Amantadine and Rimantadine Analogues—Single-Crystal Analysis and Anti-Coronaviral Activity
Abstract
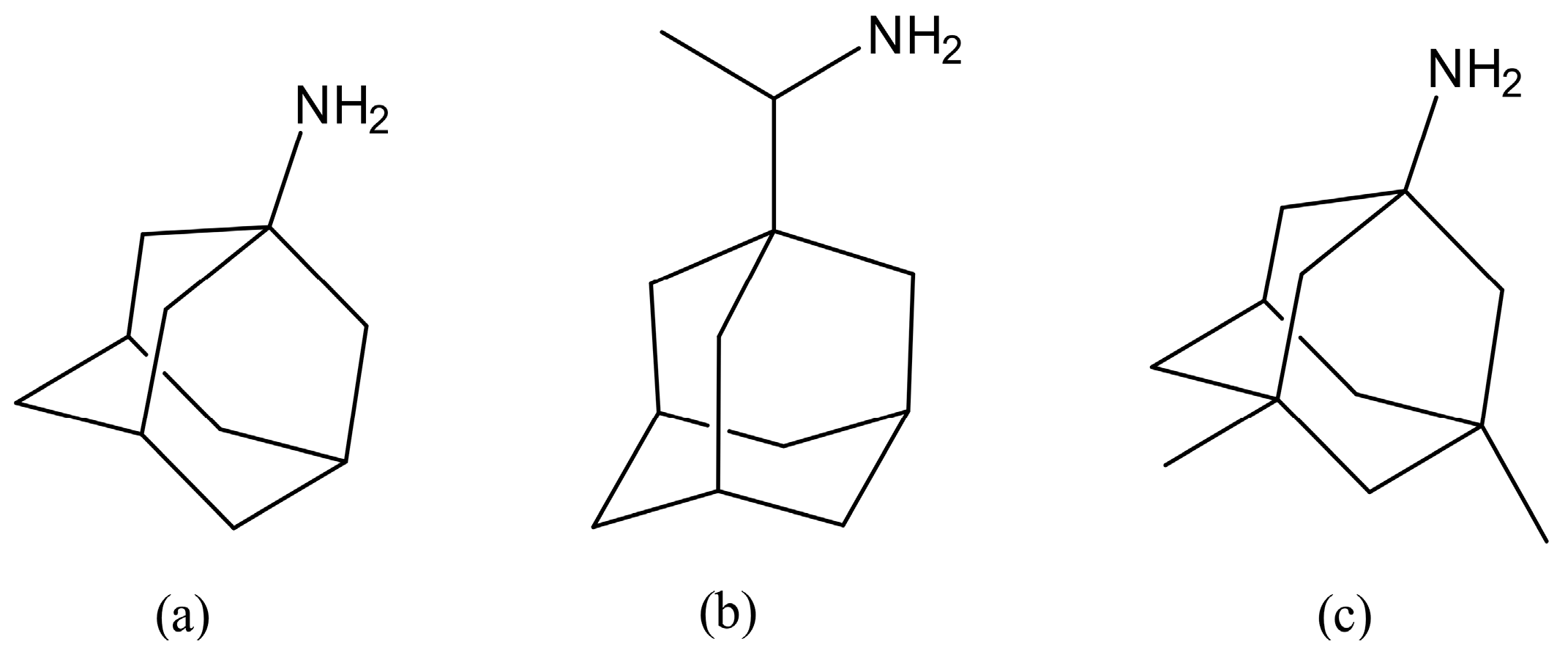
:1. Introduction
