The Short Series of the Oxygen-Poor Lanthanide Oxide Selenides M10OSe14 with M = La–Nd
1. Introduction
2. Results and Discussion
2.1. Structure Description
 ..), Se4 at 16e (.2.), M3 at 16f (..2) along with M1, M2, Se1, Se2 and Se3 all at the general 32g site with symmetry 1. The coordination spheres of the trivalent lanthanide cations M3+ exhibit a trigonal prismatic shape with two caps each (see Figure 1). From these, just (M2)3+ binds the light O2– anion apart from seven contacts to Se2–, while (M1)3+ and (M3)3+ show eight bonds to only Se2– anions.
..), Se4 at 16e (.2.), M3 at 16f (..2) along with M1, M2, Se1, Se2 and Se3 all at the general 32g site with symmetry 1. The coordination spheres of the trivalent lanthanide cations M3+ exhibit a trigonal prismatic shape with two caps each (see Figure 1). From these, just (M2)3+ binds the light O2– anion apart from seven contacts to Se2–, while (M1)3+ and (M3)3+ show eight bonds to only Se2– anions. 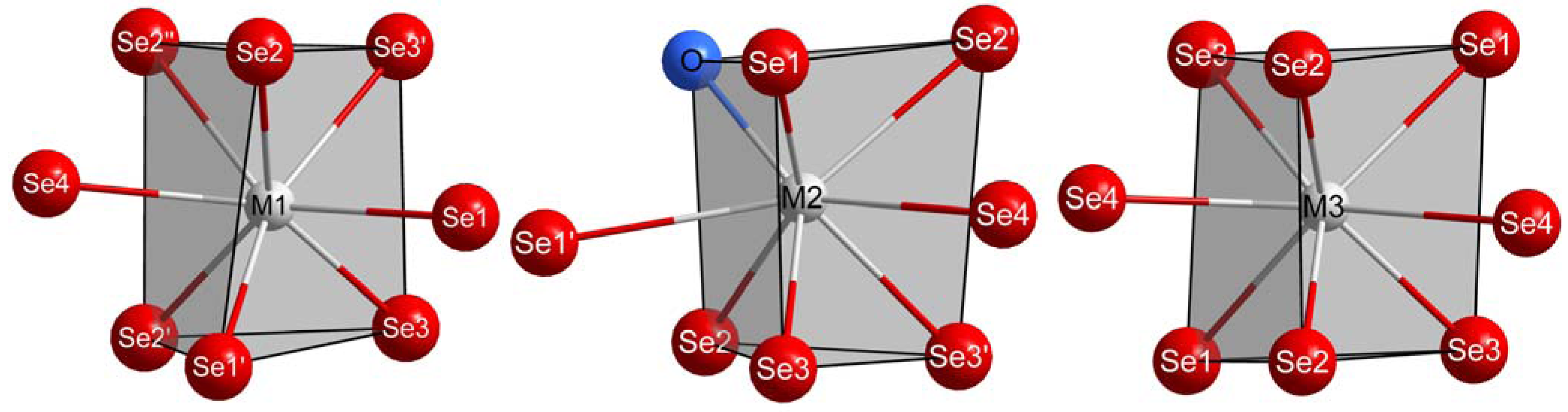
| Atom | x/a | y/b | z/c | Atom | x/a | y/b | z/c | ||
|---|---|---|---|---|---|---|---|---|---|
| La10OSe14 | Pr10OSe14 | ||||||||
| La1 | 32g | 0.13003(4) | 0.02695(4) | 0.04721(2) | Pr1 | 32g | 0.12991(3) | 0.02686(3) | 0.04742(2) |
| La2 | 32g | 0.37036(4) | 0.25434(4) | 0.05965(2) | Pr2 | 32g | 0.37071(3) | 0.25436(3) | 0.05951(2) |
| La3 | 16f | 0.13330(4) | x + 1/4 | 1/8 | Pr3 | 16f | 0.13364(4) | x + 1/4 | 1/8 |
| O | 8a | 0 | 1/4 | 3/8 | O | 8a | 0 | 1/4 | 3/8 |
| Se1 | 32g | 0.02192(6) | 0.38134(7) | 0.00124(4) | Se1 | 32g | 0.02218(5) | 0.38111(5) | 0.00199(4) |
| Se2 | 32g | 0.34224(7) | 0.07046(7) | 0.09270(4) | Se2 | 32g | 0.34290(5) | 0.07081(5) | 0.09262(4) |
| Se3 | 32g | 0.03914(6) | 0.07100(7) | 0.17174(4) | Se3 | 32g | 0.03885(5) | 0.07057(5) | 0.17167(4) |
| Se4 | 16e | 0.35523(9) | 0 | 1/4 | Se4 | 16e | 0.35463(7) | 0 | 1/4 |
| Ce10OSe14 | Nd10OSe14 | ||||||||
| Ce1 | 32g | 0.13000(3) | 0.02686(3) | 0.04741(2) | Nd1 | 32g | 0.12963(3) | 0.02694(3) | 0.04743(2) |
| Ce2 | 32g | 0.37049(3) | 0.25428(3) | 0.05949(2) | Nd2 | 32g | 0.37075(3) | 0.25459(3) | 0.05933(2) |
| Ce3 | 16f | 0.13347(3) | x + 1/4 | 1/8 | Nd3 | 16f | 0.13381(3) | x + 1/4 | 1/8 |
| O | 8a | 0 | 1/4 | 3/8 | O | 8a | 0 | 1/4 | 3/8 |
| Se1 | 32g | 0.02201(4) | 0.38106(5) | 0.00168(3) | Se1 | 32g | 0.02249(5) | 0.38122(5) | 0.00236(4) |
