Abstract
Five new 5,5'-azotetrazolate salts (amminsilver, trimethylsulfonium, tetramethyl-phosphonium, trimethylsulfoxonium, 2-(hydroxyethyl)trimethylammonium) were prepared and characterized. The crystal structures were determined by X-ray diffraction. Interactions between the ions are identified and discussed. The sensitivities of the highly energetic silver salt were measured by BAM (Bundesanstalt für Materialforschung und-prüfung) methods.
1. Introduction
Nitrogen-rich “energetic salts” have received considerable attention as propellants or gas generators [1,2]. These salts typically contain cations such as hydrazinium [3,4,5], ammonium and guanidinium [6], triazolium [7,8,9,10], tetrazolium [11,12], or tetraaminopiperazinium [13], sometimes involving additional azido groups [6,10]. Preferred anions are—beside azide, nitrate, perchlorate and picrate—dinitramide [7], nitroazolates [7,10,14,15], dianions such as 5,5'-bis(tetrazolate) [16] and, in particular, 5,5'-azotetrazolate [3,4,5,6,11,12,16]. The synthesis of 5,5'-azotetrazolates was first reported by Thiele [17]. Beyond the much acclaimed use as explosives, nitrogen-rich heterocycles are also of interest as ligands in coordination chemistry. With respect to their performance as potential explosives or propellants powerful materials are obtained when nitrogen-rich cations (e.g., hydrazinium, guanidinium) are used. When ions with lower nitrogen and higher carbon content are employed, the resulting less energetic materials are still relevant. Due to their electrochemical or optical properties, technical applications such as molecular electronics are envisioned [18]. They also have potential as precursors of functional materials, for example in the synthesis of low-density, nanoporous metal foams [19].
Numerous crystal structures of 5,5'-azotetrazolate salts [3,4,5,6,7,8,11,12,13,16,20,21] have been reported, including a series of metal salts; specifically, alkali and alkaline earth metals [22,23], lanthanoids [24,25,26], Pb [27], Tl [28], Mn [29], Fe [30], Cu and Cd [31] form crystalline salts.
In the present work 5,5'-azotetrazolate salts comprising cations based on sulfur or phosphorus are reported. A not yet described salt of choline and a new energetic silver complex are also disclosed, the latter one showing high sensitivities belonging to the class of primary explosives.
2. Results and Discussion
The silver complex was prepared by slow diffusion of the components. The incorporated ammonia molecule has a phlegmatizing effect causing the product to be less sensitive than the pure silver salt. The other salts were synthesized by two metathetical steps (the Ag2SO4/Ba azotetrazolate method) starting from the respective halogenides. Satisfactory crystals could be obtained with little effort by slow evaporation of solutions in water or methanol. The structures reported herein are centrosymmetric. In all cases the asymmetric unit contained one half of the planar azotetrazolate ion which is completed by inversion. The crystallographic data and structure refinement parameters of all structures 1–5 are gathered in Table 1.