2. Materials and Methods
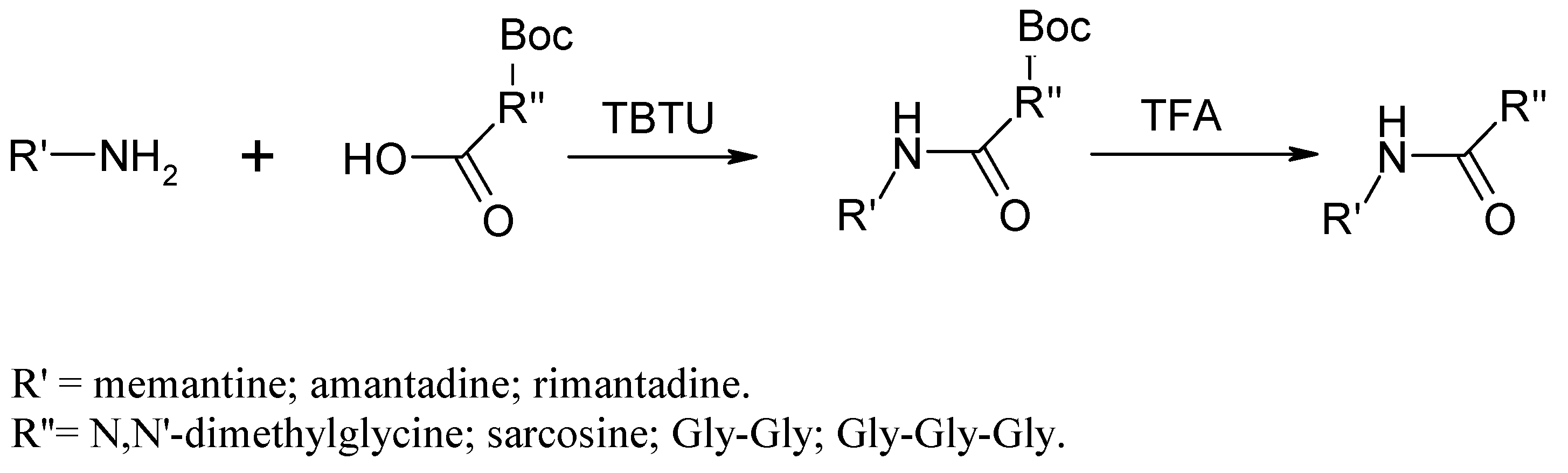
2.1. Chemistry
Chemical Synthesis
2.2. Single Crystal X-ray Diffraction
2.3. Biology
2.3.1. Cell Viability Assays