| Se2 | 32g | 0.34256(5) | 0.07063(5) | 0.09262(3) | Se2 | 32g | 0.34298(5) | 0.07106(5) | 0.09269(4) |
| Se3 | 32g | 0.03891(5) | 0.07076(5) | 0.17178(3) | Se3 | 32g | 0.03876(5) | 0.07060(5) | 0.17159(4) |
| Se4 | 16e | 0.35487(6) | 0 | 1/4 | Se4 | 16e | 0.35395(7) | 0 | 1/4 |
| M10OSe14 | M = La | M = Ce | M = Pr | M = Nd | ||
|---|---|---|---|---|---|---|
| M1 | –Se1 | (1×) | 298.7(1) | 296.4(1) | 294.4(1) | 292.4(1) |
| –Se4 | (1×) | 301.1(1) | 298.2(1) | 296.2(1) | 294.1(1) | |
| –Se2 | (1×) | 306.0(1) | 303.4(1) | 301.5(1) | 300.2(1) | |
| –Se1' | (1×) | 306.2(1) | 304.2(1) | 302.2(1) | 300.2(1) | |
| –Se3 | (1×) | 307.7(1) | 304.7(1) | 302.5(1) | 300.6(1) | |
| –Se2' | (1×) | 309.8(1) | 307.1(1) | 305.4(1) | 303.8(1) | |
| –Se3' | (1×) | 310.0(1) | 307.9(1) | 306.1(1) | 304.6(1) | |
| –Se2'' | (1×) | 357.9(1) | 355.4(1) | 353.8(1) | 352.4(1) | |
| M2 | –O | (1×) | 248.2(1) | 246.1(1) | 244.2(1) | 243.0(1) |
| –Se1 | (1×) | 294.5(1) | 291.9(1) | 289.9(1) | 288.0(1) | |
| –Se2 | (1×) | 304.2(1) | 301.4(1) | 299.2(1) | 297.6(1) | |
| –Se3 | (1×) | 308.8(1) | 305.6(1) | 304.1(1) | 302.4(1) | |
| –Se3' | (1×) | 314.9(1) | 312.0(1) | 310.1(1) | 308.6(1) | |
| –Se4 | (1×) | 320.1(1) | 317.9(1) | 316.3(1) | 314.9(1) | |
| – Se2' | (1×) | 329.1(1) | 326.2(1) | 323.6(1) | 321.6(1) | |
| –Se1' | (1×) | 348.3(1) | 345.3(1) | 343.3(1) | 342.2(1) | |
| M3 | –Se3 | (2×) | 300.6(1) | 298.1(1) | 296.2(1) | 294.5(1) |
| –Se2 | (2×) | 308.1(1) | 305.6(1) | 303.4(1) | 301.8(1) | |
| – Se1 | (2×) | 315.3(1) | 311.8(1) | 309.3(1) | 306.9(1) | |
| – Se4 | (2×) | 322.8(1) | 319.7(1) | 317.5(1) | 315.8(1) | |
| Distances/angles/examples | La10OSe14 | Ce10OSe14 | Pr10OSe14 | Nd10OSe14 | |
|---|---|---|---|---|---|
| d(Se2––M3+) | 295–358 | 292–355 | 290–354 | 288–352 | |
| example 1 | C-La2Se3 [15] | C-Ce2Se3 [16] | C-Pr2Se3 [15] | C-Nd2Se3 [17] | |
| 304–323 | 302–320 | 299–318 | 297–317 | ||
| example 2 | La5NSe6 [18] | Ce3ONSe2 [19] | Pr2OSe2 [1] | Nd3ONSe2 [19] | |
| 289–355 | 293–355 | 293–331 | 289–347 | ||
| d(O2––M3+) | (4×) | 248.2 | 246.1 | 244.2 | 243.0 |
| example | La10OS14 [8] | Ce10OS14 [8] | Pr10OS14 [8] | Nd10OS14 [8] | |
| 245.4 | 243.0 | 242.1 | 240.8 | ||
| ∢M2-O-M2 | (4×) | 107.9 | 108.0 | 108.0 | 108.1 |
| ∢M2-O-M2' | (2×) | 112.6 | 112.5 | 112.4 | 112.2 |