Table 1.
Crystal data and structure refinement details for compounds 1–5.
| Compound | 1 | 2 | 3 | 4 | 5 |
| CCDC no. | 846911 | 846912 | 846913 | 846914 | 846915 |
| Chemical formula | Ag2(NH3)2 (C2N10) | (C3H9S)2 (C2N10) | (C4H12P)2·(C2N10) | (C3H9OS)2·(C2N10) | (C5H14NO)2·(C2N10) |
| Mr | 413.89 | 318.43 | 346.31 | 350.42 | 372.43 |
| Crystal shape, color | plate, orange | prism, yellow | plate, orange | fragment, yellow | fragment, yellow |
| Crystal size/mm3 | 0.1 × 0.1 × 0.06 | 0.44 × 0.36 × 0.24 | 0.36 × 0.32 × 0.12 | 0.2 × 0.2 × 0.2 | 0.40 × 0.24 × 0.24 |
| Crystal system | monoclinic | triclinic | monoclinic | monoclinic | triclinic |
| Space group | C2/c | P  | P21/c | P21/n | P  |
| a/Å | 18.0338(7) | 5.9032(7) | 5.9035(9) | 5.2452(2) | 5.4900(4) |
| b/Å | 3.601(2) | 7.4591(8) | 13.388(2) | 14.0290(4) | 8.4206(6) |
| c/Å | 14.906(3) | 9.2538(8) | 11.3173(19) | 10.7735(4) | 10.3003(6) |
| α/° | 90 | 113.598(9) | 90 | 90 | 78.564(5) |
| β/° | 91.94(1) | 98.370(8) | 93.941(17) | 102.036(3) | 85.796(6) |
| γ/° | 90 | 99.017(9) | 90 | 90 | 81.876(6) |
| V/Å3 | 967.4(6) | 358.88(7) | 892.4 (2) | 775.34 (5) | 461.53(6) |
| Z | 8 | 2 | 4 | 4 | 2 |
| Dx/g cm–3 | 2.84 | 1.47 | 1.29 | 1.50 | 1.34 |
| µ/mm–1 | 4.04 | 0.38 | 0.26 | 0.37 | 0.10 |
| F(000)/e | 784 | 168 | 368 | 368 | 200 |
| Diffractometer | Nonius KappaCCD | Gemini Ultra | Gemini-R Ultra | Gemini-R Ultra | Gemini-R Ultra |
| Data collection method | φand ω scans | ω scans | ω scans | Ω scans | ω scans |
| Temperature/K | 233 | 173 | 173 | 173 | 173 |
| θmax/° | 24 | 25.4 | 25 | 28 | 25 |
| h, k, l range | –19 ≤ h ≤ 20 | –6 ≤ h ≤ 7 | –5 ≤ h ≤ 7 | –6 ≤ h ≤ 6 | –6 ≤ h ≤ 5 |
| –4 ≤ k ≤ 3 | –8 ≤ k ≤ 5 | –14 ≤ k ≤ 16 | –18 ≤ k ≤ 17 | –10 ≤ k ≤ 10 | |
| –16 ≤ l ≤ 17 | –10 ≤ l ≤ 11 | –15 ≤ l ≤ 13 | –13 ≤ l ≤ 13 | –12 ≤ l ≤ 10 | |
| Absorption correction | none | multi-scan | multi-scan | multi-scan | none |
| Measured reflections | 1927 | 2168 | 3626 | 6332 | 3070 |
| Independent reflections (Rint) | 739 (0.028) | 1288 (0.024) | 1760 (0.021) | 1723 (0.021) | 1686 (0.030) |
| Observed reflections [I≥ 2σ(I)] | 620 | 1162 | 1461 | 1573 | 1449 |
| Restraints / parameters | 0/75 | 0/94 | 0/104 | 0/103 | 0/123 |
| R1/wR2[I ≥ 2σ(I)] | 0.037/0.097 | 0.030/0.070 | 0.030/0.079 | 0.028/0.070 | 0.035/0.095 |
| R1/wR2 (all data) | 0.046/0.104 | 0.035/ 0.073 | 0.039/0.082 | 0.031/0.072 | 0.042/0.098 |
| Goodness of fit | 1.09 | 1.06 | 1.08 | 1.05 | 1.03 |
| Δρmax/min/e Å–3 | 1.38/–0.81 | 0.24/–0.25 | 0.27/–0.24 | 0.34/0.31 | 0.19/–0.17 |
2.1. Bis(amminsilver(I)) 5,5'-Azotetrazolate (1)
The silver ion in compound 1 coordinates to two nitrogen atoms of two azotetrazolate anions and to the ammonia molecule. Short contacts observed are Ag...N1 (2.255(5) Å), Ag...N2i (2.342(5) Å), and Ag...N6 (2.205(6) Å), respectively (Figure 1). Symmetry operation i: 1/2 – x, 1/2 + y, 1/2 – z. These interionic contacts assemble a layer structure parallel to the bc-plane.
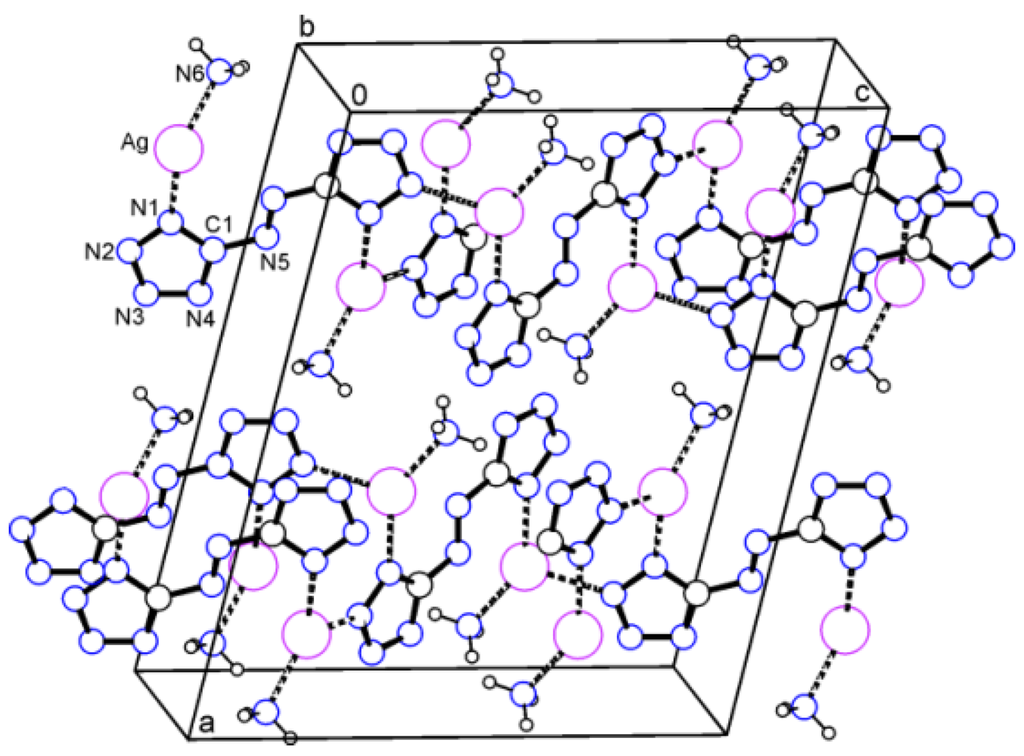
Figure 1.
