Structural and Optical Characterization of a New Tetra- and Hexa-Coordinated Cd-Based Hybrid Compound with White Light Emission
Abstract
:1. Introduction
2. Experimental Analysis
2.1. Synthesis Method
2.2. Structure Determination
2.3. Physico-Chemical Characterizations
3. Results and Discussion
3.1. Crystal Structure
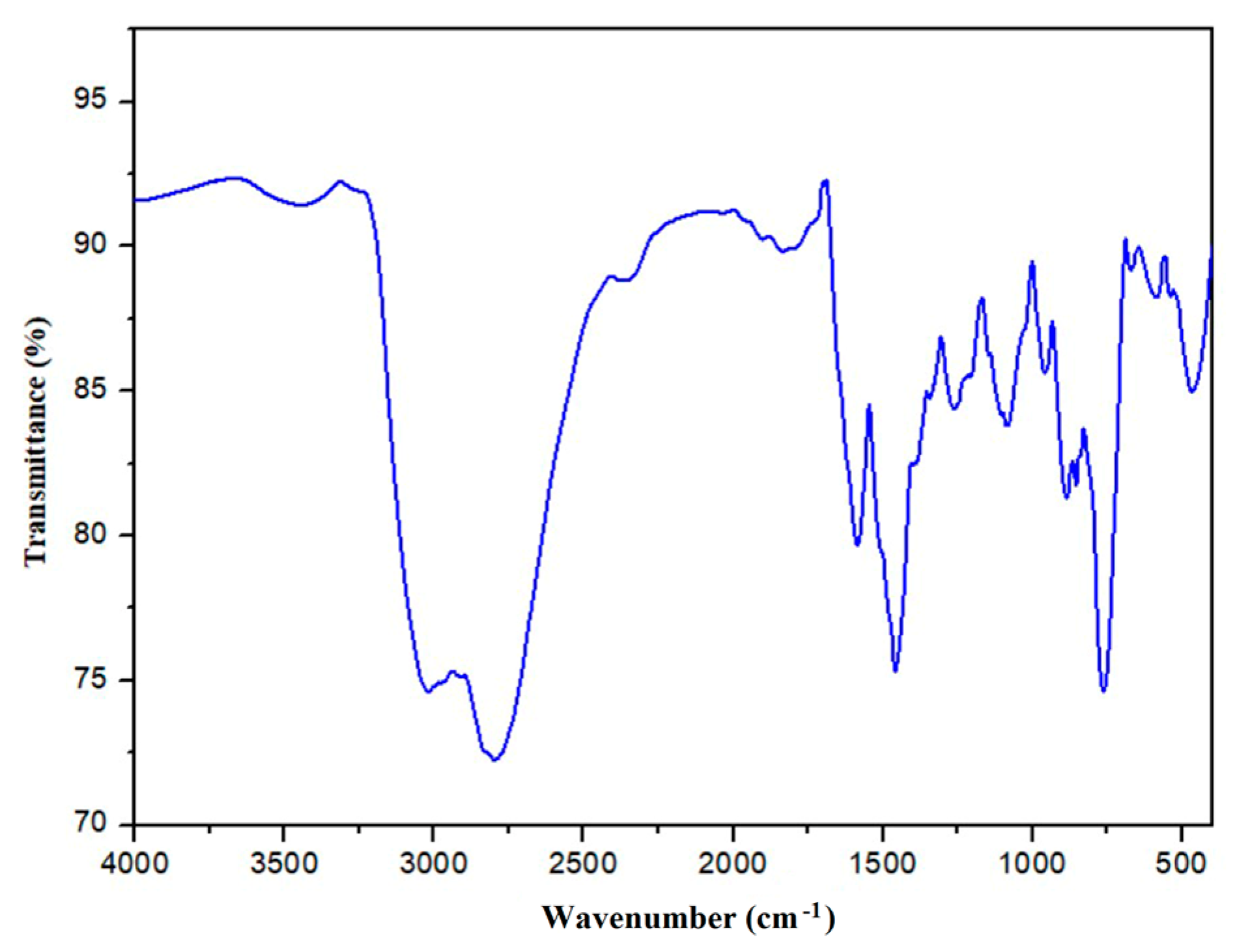
3.2. Infrared Spectroscopy
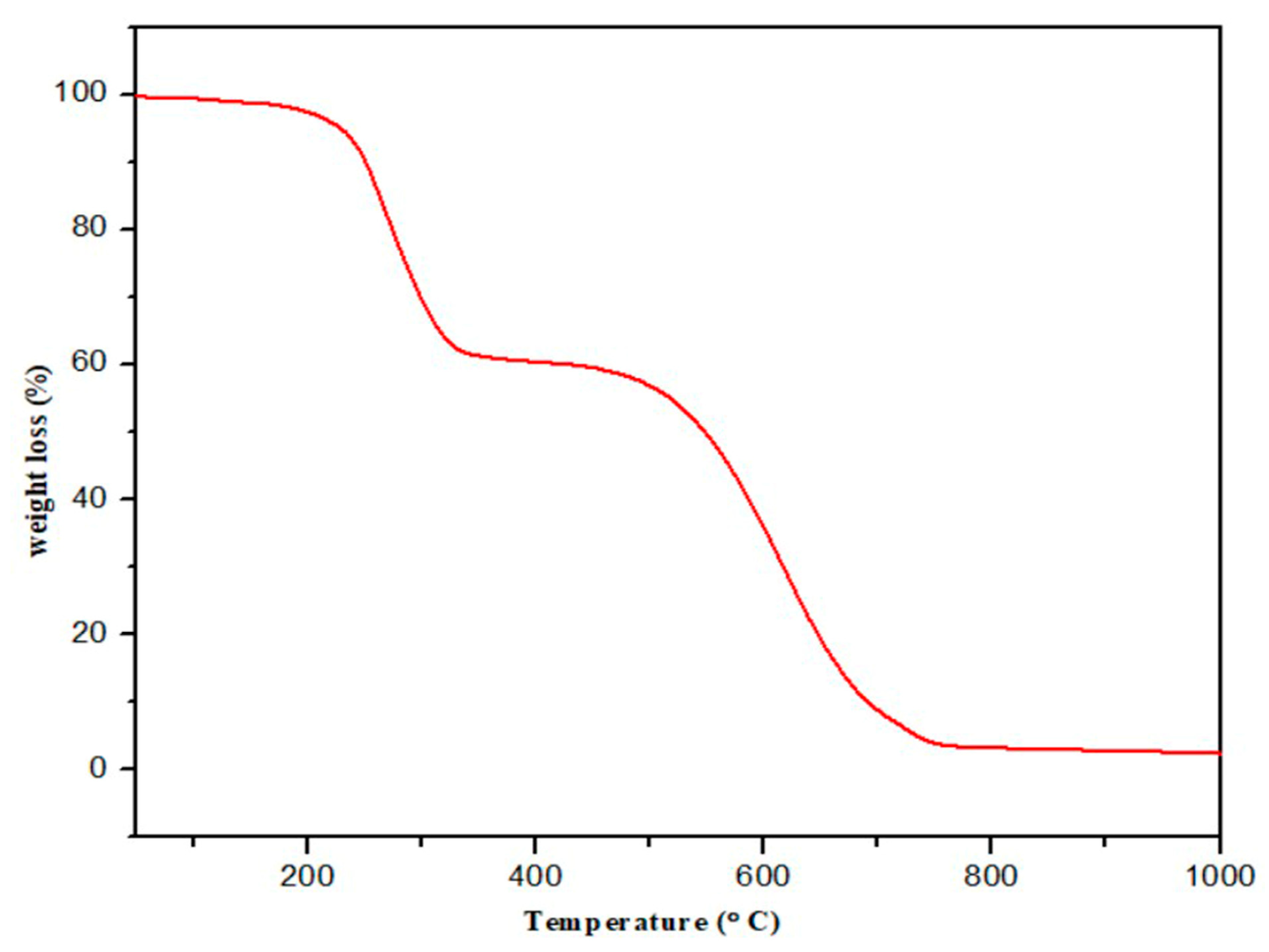
3.3. Thermal Analysis
3.4. Optical Analysis
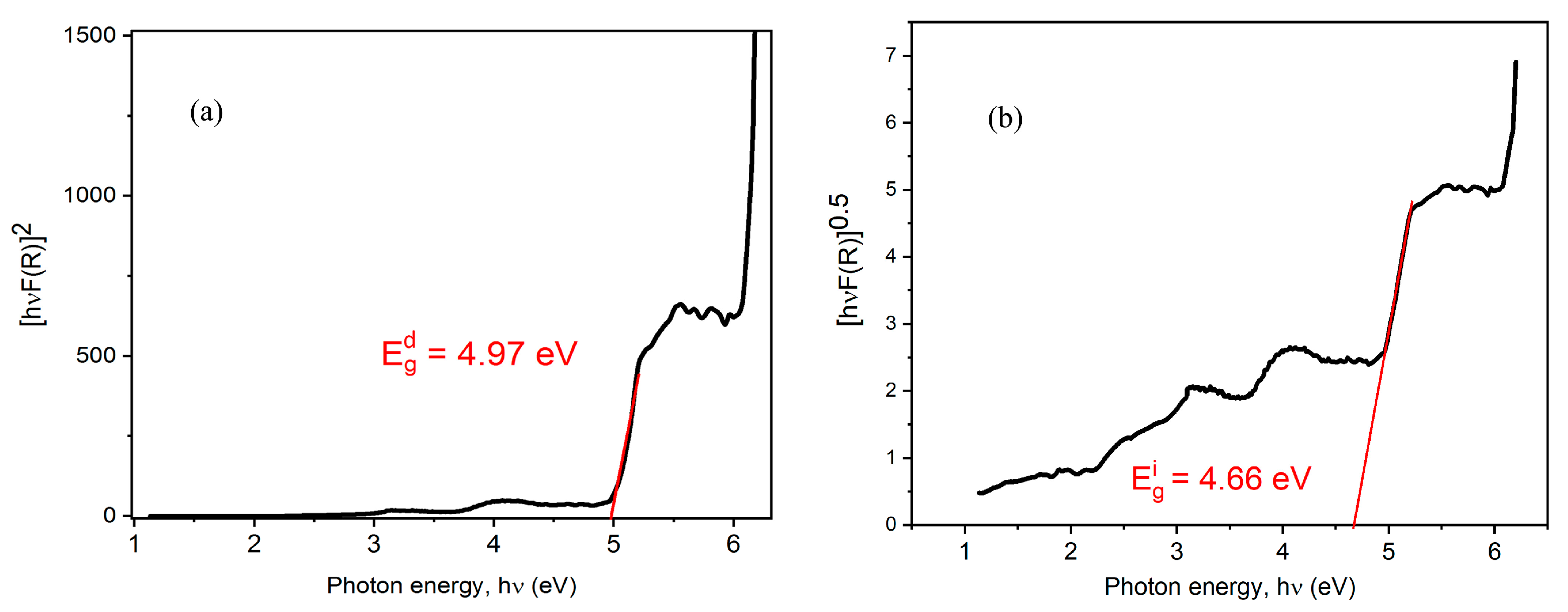
3.4.1. UV–Vis Absorption Properties and Fundamental Gap Energy
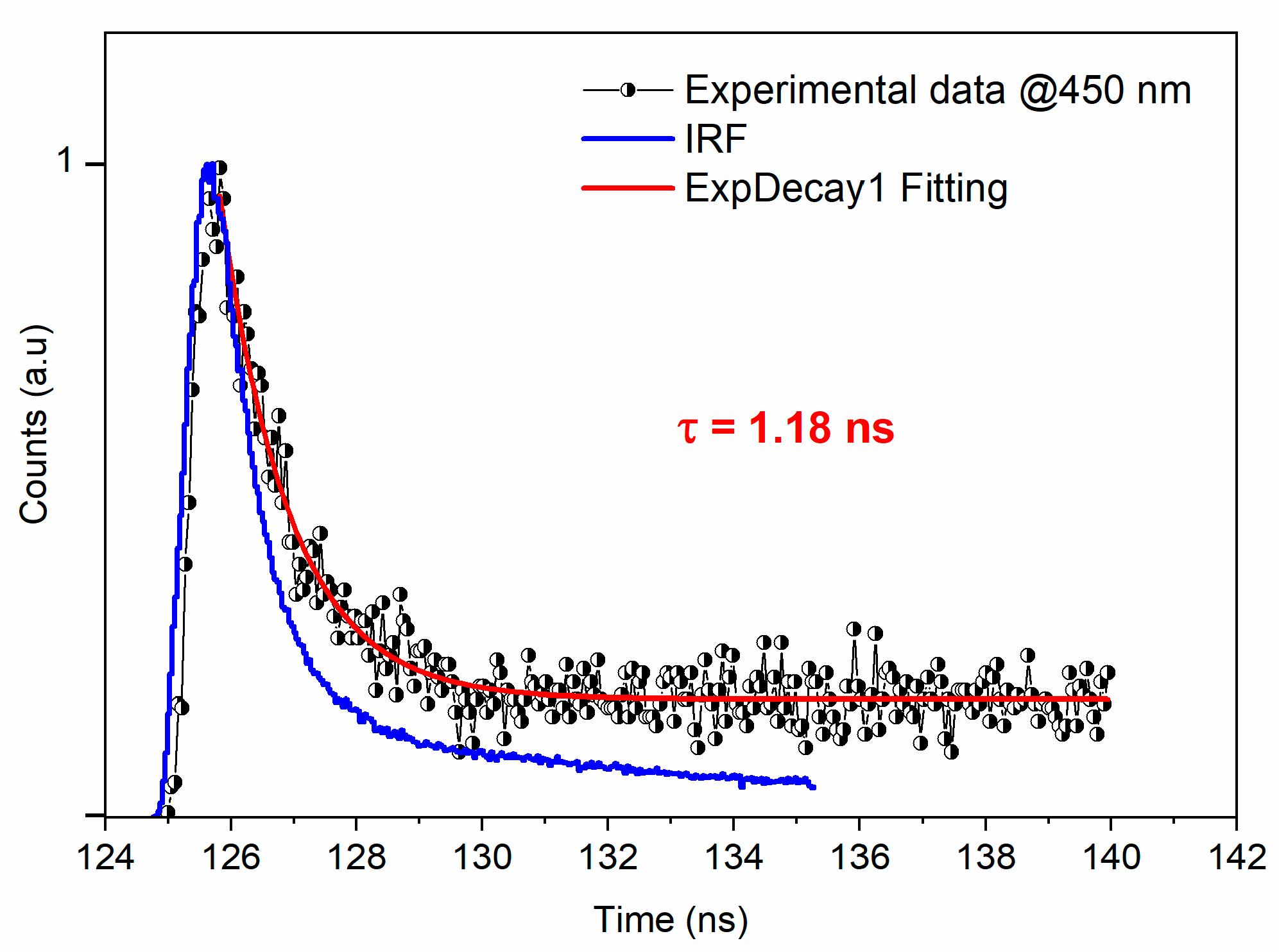
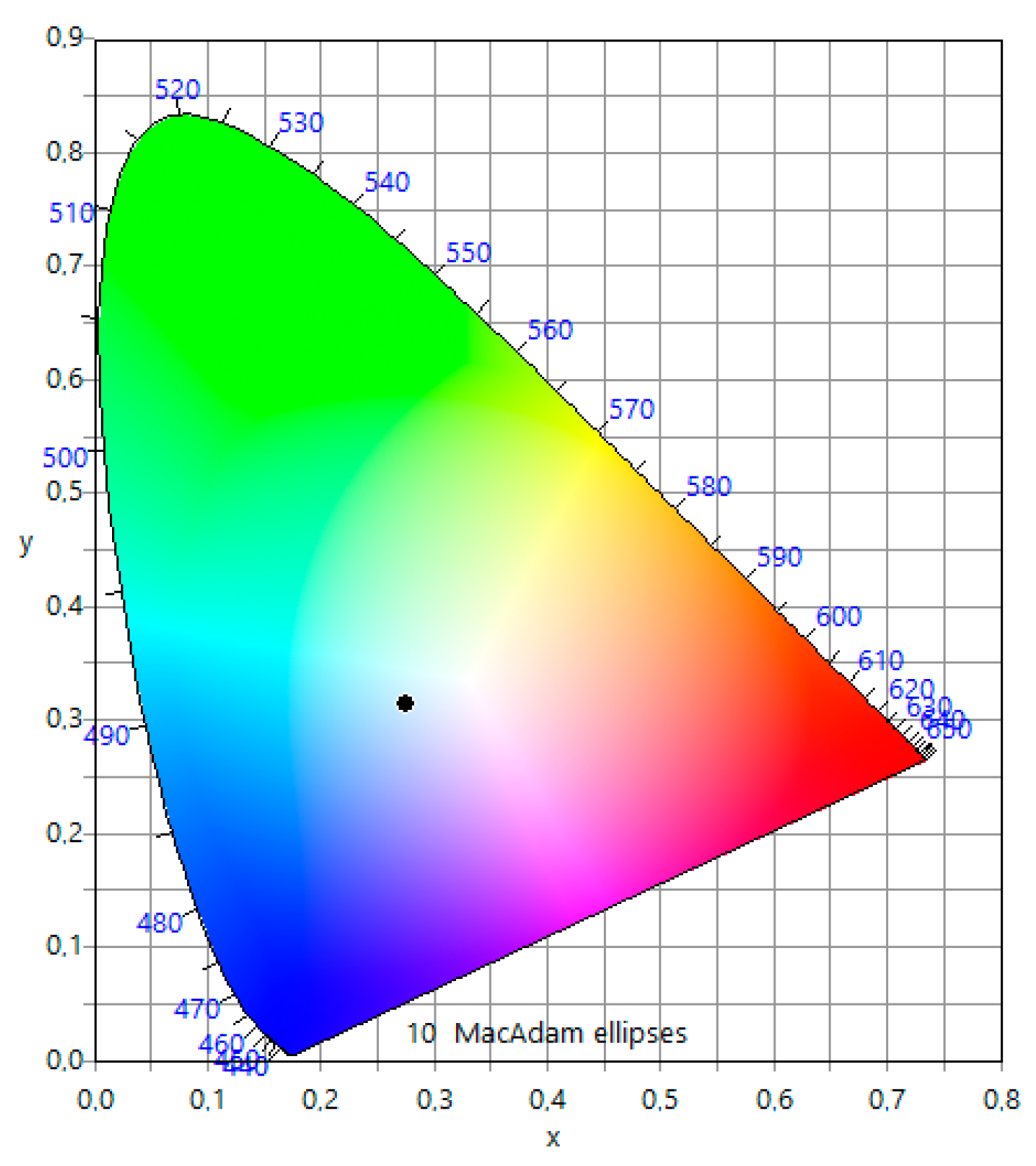
3.4.2. Photoluminescence Properties: White Light Emission
3.4.3. Theoretical Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.S.; Guo, S.P.; Li, Y.; Cai, L.Z.; Zou, J.P.; Xu, G.; Zhou, W.W.; Zheng, F.K.; Guo, G.C. A Direct White-Light-Emitting Metal-Organic Framework with Tunable Yellow-to-White Photoluminescence by Variation of Excitation Light. J. Am. Chem. Soc. 2009, 131, 13572–13573. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J. Optical Properties of Functional Hybrid Organic-Inorganic Nanocomposites. Adv. Mater. 2004, 15, 1969–1994. [Google Scholar] [CrossRef]
- Jin, J.-C.; Lin, Y.-P.; Chen, D.-Y.; Lin, B.-Y.; Zhuang, T.-H.; Ma, W.; Gong, L.-K.; Du, K.-Z.; Jiang, J.; Huang, X.-Y. X-Ray Scintillation and Photoluminescence of Isomorphic Ionic Bismuth Halides with [Amim]+ or [Ammim]+ Cations. Inorg. Chem. Front. 2021, 8, 4474–4481. [Google Scholar] [CrossRef]
- Borysova, K.V.; Sorg, J.R.; Mikhalyova, E.A.; Oberst, K.; Würtele, C.; Müller-Buschbaum, K. Bismuth Trihalide Based Coordination Polymers with the N- Donor Cyanopyridine as Source for Charge Transfer Based Luminescence. J. Inorg. Gen. Chem. 2023, 649, e202300131. [Google Scholar] [CrossRef]