| example | La10OS14 [8] | Ce10OS14 [8] | Pr10OS14 [8] | Nd10OS14 [8] | |
| ∢M2-O-M2 | (4×) | 108.1 | 108.2 | 108.2 | 108.3 |
| ∢M2-O-M2' | (2×) | 112.3 | 112.1 | 112.1 | 111.9 |
 {[(M1)3(M3)3Se14]10–} dominate the crystal structure of the title compounds (Figure 2a). The distances between O2– and (M2)3+ in these oxide selenides M10OSe14 decrease from 248 pm for M = La to 243 pm for M = Nd caused by the lanthanide contraction, but they also amount to values slightly higher than in the corresponding lanthanide oxide sulfides M10OS14 (Table 3, M = La–Nd). Although most trends remain the same in the oxide chalcogenides M10OCh14 from M = La to M = Nd, the angles M2-O-M2 exhibit lower values in the selenide compounds, while the angles M2-O-M2' show higher values as compared to the sulfide representatives. These effects certainly originate from the different sizes of the chalcogenide anions within the complex anionic lanthanide chalcogenide matrix
{[(M1)3(M3)3Se14]10–} dominate the crystal structure of the title compounds (Figure 2a). The distances between O2– and (M2)3+ in these oxide selenides M10OSe14 decrease from 248 pm for M = La to 243 pm for M = Nd caused by the lanthanide contraction, but they also amount to values slightly higher than in the corresponding lanthanide oxide sulfides M10OS14 (Table 3, M = La–Nd). Although most trends remain the same in the oxide chalcogenides M10OCh14 from M = La to M = Nd, the angles M2-O-M2 exhibit lower values in the selenide compounds, while the angles M2-O-M2' show higher values as compared to the sulfide representatives. These effects certainly originate from the different sizes of the chalcogenide anions within the complex anionic lanthanide chalcogenide matrix  {[(M1)3(M3)3Ch14]10–} (S2–vs. Se2–). Similar to the M10OS14-type compounds (M = La-Nd, Sm and Gd) [8,9], most of the oxygen-free part in this crystal structure of the M10OSe14 series (M10Se14 ≡ M2Se2.8, M = La–Nd) can be interpreted as closely related to the cation-defective Th3P4-type structure [20] of the corresponding lanthanide sesquiselenides M2Se3 known as their C-type modification [15,16,17]. Hence the internuclear distances between Se2– and M3+ do not differ significantly, with the exception of the contacts M1-Se2'' and M2-Se1' in the title compounds (compare Table 2 and Table 3).