Packing diagram of Ag salt 1. Dashed lines represent the Ag...N interactions. Layers are arranged parallel to the bc-plane.
2.2. Bis(trimethylsulfonium) 5,5'-Azotetrazolate (2)
In contrast, no directional interactions are found in salt 2. This aggregate consists of discrete ions and is predominantly stabilized by electrostatic forces. The (CH3)3S+ cation is pyramidal and exhibits approximately 3m symmetry as found in other trimethylsulfonium salts in the literature [32]. The C–S bond lengths are equal within the experimental error (1.782(2) Å), and the C–S–C angles differ only slightly (100.3, 101.6 and 102.4°). As discussed previously [33], the electron lone pair is not a structure-determining factor. The shortest distance between neighbouring S atoms is 3.742 Å. The unit cell is shown in Figure 2.
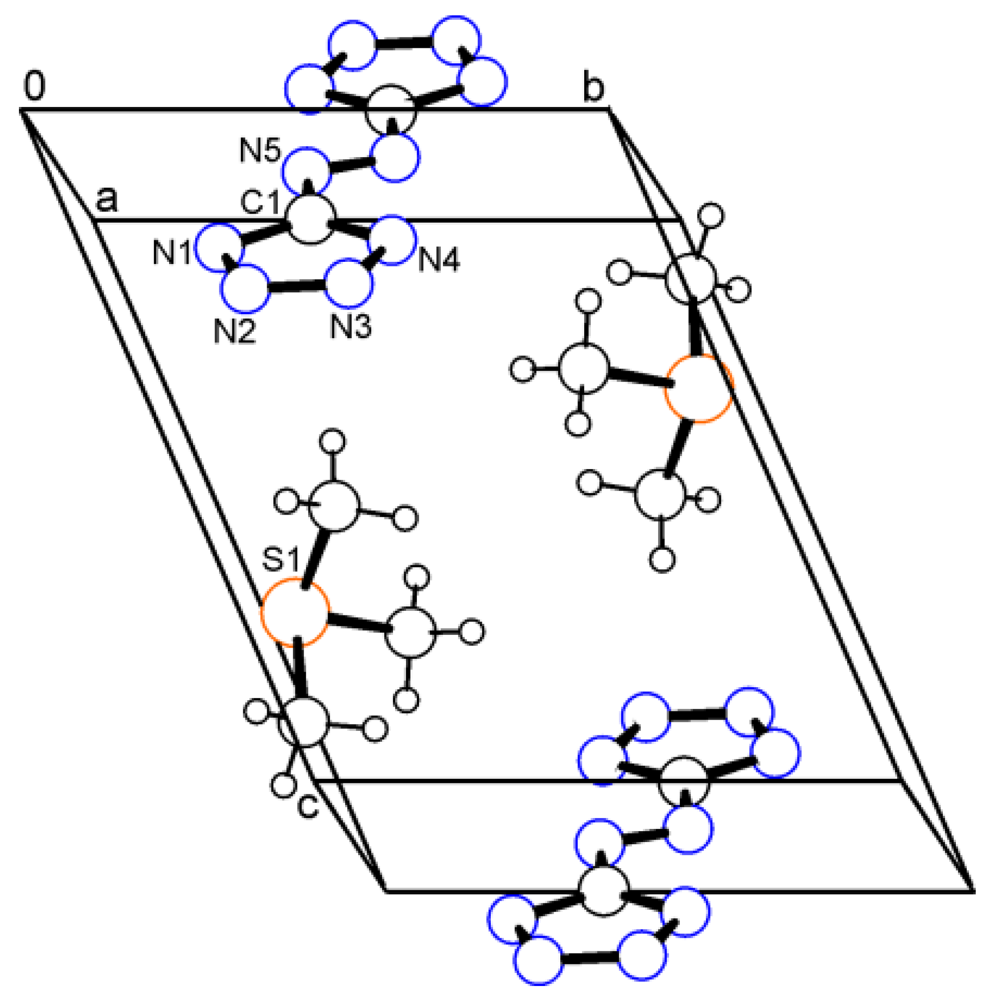
Figure 2.
Packing diagram of trimethylsulfonium salt 2.