- Jin, J.-C.; Lin, Y.-P.; Lin, L.-F.; Xiao, C.; Song, Y.; Shen, N.-N.; Gong, L.-K.; Zhang, Z.-Z.; Du, K.-Z.; Huang, X.-Y. 2,2′-Bipyridyl-1,1′-Dioxide Based Bismuth(Iii) Bromide Hybrids: Studies on Crystal Structure and Luminescence. CrystEngComm 2021, 23, 3744–3752. [Google Scholar] [CrossRef]
- Zhuang, T.H.; Lin, Y.M.; Lin, H.W.; Guo, Y.L.; Li, Z.W.; Du, K.Z.; Wang, Z.P.; Huang, X.Y. Luminescence Enhancement and Temperature Sensing Properties of Hybrid Bismuth Halides Achieved via Tuning Organic Cations. Molecules 2023, 28, 2380. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-N.; Gao, C.-C.; Guo, J.-S.; Yuan, M.-Y.; Fan, S.-H.; Li, C.; Liu, G.-N. Crystal Structure and Thermophosphorochromic Property of One-Dimensional Hybrid Haloplumbates with Alkylated Benzothiazole Counter Cations. Cryst. Growth Des. 2023, 23, 7982–7991. [Google Scholar] [CrossRef]
- Adonin, S.A.; Rakhmanova, M.E.; Samsonenko, D.G.; Sokolov, M.N.; Fedin, V.P. Bi(III) Halide Complexes Containing 4,4′-Vinylenedipyridinium Cation: Synthesis, Structure and Luminescence in Solid State. Polyhedron 2015, 98, 1–4. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, Z.; Cai, S.; An, Z.; Huang, W. Recent Advances in Room-Temperature Phosphorescence Metal-Organic Hybrids: Structures, Properties, and Applications. Adv. Mater. 2024, 36, e2308290. [Google Scholar] [CrossRef]
- Chen, K.; Lai, Y.-C.; Lin, B.C.; Lin, C.C.; Chiu, S.H.; Tu, Z.-Y.; Shih, M.; Yu, P.; Lee, P.-T.; Li, X.; et al. Efficient Hybrid White Light-Emitting Diodes by Organic-Inorganic Materials at Different CCT from 3000K to 9000K. Opt. Express 2015, 23, A204–A210. [Google Scholar] [CrossRef]