{[(M1)3(M3)3Ch14]10–} (S2–vs. Se2–). Similar to the M10OS14-type compounds (M = La-Nd, Sm and Gd) [8,9], most of the oxygen-free part in this crystal structure of the M10OSe14 series (M10Se14 ≡ M2Se2.8, M = La–Nd) can be interpreted as closely related to the cation-defective Th3P4-type structure [20] of the corresponding lanthanide sesquiselenides M2Se3 known as their C-type modification [15,16,17]. Hence the internuclear distances between Se2– and M3+ do not differ significantly, with the exception of the contacts M1-Se2'' and M2-Se1' in the title compounds (compare Table 2 and Table 3).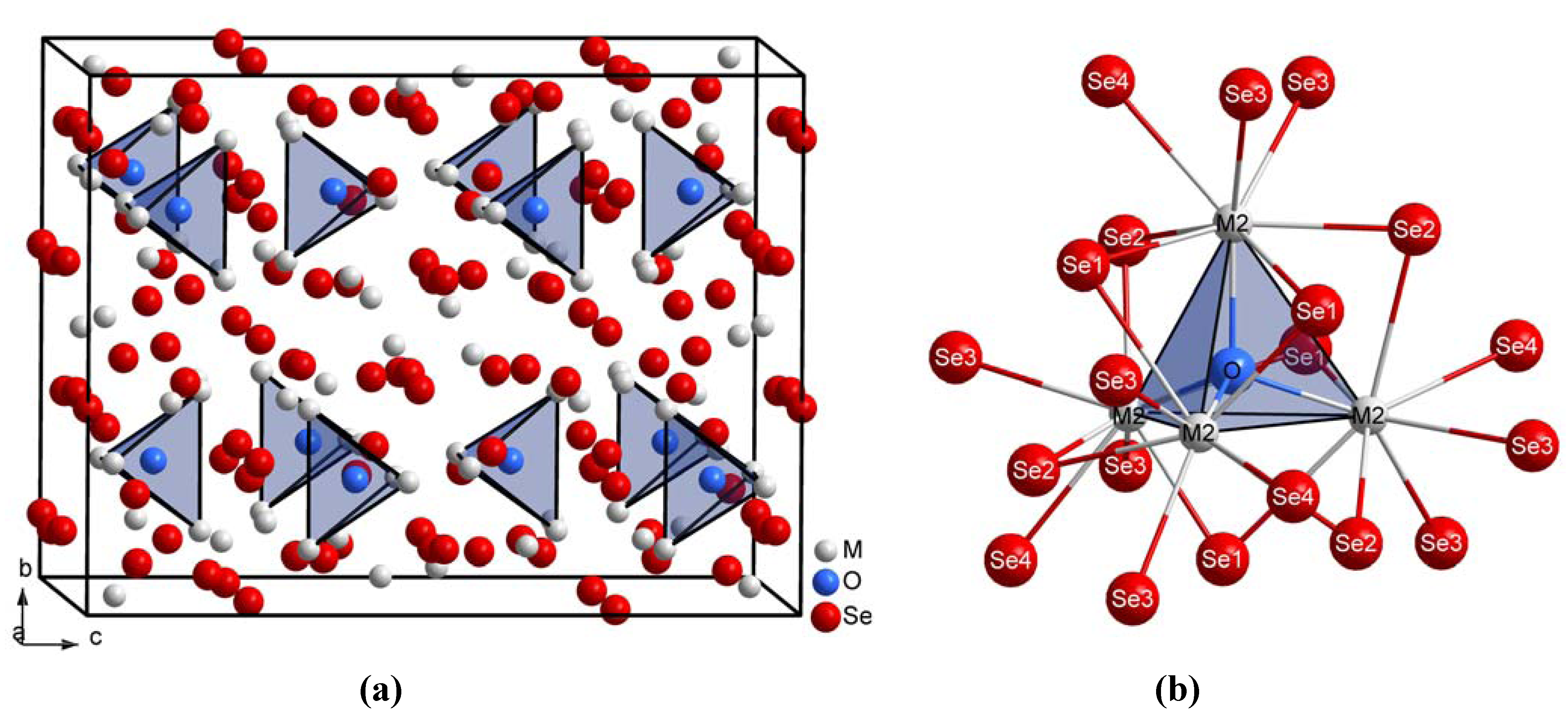
2.2. Optical Band Gaps
| General formula | Reference | M = La | M = Ce | M = Pr | M = Nd |
|---|---|---|---|---|---|
| M10OSe14 | this work | 2.04 eV | 1.97 eV | 1.89 eV | 1.98 eV |
| M4O4Se[Se2] | [7] | 1.89 eV | 1.69 eV | 1.87 eV | 1.87 eV |
| M2Se3 | [21] | 2.3 eV | 1.85 eV | 2.0 eV | 2.0 eV |

3. Experimental Section
| M10OSe14 | M = La | M = Ce | M = Pr | M = Nd |
|---|---|---|---|---|
| Colour | red | ruby red | ruby red | ruby red |
| Crystal system | tetragonal | tetragonal | tetragonal | tetragonal |
| Space group/Formula units | I41/acd/Z = 8 | I41/acd/Z = 8 | I41/acd/Z = 8 | I41/acd/Z = 8 |
| a (pm) | 1592.04(9) | 1578.96(9) | 1568.74(8) | 1559.83(8) |