2.3. Bis(tetramethylphosphonium) 5,5'-Azotetrazolate (3)
The (CH3)4P+ cation in 3 shows only small deviations from ideal tetrahedral geometry. The C–P bond lengths range from 1.772 to 1.777 Å, and the C–P–C angles from 107.9 to 110.4°. The shortest distance between neighbouring P atoms is 5.736 Å. The crystal packing is shown in Figure 3a. There is a number of weak C–H...N contacts in this structure with three of them being considerably shorter than the sum of van der Waals radii. Thus, C4–H...N5 (d(H...A) = 2.494 and d(D...A) = 3.404 Å, <(D–H...A) = 154.4°), C5–H...N1 (2.566 and 3.540 Å, 172.4°), and C5–H...N5ii (2.517 and 3.403 Å, 150.2°) are the major interactions (Figure 3b). Symmetry operation ii: –1 + x, y, z.
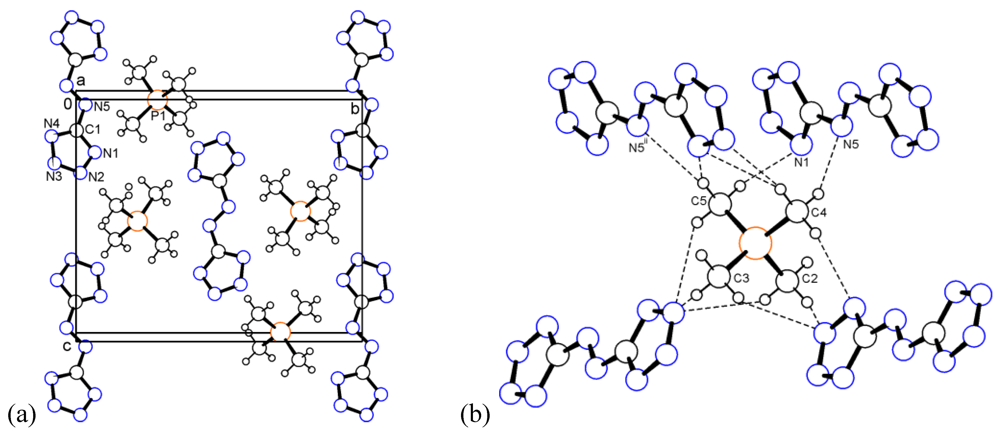
Figure 3.
(a) Packing diagram of tetramethylphosphonium salt 3. (b) Dashed lines represent the C–H...N contacts. Only N atoms engaged in major interactions are numbered.
2.4. Bis(trimethylsulfoxonium) 5,5'-Azotetrazolate (4)
The (CH3)3SO cation in 4 again adopts a pyramidal geometry. It has neither a mirror plane nor a rotation axis. Nevertheless, the 3m symmetry is approximately fulfilled in good agreement with known trimethylsulfoxonium salts [34]. The shortest S–S distance is 5.245 Å. The packing is presented in Figure 4a. Probably due to the enhanced dipolar character of this cation, it participates in a series of significant C–H...N interactions with the anion. All nitrogen atoms of the anion serve as acceptors building a three-dimensional network (Figure 4b): C3–H...N4 (2.477 and 3.289 Å, 140.0°), C2–H...N5iii (2.411 and 3.352 Å, 160.8°), C4–H...N1iv (2.634 and 3.407 Å, 136.1°), C2–H...N3v (2.497 and 3.428 Å, 158.6°), C4–H...N2v (2.563 and 3.499 Å, 159.8°), C3–H...N1vi (2.435 and 3.334 Å, 152.3°), C4–H...N2vi (2.446 and 3.320 Å, 148.4°). Symmetry codes iii: 1 – x, 1 – y, 1 – z; iv: 1/2 – x, -1/2 + y, 1/2 – z; v: –1/2 + x, 1/2 – y,1/2 + z; vi: 3/2 – x, –1/2 + y, 1/2 – z.
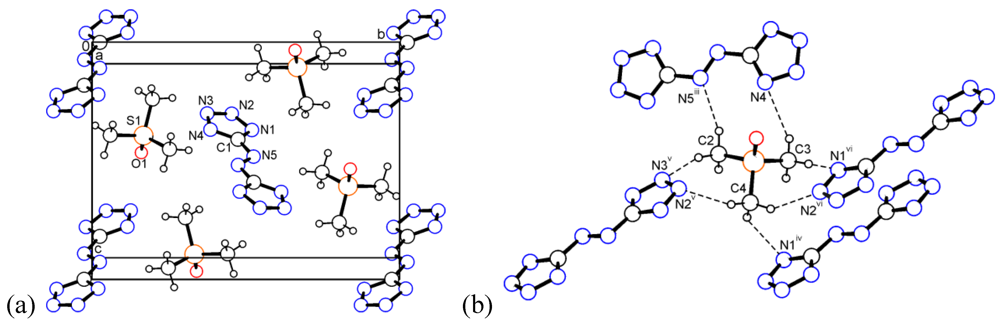
Figure 4.
(a) Packing diagram of trimethylsulfoxonium salt 4. (b) Dashed lines represent the short C–H...N contacts (for symmetry operators see text).