- Bai, T.; Wang, X.; Wang, Z.; Ji, S.; Meng, X.; Wang, Q.; Zhang, R.; Han, P.; Han, K.-L.; Chen, J.; et al. Highly Luminescent One-Dimensional Organic-Inorganic Hybrid Double-Perovskite-Inspired Materials for Single-Component Warm White-Light-Emitting Diodes. Angew. Chem. Int. Ed. Engl. 2023, 62, e202213240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yue, S.; Chen, R.; Yin, J.; Cui, B.-B. White Light-Emitting Diodes Based on One-Dimensional Organic-Inorganic Hybrid Metal Chloride with Dual Emission. Inorg. Chem. 2022, 61, 15475–15483. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.S.; Yoo, S.; et al. Overcoming the Electroluminescence Efficiency Limitations of Perovskite Light-Emitting Diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Park, J.; Han, T.H.; Zhang, F.; Zhu, K.; Kim, J.S.; Park, M.H.; Reese, M.O.; Yoo, S.; Lee, T.W. Characterizing the Efficiency of Perovskite Solar Cells and Light-Emitting Diodes. Joule 2020, 4, 1206–1235. [Google Scholar] [CrossRef]
- Jüstel, T.; Nikol, H.; Ronda, C. New Developments in the Field of Luminescent Materials for Lighting and Displays. Angew. Chemie Int. Ed. 1998, 37, 3084–3103. [Google Scholar] [CrossRef]
- Pan, X.; Liu, H.; Huynh, U.; Vardeny, Z.V. Magneto-Electroluminescence Response in 2D and 3D Hybrid Organic—Inorganic Perovskite Light Emitting Diodes Magneto-Electroluminescence Response in 2D and 3D Hybrid Organic—Inorganic Perovskite Light Emitting Diodes. J. Chem. Phys. 2020, 152, 44714. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Huang, Q.; Yang, S.; Lin, Z.; Ling, Q. A Metal-Free 2D Layered Organic Ammonium Halide Framework Realizing Full-Color Persistent Room-Temperature Phosphorescence. Chem. Sci. 2021, 12, 14451–14458. [Google Scholar] [CrossRef] [PubMed]