| c (pm) | 2106.48(14) | 2086.59(14) | 2073.42(13) | 2062.91(12) |
| c/a | 1.323 | 1.321 | 1.322 | 1.322 |
| Vm (cm3/mol)/Dx (g/cm3) | 401.91/6.247 | 391.60/6.442 | 384.11/6.588 | 377.83/6.786 |
| F(000)/ θmax | 8432/30.2 | 8512/31.6 | 8592/30.4 | 8672/31.6 |
| ±h/±k/±l | 22/22/29 | 23/23/30 | 22/22/29 | 22/22/30 |
| Reflections (all/independent) | 36621/1999 | 52742/2178 | 43025/1926 | 39083/2117 |
| μ/mm–1 | 34.70 | 36.69 | 38.66 | 40.58 |
| Rint/Rσ | 0.118/0.051 | 0.127/0.047 | 0.101/0.034 | 0.087/0.026 |
| R1/wR2 | 0.069/0.078 | 0.066/0.057 | 0.052/0.067 | 0.059/0.096 |
| GooF | 0.986 | 0.973 | 0.986 | 1.037 |
4. Conclusions
 {[(M1)3(M3)3Se14]10–} embed isolated [O(M2)4]10+ tetrahedra. It should be noted that no hint of the existence of representatives with heavier lanthanides (M = Sm–Lu) could be obtained so far, but we are still busy trying to synthesize them. The optical band gaps amount to values between 1.89 and 2.04 eV encouraging investigations in their ability to be used for application as red pigments. Based on the interplay of the light anions O2– and N3– in the perovskite-type compounds Ca(1–x)LaxTaO(2–x)N(1+x) [26], we are also actively investigating the band-gap changes in correlation with nitride incorporation in this structure type represented by the recently published compound La10.25O0.25N0.75Se14 [27,28].