2.5. Bis(2-(hydroxyethyl)trimethylammonium) 5,5'-azotetrazolate (5)
In the structure of 5, a strong hydrogen bond with the parameters O1–H...N1 (1.984 and 2.791 Å, 160.8°) between the cation and the dianion is observed. Figure 5 shows the unit cell of 5.
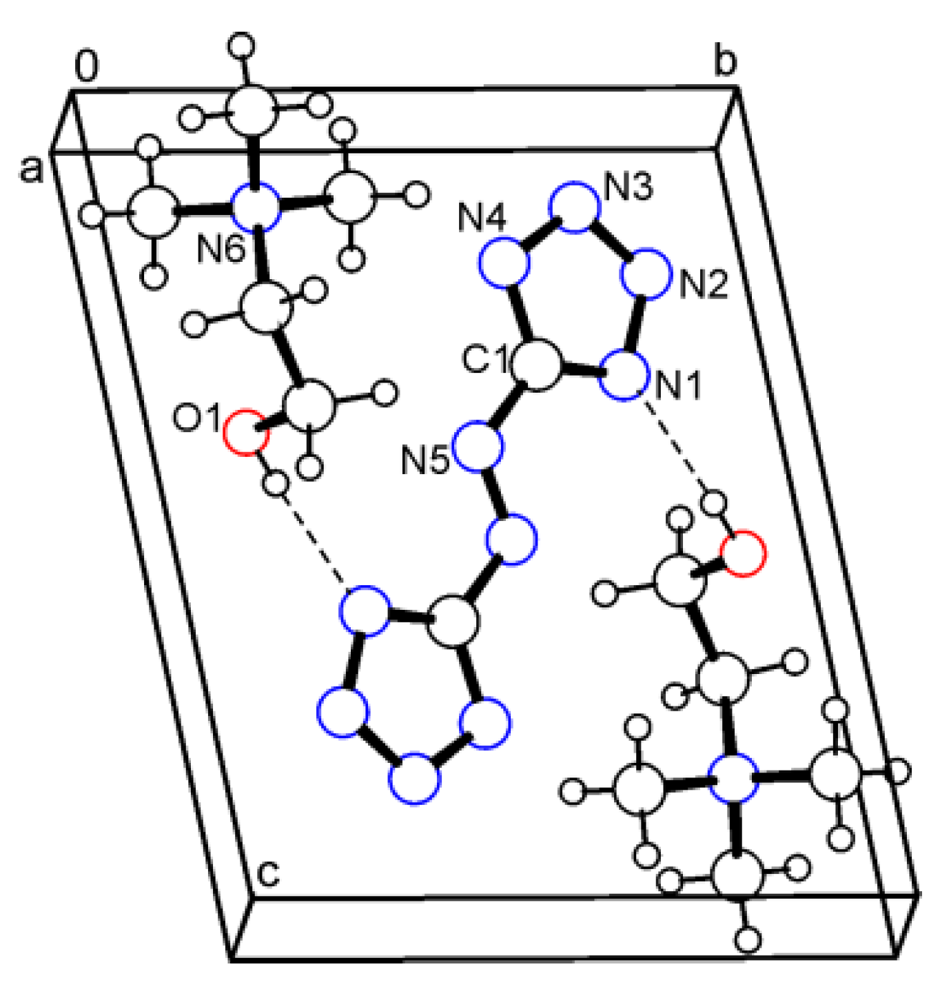
Figure 5.
Packing diagram of cholinium salt 5. Dashed lines represent the strong intermolecular O–H···N hydrogen bonds.
3. Experimental Section
The starting materials were obtained from Sigma-Aldrich and used as received. The NMR spectra were recorded with a Bruker AC 300 spectrometer. IR spectra were obtained with a Nicolet 5700 FT instrument in ATR mode. The impact and friction sensitivity of silver salt 1 was determined by the BAM drophammer (method 1 of 6) [35,36,37] and BAM friction tester [35,36,37] respectively. The sensitivity towards electrostatic discharge was measured using an OZM small scale electrostatic discharge device [38]. X-Ray diffraction data were collected on Oxford Diffraction Gemini-R Ultra and Nonius Kappa CCD diffractometers using Mo-Kα radiation. The structures were solved by direct methods and refined by full-matrix least-squares methods on F2 [39,40]. CCDC 846911-846915 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
3.1. Bis(amminsilver(I)) 5,5'-Azotetrazolate (1)
Caution: this compound is a primary explosive with extremely high sensitivities towards friction and also electrostatic discharge when dry. Proper protective measures (safety glasses, face shield, leather coat, earthened equipment and shoes, Kevlar® gloves and ear plugs) should be used during the handling of this compound. Concentrated NH3 (2 mL) was layered over a solution of AgNO3 (51 mg, 0.30 mmol) in H2O (2 mL) and concentrated NH3 (1 mL). A solution of sodium azotetrazolate pentahydrate (45 mg, 0.15 mmol) in H2O (5 mL) and concentrated NH3 (1 mL) was added cautiously on top of the mixture. The mixture was set aside, and orange-red crystals grew overnight at 20 °C. Yield: 10 mg (16%). No melting below 230 °C (dec). No NMR spectra could be recorded due to insolubility in common solvents. IR (neat, cm−1): 1396 (s), 1214 (w), 1184 (m), 1168 (m), 1049 (w), 1032 (w), 765 (m), 737 (s), 719 (m). BAM drophammer: 2 J. BAM friction tester (<5 N). ESD: 5 mJ.