- Msalmi, R.; Elleuch, S.; Hamdi, B.; Zouari, R.; Naïli, H. Synthesis, DFT Calculations, Intermolecular Interactions and Third Order Nonlinear Optical Properties of New Organoammonium Tetrabromocadmate (II): (C5H6N2Cl)2[CdBr4]·H2O. J. Mol. Struct. 2020, 1222, 128853. [Google Scholar] [CrossRef]
- Liu, W.; Lustig, W.P.; Li, J. Luminescent Inorganic-Organic Hybrid Semiconductor Materials for Energy-Saving Lighting Applications. EnergyChem 2019, 1, 100008. [Google Scholar] [CrossRef]
- Vargas, B.; Coutiño-Gonzalez, E.; Ovalle-Encinia, O.; Sánchez-Aké, C.; Solis-Ibarra, D. Efficient Emission in Halide Layered Double Perovskites: The Role of Sb3+ Substitution in Cs4Cd1–xMnxBi2Cl12 Phosphors. J. Phys. Chem. Lett. 2020, 24, 10362–10367. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, G.E.; Xu, G.; Long, X.; Xia, Y. Luminescent Inorganic–Organic Hybrid with Tunable Red Light Emissions by Neutral Molecule Modification. Inorg. Chem. Commun. 2020, 116, 107909. [Google Scholar] [CrossRef]
- Uchida, Y. Lighting Theory and Luminous Characteristics of White Light-Emitting Diodes. Opt. Eng. 2005, 44, 124003. [Google Scholar] [CrossRef]
- Taguchi, T. Present Status of White LED Lighting Technologies in Japan. J. Light Vis. Environ. 2003, 27, 131–139. [Google Scholar] [CrossRef]
- Temerova, D.; Chou, T.C.; Kisel, K.S.; Eskelinen, T.; Kinnunen, N.; Jänis, J.; Karttunen, A.J.; Chou, P.T.; Koshevoy, I.O. Hybrid Inorganic-Organic Complexes of Zn, Cd, and Pb with a Cationic Phenanthro-Diimine Ligand. Inorg. Chem. 2022, 61, 19220–19231. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; He, W.-L.; Liu, M.-J.; Pan, W.-J.; Dong, L.-F.; Chen, G.; Liu, G.-D.; Lei, X.-W. Zero-Dimensional Hybrid Cd-Based Perovskites with Broadband Bluish White-Light Emissions. Chem. Asian J. 2020, 15, 3050–3058. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Chen, Y.; Guo, Y.; Yang, X.; Zhang, F.Q.; Zhou, G.; Zhang, X.M. Broadband White-Light Emission in a One-Dimensional Organic-Inorganic Hybrid Cadmium Chloride with Face-Sharing CdCl6octahedral Chains. J. Mater. Chem. C 2021, 9, 88–94. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Han, S.; Li, Y.; Xu, Z.; Luo, J.; Hong, M.; Sun, Z.; Liu, Y.; Han, S.; et al. Ultrasensitive Polarized-Light Photodetectors Based on 2D Hybrid. Sci. Bull. 2020, 66, 158–163. [Google Scholar] [CrossRef]
- Jellali, H.; Msalmi, R.; Smaoui, H.; Elleuch, S.; Tozri, A.; Roisnel, T.; Mosconi, E.; Althubiti, N.A.; Naïli, H. Zn2+ and Cu2+ Doping of One-Dimensional Lead-Free Hybrid Perovskite ABX3 for White Light Emission and Green Solar Cell Applications. Mater. Res. Bull. 2022, 151, 111819. [Google Scholar] [CrossRef]
- Msalmi, R.; Elleuch, S.; Hamdi, B.; Abd El-Fattah, W.; Ben Hamadi, N.; Naili, H. Organically Tuned White-Light Emission from Two Zero-Dimensional Cd-Based Hybrids. RSC Adv. 2022, 12, 10431. [Google Scholar] [CrossRef]
- Sasa, W.; Li, L.; Sun, Z.; Ji, C.; Liu, S.; Wu, Z.; Zhao, S.; Hong, M.; Luo, J. A Semi-Conductive Organic-Inorganic Hybrid Emits Pure White Light with Ultrahigh Color Rendering Index. J. Mater. Chem. C 2017, 5, 4731–4735. [Google Scholar] [CrossRef]
- Gassara, M.; Msalmi, R.; Liu, X.; Hassen, F.; Moliterni, A.; Ben Hamadi, N.; Guesmi, A.; Khezami, L.; Soltani, T.; Naïli, H. A Promising 1D Cd-Based Hybrid Perovskite-Type for White-Light Emission with High-Color-Rendering Index. RSC Adv. 2022, 12, 33516–33524. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, A.; Yamada, T.; Yoshinari, T.; Akimoto, I.; Kanno, K.; Kamikawa, T. Emission Spectra and Decay Characteristics in Photo-Stimulated (CnH2n+,NHa)2CdCh: N = l, 2, 3. J. Electron Spectros. Relat. Phenomena 1996, 79, 163–166. [Google Scholar] [CrossRef]