{[(M1)3(M3)3Se14]10–} embed isolated [O(M2)4]10+ tetrahedra. It should be noted that no hint of the existence of representatives with heavier lanthanides (M = Sm–Lu) could be obtained so far, but we are still busy trying to synthesize them. The optical band gaps amount to values between 1.89 and 2.04 eV encouraging investigations in their ability to be used for application as red pigments. Based on the interplay of the light anions O2– and N3– in the perovskite-type compounds Ca(1–x)LaxTaO(2–x)N(1+x) [26], we are also actively investigating the band-gap changes in correlation with nitride incorporation in this structure type represented by the recently published compound La10.25O0.25N0.75Se14 [27,28].Acknowledgments
References
- Weber, F.A.; Schleid, T. Vier oxidselenide des praseodyms: Pr10OSe14, Pr2OSe2, Pr2O2Se und Pr4O4Se3 (In German). Z. Anorg. Allg. Chem. 2001, 627, 1383–1388. [Google Scholar] [CrossRef]
- Weber, F.A. Präparative Studien in den Mehrstoffsystemen Selten-Erd-Metall–Selen bzw. Tellur und Sauerstoff (In German). Ph.D. Dissertation, University of Stuttgart, Stuttgart, Germany, 1999. [Google Scholar]
- Li, B.W.; Huang, F. Refinement of the crystal structure of decalanthanum monooxide tetradecaselenide La10OSe14 at 153 K. Z. Kristallogr. New Cryst. Struct. 2007, 222, 175–176. [Google Scholar]
- Tougaît, O.; Ibers, J.A. Gd2OSe2. Acta Crystallogr. 2000, A56, 623–624. [Google Scholar]
- Eick, H.A. The crystal structures and lattice parameters of some rare earth mono-seleno oxides. Acta Crystallogr. 1960, 13, 161. [Google Scholar] [CrossRef]
- Dugué, J.; Adolphe, C.; Khodadad, P. Structure cristalline de l’oxyséléniure de lanthane La4O4Se3 (In French). Acta Crystallogr. 1970, B26, 1627–1628. [Google Scholar]
- Strobel, S.; Choudhury, A.; Dorhout, P.K.; Lipp, C.; Schleid, T. Rare-earth metall(III) oxide selenides M4O4Se[Se2] (M = La, Ce, Pr, Nd, Sm) with discrete diselenide units: Crystal structures, magnetic frustration and other properties. Inorg.Chem. 2008, 47, 4936–4944. [Google Scholar] [CrossRef]
- Schleid, T.; Lissner, F. M10S14O-type oxysulphides (M ≡ La, Ce, Pr, Nd, Sm) as an “oxygen trap” in oxidation reactions of reduced lanthanide chlorides with sulphur. J. Less Common Met. 1991, 175, 309–319. [Google Scholar] [CrossRef]
- Schleid, T.; Weber, F.A. Crystal structure of dekagadolinium(III) oxide tetradekasulfide, Gd10OS14. Z. Kristallogr. New Cryst. Struct. 1998, 213, 32. [Google Scholar]
- Picon, M.; Domange, L.; Flahaut, J.; Guittard, M.; Patrie, M. Les sulfures Me2S3 et Me3S4 des elements des TerresRares (In French). Bull. Soc. Chim. Fr. 1960, 1960, 221–228. [Google Scholar]
- Flahaut, J. Les Éléments des Terres Rares, Collection de Monographies de Chimie (In French); Masson: Paris, France, 1969; p. 127. [Google Scholar]
- Carré, D.; Laurelle, P.; Besançon, P. Structure cristalline de la prétenduevariété β des sulfures de terres rares de composition Pr10S14O (In French). C. R. Hebd. Seances Acad. Sci. 1970, C270, 537–539. [Google Scholar]
- Besançon, P. Teneur en oxygéne et formule exacte d’Une famille de composés habituellement appelés “variété β” ou “phase complexe” des sulfures de terres rares (In French). J. Solid State Chem. 1973, 7, 232–240. [Google Scholar] [CrossRef]