3.2. Preparation of 5,5'-Azotetrazolates (2–5) (General Procedure)
Ag2SO4 (78 mg, 0.25 mmol) was added to a solution of the respective organic halide (0.50 mmol) in H2O (10 mL). The mixture was stirred at 50 °C for 10 min and ultrasonicated for 5 min. Subsequently, the precipitate was removed by centrifugation. Barium azotetrazolate pentahydrate (94 mg, 0.24 mmol) was added to the supernatant, and the mixture was again stirred at 50 °C for 10 min and ultrasonicated for 5 min. After centrifugation, the supernatant solution was brought to dryness in a rotary evaporator, the temperature not exceeding 50 °C. The yellow residue was recrystallized from MeOH, collected by filtration and vacuum-dried.
3.3. Bis(trimethylsulfonium) 5,5'-Azotetrazolate (2)
Yield: 61 mg (80%), m.p. 155 °C (dec.). 1H NMR (DMSO-d6, 300 MHz): δ 2.90 (s). 13C NMR (DMSO-d6, 75 MHz): δ 26.2 (3C), 173.3. IR (neat): v 2992 (m), 2976 (m), 1417 (w), 1401 (s), 1196 (w), 1163 (w), 1039 (s), 773 (m), 739 (s), 733 (s) cm–1.
3.4. Bis(tetramethylphosphonium) 5,5'-Azotetrazolate (3)
Yield: 70 mg (84%), m.p. 215 °C (dec.). 1H NMR (DMSO-d6, 300 MHz, ppm): δ 1.85 (d, JH–P = 15.4 Hz). 13C NMR (DMSO-d6, 75 MHz, ppm): δ 8.9 (d, JC–P = 55 Hz, 4C), 173.4. IR (neat, cm−1): v 2985 (m), 2915 (w), 1434 (m), 1374 (m), 1289 (m), 1174 (w), 1149 (w), 1015 (m), 973 (s), 772 (m), 725 (m).
3.5. Bis(trimethylsulfoxonium) 5,5'-Azotetrazolate (4)
Yield: 75 mg (89%), m.p. 192–193 °C (dec.). 1H NMR (DMSO-d6, 300 MHz, ppm): δ 3.92 (s). 13C NMR (DMSO-d6, 75 MHz, ppm): δ 39.2 (3C), 173.2. IR (neat, cm–1): v 2959 (s), 2878 (m), 1403 (s), 1225 (s), 1036 (s), 950 (s), 756 (m), 741 (m).
3.6. Bis(2-(hydroxyethyl)trimethylammonium) 5,5'-Azotetrazolate (5)
Yield: 65 mg (73%), m.p. 126–129 °C. 1H NMR (DMSO-d6, 300 MHz, ppm): δ 3.15 (s, 9H), 3.45 (m, 2H), 3.82 (m, 2H), 5.76 (s, 1H). 13C NMR (DMSO-d6, 75 MHz, ppm): δ 53.2 (3C), 55.1, 66.9, 173.3. IR (neat, cm–1): v 3177 (w), 3028 (w), 2902 (w), 1471 (m), 1384 (s), 1338 (w), 1179 (w), 1146 (w), 1095 (s), 1028 (w), 950 (s), 875 (m), 722 (m).
4. Conclusions
A large number of crystalline azotetrazolates is known today and can also be found in a recently published review article [41]. This versatile dianion grants access to an almost unlimited diversity of intriguing structures. Certainly, the five new salts presented in this work will be succeeded by others in due course, stimulating both materials science and crystallography. Silver salt 1 was characterized to be a thermally stable primary explosive which detonates in flame. The sensitivities towards outer stimuli are in the range of those observed for lead azide.
