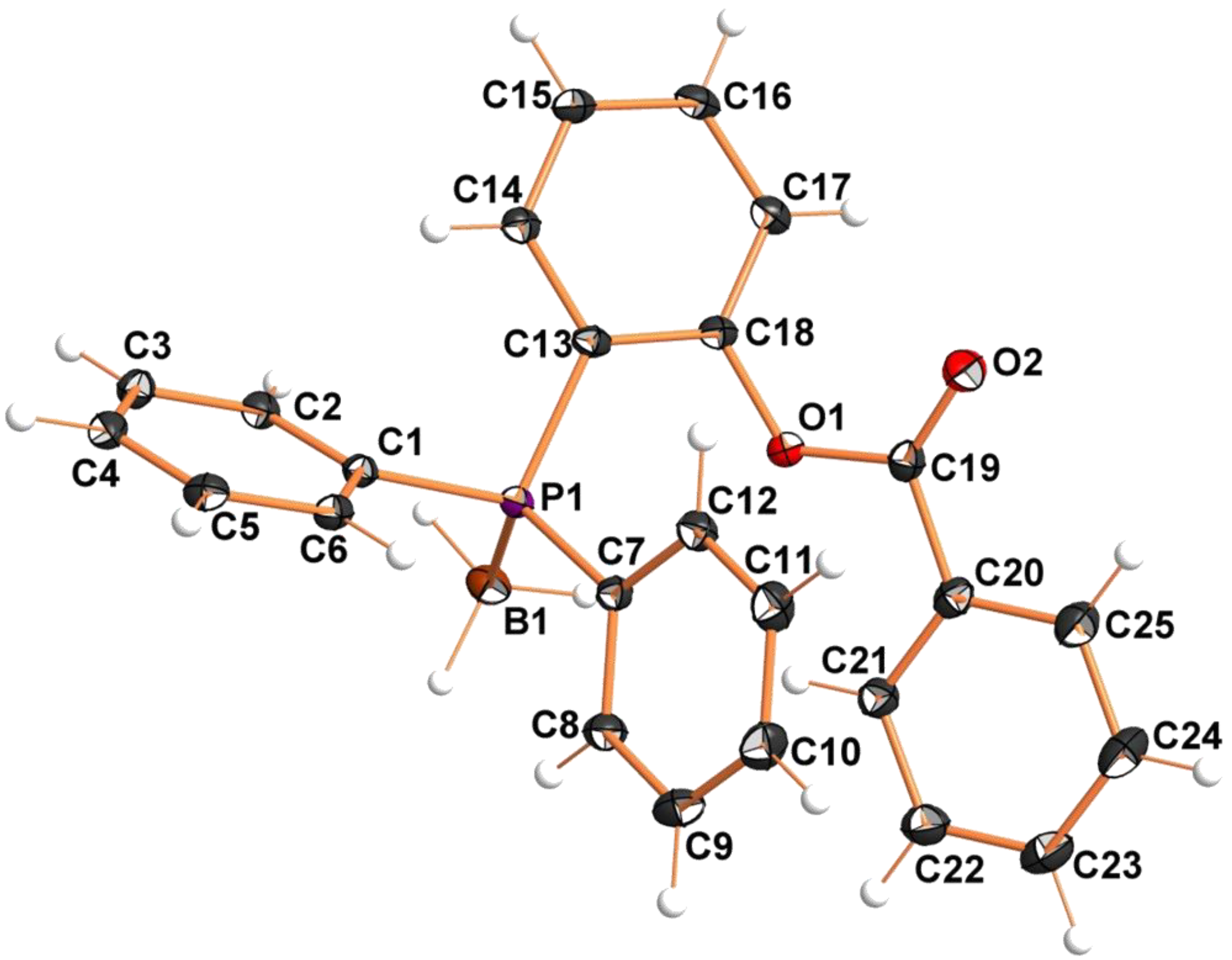
| Atoms | Distance | Atoms | Distance |
|---|---|---|---|
| P1−C1 | 1.815(1) | C18−O1 | 1.397(1) |
| P1−C7 | 1.808(1) | O1−C19 | 1.374(1) |
| P1−C13 | 1.822(1) | C19−O2 | 1.203(1) |
| P1−B1 | 1.932(1) | C19−C20 | 1.481(2) |

3. Experimental Section
3.1. Synthesis of 2-(Diphenylphosphano)phenyl Benzoate Borane Adduct (4)
3.2. Data Collection and Refinement
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Köhn, M.; Breinbauer, R. The Staudinger ligation—A gift to chemical biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef]
- Schilling, C.I.; Jung, N.; Biskup, M.; Schepers, U.; Bräse, S. Bioconjugation via azide–Staudinger ligation: An overview. Chem. Soc. Rev. 2011, 40, 4840–4871. [Google Scholar] [CrossRef]
- Van Berkel, S.S.; van Eldijk, M.B.; van Hest, J.C.M. Staudinger Ligation as a Method for Bioconjugation. Angew. Chem. Int. Ed. 2011, 50, 8806–8827. [Google Scholar] [CrossRef]
- Mamat, C.; Flemming, A.; Köckerling, M.; Steinbach, J.; Wuest, F.R. Synthesis of Benzoate-Functionalized Phosphanes as Novel Building Blocks for the Traceless Staudinger Ligation. Synthesis 2009, 3311–3321. [Google Scholar]
- Mamat, C.; Franke, M.; Peppel, T.; Köckerling, M.; Steinbach, J. Synthesis, structure determination, and (radio-)fluorination of novel functionalized phosphanes suitable for the traceless Staudinger ligation. Tetrahedron 2011, 67, 4521–4529. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Sloan, M.E.; Manners, I. Amine- and Phosphine-Borane Adducts: New Interest in Old Molecules. Chem. Rev. 2010, 110, 4023–4078. [Google Scholar]
- Pretze, M.; Wuest, F.; Peppel, T.; Köckerling, M.; Mamat, C. The traceless Staudinger ligation with fluorine-18: A novel and versatile labeling technique for the synthesis of PET-radiotracers. Tetrahedron Lett. 2010, 51, 6410–6414. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97 Programs for the Solution and Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL2014/1 Programs for the Solution and Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamat, C.; Köckerling, M. Synthesis and Molecular Structure of 2-(Diphenylphosphano)phenyl Benzoate Borane Adduct. Crystals 2015, 5, 9-13. https://doi.org/10.3390/cryst5010009
Mamat C, Köckerling M. Synthesis and Molecular Structure of 2-(Diphenylphosphano)phenyl Benzoate Borane Adduct. Crystals. 2015; 5(1):9-13. https://doi.org/10.3390/cryst5010009
Chicago/Turabian StyleMamat, Constantin, and Martin Köckerling. 2015. "Synthesis and Molecular Structure of 2-(Diphenylphosphano)phenyl Benzoate Borane Adduct" Crystals 5, no. 1: 9-13. https://doi.org/10.3390/cryst5010009