- Meerschaut, A.; Lafond, A.; Palvadeau, P.; Deudon, C.; Cario, L. Synthesis and crystal structure of two new oxychalcogenides: Eu5V3S6O7 and La10Se14O. Mater. Res. Bull. 2002, 37, 1895–1905. [Google Scholar] [CrossRef]
- Folchnandt, M.; Schleid, T. Single crystals of C-La2Se3, C-Pr2Se3 and C-Gd2Se3 with cation-deficient Th3P4-type structure. Z. Anorg. Allg. Chem. 2001, 627, 1411–1413. [Google Scholar] [CrossRef]
- Folchnandt, M.; Schneck, C.; Schleid, T. Über Sesquiselenide der Lanthanoide: Einkristalle von Ce2Se3 im C-, Gd2Se3 im U- und Lu2Se3 im Z-Typ (In German). Z. Anorg. Allg. Chem. 2004, 630, 149–155. [Google Scholar] [CrossRef]
- Schneck, C.; Höss, P.; Schleid, T. C-type Nd2Se3. ActaCrystallogr. 2009, 65, i20. [Google Scholar]
- Schurz, C.M.; Lissner, F.; Janka, O.; Schleid, T. Synthese und kristallstruktur der N3–-armen Nitridselenide des Formeltyps M5NSe6 (M = La–Pr) mit isolierten Tetraederdoppeln [N2M6]12+ (In German). Z. Anorg. Allg. Chem. 2011, 637, 1045–1051. [Google Scholar] [CrossRef]
- Lissner, F.; Schleid, T. Die Oxidnitridselenide M3ONSe2 dreiwertiger Lanthanide (M = Ce–Nd) (In German). Z. Anorg. Allg. Chem. 2008, 634, 2799–2804. [Google Scholar] [CrossRef]
- Meisel, K. Kristallstrukturen von thoriumphospiden (In German). Z. Anorg. Allg. Chem. 1939, 240, 300–312. [Google Scholar] [CrossRef]
- Prokofiey, A.V.; Shelykh, A.I.; Golubkov, A.V.; Smirnov, I.A. Crystal growth and optical properties of rare earth sesquiselenides and sesquisulphides—New magneto-optic materials. J. Alloys Compds. 1995, 219, 172–175. [Google Scholar] [CrossRef]
- Wirth, W.; Gloxhuber, C. Toxikologie (In German), 5th ed; Urban and Fischer: München, Germany, 2000. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Herrendorf, W.; Bärnighausen, H. HABITUS: A Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE, Version 1.06; Darmstadt: Karlsruhe, Germany, 1996. [Google Scholar]
- Hahn, T.; Wilson, A.J.C. International Tables for Crystallography, Volume C, 2nd ed; Kluwer Academic Publishers: Boston, MA, USA, 1992. [Google Scholar]
- Jansen, M.; Letschert, H.P. Inorganic yellow-red pigments without toxic metals. Nature 2000, 404, 980–982. [Google Scholar] [CrossRef]
- Schurz, C.M.; Schleid, T. La10.25O0.25N0.75Se14: The first lanthanum oxide nitride selenide with a stuffed Pr10OS14-Type structure and subtle interactions of La3+ Cations. Z. Kristallogr. 2012, 104. [Google Scholar]
- Schurz, C.M. Über Nitridhalogenide und -Chalkogenide Dreiwertiger Seltenerdmetalle: Synthese, Kristallstrukturen, Optische Spektroskopie und Oxidische Derivate (In German). Ph.D. Dissertation, University of Stuttgart, Stuttgart, Germany, 2011. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Weber, F.A.; Schurz, C.M.; Frunder, S.; Kuhn, C.F.; Schleid, T. The Short Series of the Oxygen-Poor Lanthanide Oxide Selenides M10OSe14 with M = La–Nd. Crystals 2012, 2, 1136-1145. https://doi.org/10.3390/cryst2031136
Weber FA, Schurz CM, Frunder S, Kuhn CF, Schleid T. The Short Series of the Oxygen-Poor Lanthanide Oxide Selenides M10OSe14 with M = La–Nd. Crystals. 2012; 2(3):1136-1145. https://doi.org/10.3390/cryst2031136
Chicago/Turabian StyleWeber, Frank A., Christian M. Schurz, Susanne Frunder, Charlotte F. Kuhn, and Thomas Schleid. 2012. "The Short Series of the Oxygen-Poor Lanthanide Oxide Selenides M10OSe14 with M = La–Nd" Crystals 2, no. 3: 1136-1145. https://doi.org/10.3390/cryst2031136