References and Notes
- Singh, R.P.; Verma, R.D.; Meshri, D.T.; Shreeve, J.M. Energetic Nitrogen-Rich Salts and Ionic Liquids. Angew. Chem. Int. Ed. 2006, 45, 3584–3601. [Google Scholar]
- Steinhauser, G.; Klapötke, T.M. “Green” Pyrotechnics: A Chemists’ Challenge. Angew. Chem. Int. Ed. 2008, 47, 3330–3347. [Google Scholar] [CrossRef]
- Hammerl, A.; Holl, G.; Kaiser, M.; Klapötke, T.M.; Mayer, P.; Piotrowski, H.; Vogt, M. Methylated Ammonium and Hydrazinium Salts of 5,5'-Azotetrazolate. Z. Naturforsch. 2001, 56b, 847–856. [Google Scholar]
- Hammerl, A.; Holl, G.; Kaiser, M.; Klapötke, T.M.; Mayer, P.; Nöth, H.; Piotrowski, H.; Suter, M. New Hydrazinium Salts of 5,5'-Azotetrazolate. Z. Naturforsch. 2001, 56b, 857–870. [Google Scholar]
- Hammerl, A.; Klapötke, T.M.; Nöth, H.; Warchhold, M.; Holl, G.; Kaiser, M.; Ticmanis, U. [N2H5]+2[N4C-N=N-CN4]2–: A New High-Nitrogen High-Energetic Material. Inorg. Chem. 2001, 40, 3570–3575. [Google Scholar]
- Hammerl, A.; Hiskey, M.A.; Holl, G.; Klapötke, T.M.; Polborn, K.; Stierstorfer, J.; Weigand, J.J. Azidoformamidinium and Guanidinium 5,5'-Azotetrazolate Salts. Chem. Mater. 2005, 17, 3784–3793. [Google Scholar]
- Darwich, C.; Klapötke, T.M.; Sabaté, C.M. 1,2,4-Triazolium-Cation-Based Energetic Salts. Chem. Eur. J. 2008, 14, 5756–5771. [Google Scholar]
- Klapötke, T.M.; Sabaté, C.M. Bistetrazoles: Nitrogen-Rich, High-Performing, Insensitive Energetic Compounds. Chem. Mater. 2008, 20, 3629–3637. [Google Scholar]
- Xue, H.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Energetic Salts from N-Aminoazoles. Inorg. Chem. 2004, 43, 7972–7977. [Google Scholar]
- Xue, H.; Shreeve, J.M. Energetic Ionic Liquids from Azido Derivatives of 1,2,4-Triazole. Adv. Mater. 2005, 17, 2142–2146. [Google Scholar]
- Klapötke, T.M.; Sabaté, C.M. New energetic compounds based on the nitrogen-rich 5,5’-azotetrazolate anion ([C2N10]2-). New J. Chem. 2009, 33, 1605–1617. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M. Nitrogen-Rich TetrazoliumAzotetrazolate Salts: A New Family of Insensitive Energetic Materials. Chem. Mater. 2008, 20, 1750–1763. [Google Scholar]
- Gao, H.; Huang, Y.; Twamley, B.; Ye, C.; Shreeve, J.M. Energetic N,N,N',N'-Tetraaminopiperazinium Salts. ChemSusChem 2008, 2, 222–227. [Google Scholar]
- Katritzky, A.R.; Singh, S.; Kirichenko, K.; Holbrey, J.D.; Smiglak, M.; Reichert, W.M.; Rogers, R.D. 1-Butyl-3-methylimidazolium 3,5-dinitro-1,2,4-triazolate: a novel ionic liquid containing a rigid, planar energetic anion. Chem. Commun. 2005, 868–870. [Google Scholar]
- Gao, H.; Ye, C.; Gupta, O.D.; Xiao, J.C.; Hiskey, M.A.; Twamley, B.; Shreeve, J.M. 2,4,5-Trinitroimidazole-Based Energetic Salts. Chem. Eur. J. 2007, 13, 3853–3860. [Google Scholar]
- Ye, C.; Xiao, J.-C.; Twamley, B.; Shreeve, J.M. Energetic salts of azotetrazolate, iminobis(5-tetrazolate) and 5,5'-bis(tetrazolate). Chem. Commun. 2005, 2750–2752. [Google Scholar]
- Thiele, J. Ueber Azo- und Hydrazoverbindungen des Tetrazols. Ann. Chem. 1898, 303, 57–75. [Google Scholar] [CrossRef]
- Weibel, N.; Grunder, S.; Mayor, M. Functional molecules in electronic circuits. Org. Biomol. Chem. 2007, 5, 2343–2353. [Google Scholar]
- Tappan, B.C.; Huynh, M.H.; Hiskey, M.A.; Chavez, D.E.; Luther, E.P.; Mang, J.T.; Son, S.F. Ultralow-Density Nanostructured Metal Foams: Combustion Synthesis, Morphology, and Composition. J. Am. Chem. Soc. 2006, 128, 6589–6594. [Google Scholar]
- Bentivoglio, G.; Laus, G.; Kahlenberg, V.; Nauer, G.; Schottenberger, H. Crystal structure of bis(hydroxylammonium) 5,5'-azotetrazolate dihydrate, (NH3OH)2(C2N10)·2H2O. Z. Kristallogr. NCS 2008, 223, 425–426. [Google Scholar]