- Roccanova, R.; Ming, W.; Whiteside, V.R.; McGuire, M.A.; Sellers, I.R.; Du, M.H.; Saparov, B. Synthesis, Crystal and Electronic Structures, and Optical Properties of (CH3NH3)2CdX4 (X = Cl, Br, I). Inorg. Chem. 2017, 56, 13878–13888. [Google Scholar] [CrossRef] [PubMed]
- Roccanova, R.; Houck, M.; Yangui, A.; Han, D.; Shi, H.; Wu, Y.; Glatzhofer, D.T.; Powell, D.R.; Chen, S.; Fourati, H.; et al. Broadband Emission in Hybrid Organic—Inorganic Halides of Group 12 Metals. ACS Omega 2018, 3, 18791–18802. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, X.; Lu, B.; Yan, D. Wide Range Zero-Thermal-Quenching Ultralong Phosphorescence from Zero-Dimensional Metal Halide Hybrids. Nat. Commun. 2020, 11, 4649. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Chen, W.; Hu, R. Photoluminescence and Theoretical Study of [∞ 1(Cd2Cl6)][2(CdCl4)][3(N,N′-Dimethyl- 4,4′-Bipyridinium)]. Indian J. Chem. 2015, 54A, 489–493. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, I.; Bkhairia, I.; Roodt, A.; Roisnel, T.; Nasri, M.; Naïli, H. Synthesis, Intermolecular Interactions and Biological Activities of Two New Organic-Inorganic Hybrids C6H10N2,2Br and C6H10N2,2Cl·H2O. RSC Adv. 2020, 10, 5864–5873. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, I.; Mhadhbi, N.; Issaoui, N.; Roodt, A.; Turnbull, M.M.; Naïli, H. Design, Synthesis and Physico-Chemical Studies of a Co(II)/Co(III) Mixed-Valence Complex: An Experimental and DFT Approach. J. Mol. Struct. 2021, 1237, 130384. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Zhang, Y.; Zheng, Y.; Xu, Z.; Liu, R. Structure and Luminescent Properties of Electrodeposited Eu3+ -Doped CaF2 Thin Films. Thin Solid Films 2014, 562, 478–484. [Google Scholar] [CrossRef]