- Pan, W.-L.; Chena, X.-Y.; Hu, C.-W. Bis(amantadinium) 5,5'-diazenediylditetrazolate dihydrate. Acta Crystallogr. 2007, E63, o1606–o1608. [Google Scholar]
- Hammerl, A.; Holl, G.; Klapötke, T.M.; Mayer, P.; Nöth, H.; Piotrowski, H.; Warchhold, M. Salts of 5,5'-Azotetrazolate. Eur. J. Inorg. Chem. 2002, 834–845. [Google Scholar]
- Meng, Y. Poly[l-(5,5'-diazenediylditetrazolido)dicaesium]. Acta Crystallogr. 2011, E67, m453. [Google Scholar]
- Steinhauser, G.; Giester, G.; Leopold, N.; Wagner, C.; Villa, M. Nitrogen-Rich Compounds of the Lanthanoids: The 5,5'-Azobis[1H-tetrazol-1-ides] of the Light Rare Earths (Ce, Pr, Nd, Sm, Eu, Gd). Helv. Chim. Acta 2009, 92, 2038–2051. [Google Scholar] [CrossRef]
- Steinhauser, G.; Giester, G.; Wagner, C.; Leopold, N.; Sterba, J.H.; Lendl, B.; Bichler, M. Nitrogen-Rich Compounds of the Lanthanoids: The 5,5'-Azobis[1H-tetrazol-1-ides] of some Yttric Earths (Tb, Dy, Ho, Er, Tm, Yb, and Lu). Helv. Chim. Acta 2009, 92, 1371–1384. [Google Scholar] [CrossRef]
- Steinhauser, G.; Giester, G.; Leopold, N.; Wagner, C.; Villa, M.; Musilek, A. Nitrogen-Rich Compounds of the Lanthanoids: Highlights and Summary. Helv. Chim. Acta 2010, 93, 183–202. [Google Scholar] [CrossRef]
- Pierce-Butler, M.A. Structure of Bis[hydroxolead(II)] 5,5'-Azotetrazolediide. Acta Crystallogr. 1982, B38, 2681–2683. [Google Scholar]
- Bhandari, S.; Mahon, M.F.; Molloy, K.C.; Palmer, J.S.; Sayers, S.F. Thallium(I)- and organothallium(III)-substituted mono-, bis- and tris-tetrazoles: synthesis and supramolecular structures. J. Chem. Soc. Dalton Trans. 2000, 1053–1060. [Google Scholar]
- Jiao, B.; Chen, S.; Zhao, F.; Hu, R.; Gao, S. A new high-nitrogen compound [Mn(ATZ)(H2O)4]·2H2O: Synthesis and characterization. J. Hazard. Mater. 2007, 142, 550–554. [Google Scholar] [CrossRef]
- Jiao, B.; Yan, Z.; Fan, G.; Chen, S.; Gao, S. catena-Poly[[[tetraaquairon(II)]-µ-5,5'-diazenediyldi-tetrazolido] dihydrate]. Acta Crystallogr. 2010, E66, m1374. [Google Scholar]
- Tao, G.-H.; Twamley, B.; Shreeve, J.M. Energetic Nitrogen-Rich Cu(II) and Cd(II) 5,5'-Azobis(tetrazolate) Complexes. Inorg. Chem. 2009, 48, 9918–9923. [Google Scholar]
- Jannin, M.; Puget, R.; de Brauer, C.; Perret, R. Structures of Trimethylsulfonium Salts. I. Refinement of the Structure of the Iodide (CH3)3SI. Acta Cryst. 1991, C47, 982–984. [Google Scholar]
- Knop, O.; Linden, A.; Vincent, B.R.; Choi, S.C.; Cameron, T.S.; Boyd, R.J. The lone electron pair and crystal packing: observations on pyramidal YEL3 species, ab initio calculations, and the crystal structures of Me3SOI, Et3SI, (Me3S)2SnCl6, (Me3SO)2SnCl6, and (Et3S)2SnCl6. Can. J. Chem. 1989, 67, 1984–2008. [Google Scholar] [CrossRef]
- Jannin, M.; Puget, R.; de Brauer, C.; Perret, R. Structures of Trimethyloxosulfonium Salts. I. The Iodide and the Bromide. Acta Cryst. 1991, C47, 1687–1689. [Google Scholar]
- Available online: www.bam.de (accessed on 4 March 2012).
- Available online: www.reichel-partner.de (accessed on 4 March 2012).
- Impact: insensitive > 40 J, less sensitive > 35 J, sensitive > 4 J, very sensitive < 3 J. Friction: insensitive > 360 N, less sensitive = 360 N, sensitive < 360 N and > 80 N, very sensitive < 80 N, extremely sensitive < 10 N. According to the UN Recommendations on the Transport of Dangerous Goods, (+) indicates not safe for transport.
- Available online: http://www.ozm.cz/en/sensitivity-tests/esd-2008a-small-scale-electrostatic-spark-sensitivity-test/ (accessed on 4 March 2012).
- Burla, M.C.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G. More power for direct methods: SIR2002. Z. Kristallogr. 2002, 217, 629–635. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Gao, H.; Shreeve, J.M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).