- Msalmi, R.; Dammak, K.; Elleuch, S.; Hamdi, B.; Tozri, A.; Naïli, H. Optoelectronic, Luminescence, and Nonlinear Properties of a Non-Centrosymmetric Cd(II)-Based Complex. J. Phys. Chem. Solids 2022, 163, 110567. [Google Scholar] [CrossRef]
- Zahan, M.; Podder, J. Surface Morphology, Optical Properties and Urbach Tail of Spray Deposited Co3O4 Thin Films. J. Mater. Sci. Mater. Electron. 2019, 30, 4259–4269. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in Phosphor-Converted White Light-Emitting Diodes: Recent Advances in Materials, Techniques and Properties. Mater. Sci. Eng. R Rep. 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Rea, M.S.; Freyssinier, J.P. Color Rendering: Beyond Pride and Prejudice. Color Res. Appl. 2010, 35, 401–409. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]




| Empirical formula | (C6H10N2)4[CdBr6][CdBr4]2 |
| Formula weight (g. mol−1) | 1896.47 |
| Temperature (K) | 296 (2) |
| Crystal system | Triclinic |
| Space group | P |
| a (Å) | 7.9551 (3) |
| b (Å) | 12.7497 (6) |
| c (Å) | 13.5271 (6) |
| α (°) | 65.249 (2) |
| β (°) | 80.351 (2) |
| γ (°) | 75.455 (2) |
| V (Å3) | 1202.97 (9) |
| Z | 1 |
| Crystal size (mm3) | 0.10 × 0.03 × 0.03 |
| Color | Brown |
| λ (MoKα) (Å) | 0.71073 |
| Absorption correction | Multi- scan |
| No. of collected reflections | 46856 |
| No. of independent reflections | 4229 |
| No. of observed reflections (I > 2σ(I)) | 3213 |
| No. of parameters | 223 |
| Tmin Tmax | 0.357 0.697 |
| Index range | −9 ≤ h ≤ 9 −15 ≤ k ≤ 15 −16 ≤ l ≤ 16 |
| R1 | 0.0297 |
| wR2 | 0.0591 |
| Goof | 0.991 |
| D-H⋯A | D-H | H⋯A | D⋯A | D-H⋯A |
|---|---|---|---|---|
| N1—H3N1…Br3 a | 0.91 | 2.78 | 3.426(4) | 129 |
| N1—H3N1…Br2 b | 0.91 | 2.89 | 3.358(4) | 113 |
| N1—H1N1…Br1 a | 0.91 | 2.55 | 3.348(4) | 146 |
| N1—H2N1…Br3 b | 0.91 | 2.54 | 3.422(5) | 163 |
| N2—HN2…Br1 | 0.88 | 2.37 | 3.241(4) | 171 |
| N3—H3N3…Br4 c | 0.91 | 2.84 | 3.546(5) | 135 |
| N3—H3N3…Br6 d | 0.91 | 2.86 | 3.462(5) | 125 |
| N3—H2N3…Br5 | 0.91 | 2.50 | 3.402(5) | 172 |
| N3—H1N3…Br6 a | 0.91 | 2.67 | 3.509(5) | 153 |
| N4—Hn4…Br2 b | 0.88 | 2.41 | 3.277(5) | 169 |
| C6 —H6…Br7 e | 0.95 | 2.77 | 3.568(6) | 142 |
| C11—H10…Br1 | 0.99 | 2.77 | 3.452(7) | 127 |
| C12—H12…Br7 f | 0.95 | 2.80 | 3.640(7) | 148 |
| Symmetry codes: a = −1 + x, y, z; b = 1 − x, −y, 1 − z; c = 1 − x, 1 − y, −z; d = x, −1 + y, z; e= 2 − x, 1 − y, −z; f = 1 − x, 1 − y, 1 − z | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayer, I.; Msalmi, R.; Mosconi, E.; Guesmi, A.; Houas, A.; Ben Hamadi, N.; Naïli, H. Structural and Optical Characterization of a New Tetra- and Hexa-Coordinated Cd-Based Hybrid Compound with White Light Emission. Crystals 2024, 14, 459. https://doi.org/10.3390/cryst14050459
Sayer I, Msalmi R, Mosconi E, Guesmi A, Houas A, Ben Hamadi N, Naïli H. Structural and Optical Characterization of a New Tetra- and Hexa-Coordinated Cd-Based Hybrid Compound with White Light Emission. Crystals. 2024; 14(5):459. https://doi.org/10.3390/cryst14050459
Chicago/Turabian StyleSayer, Imen, Rawia Msalmi, Edoardo Mosconi, Ahlem Guesmi, Ammar Houas, Naoufel Ben Hamadi, and Houcine Naïli. 2024. "Structural and Optical Characterization of a New Tetra- and Hexa-Coordinated Cd-Based Hybrid Compound with White Light Emission" Crystals 14, no. 5: 459. https://doi.org/10.3390/cryst14050459