2.3.2. HCoV-229E Replication Inhibition
2.4. Molecular Docking
3. Results and Discussion
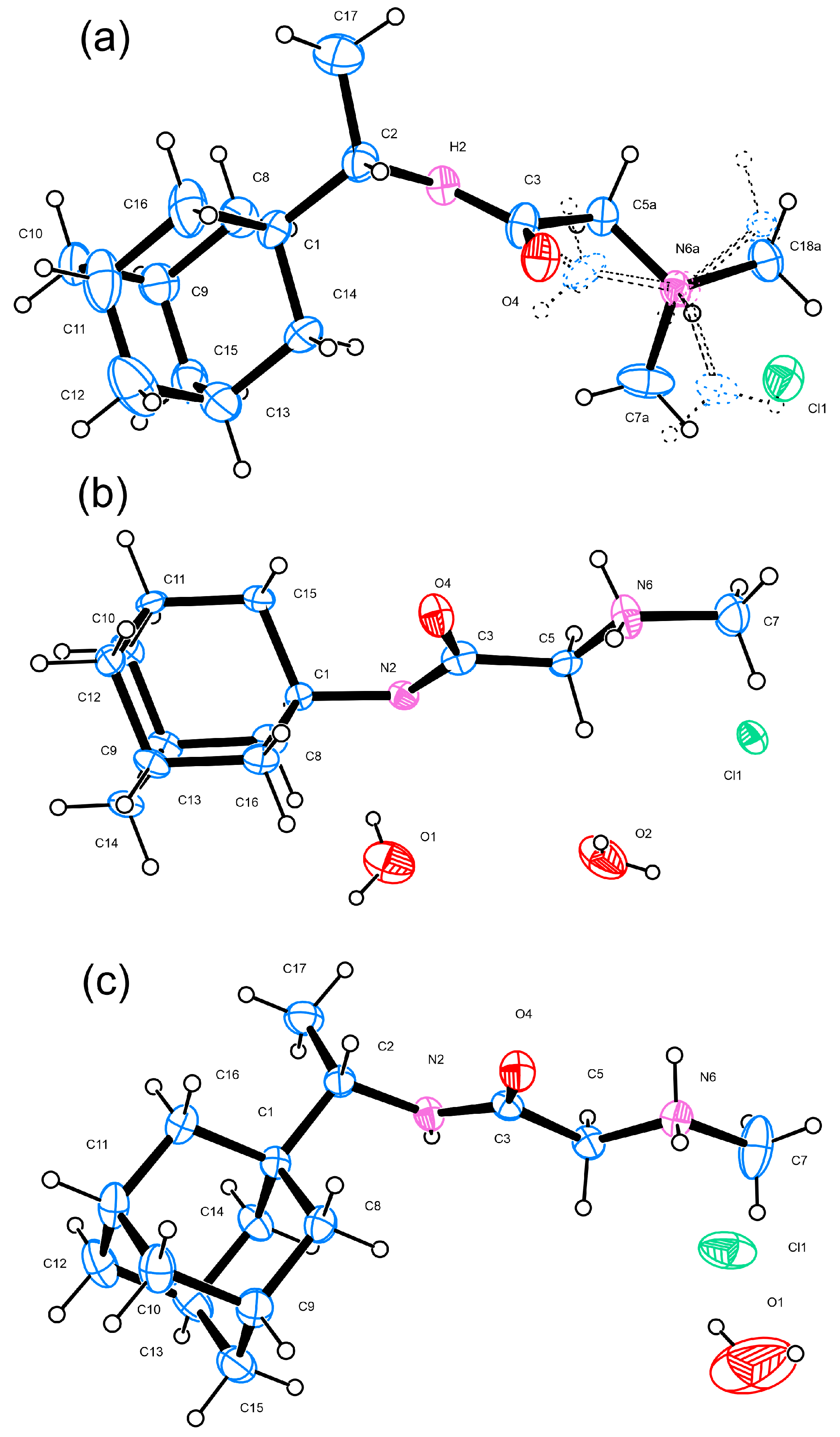
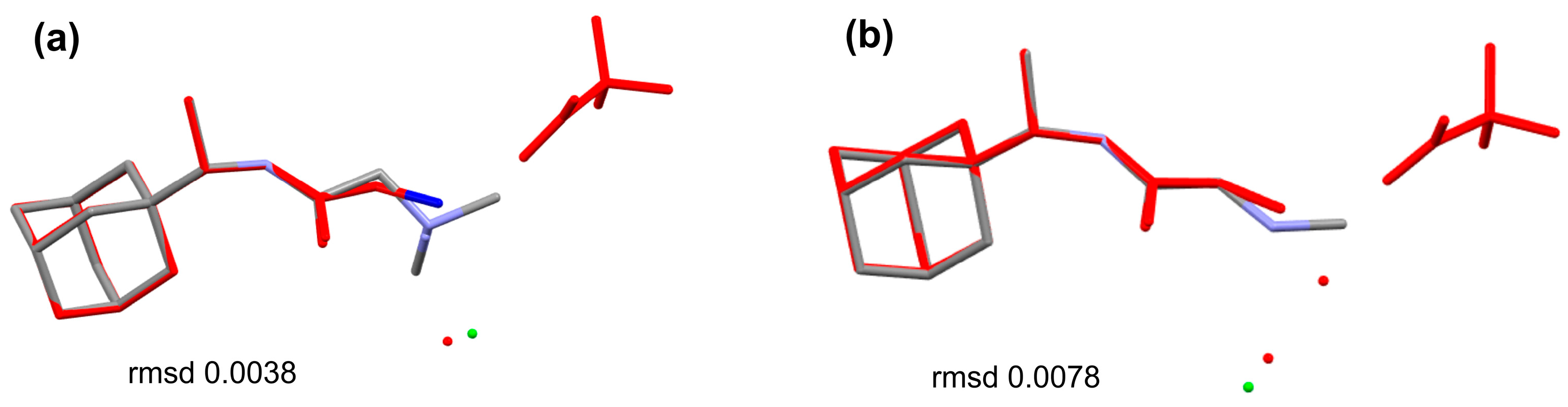
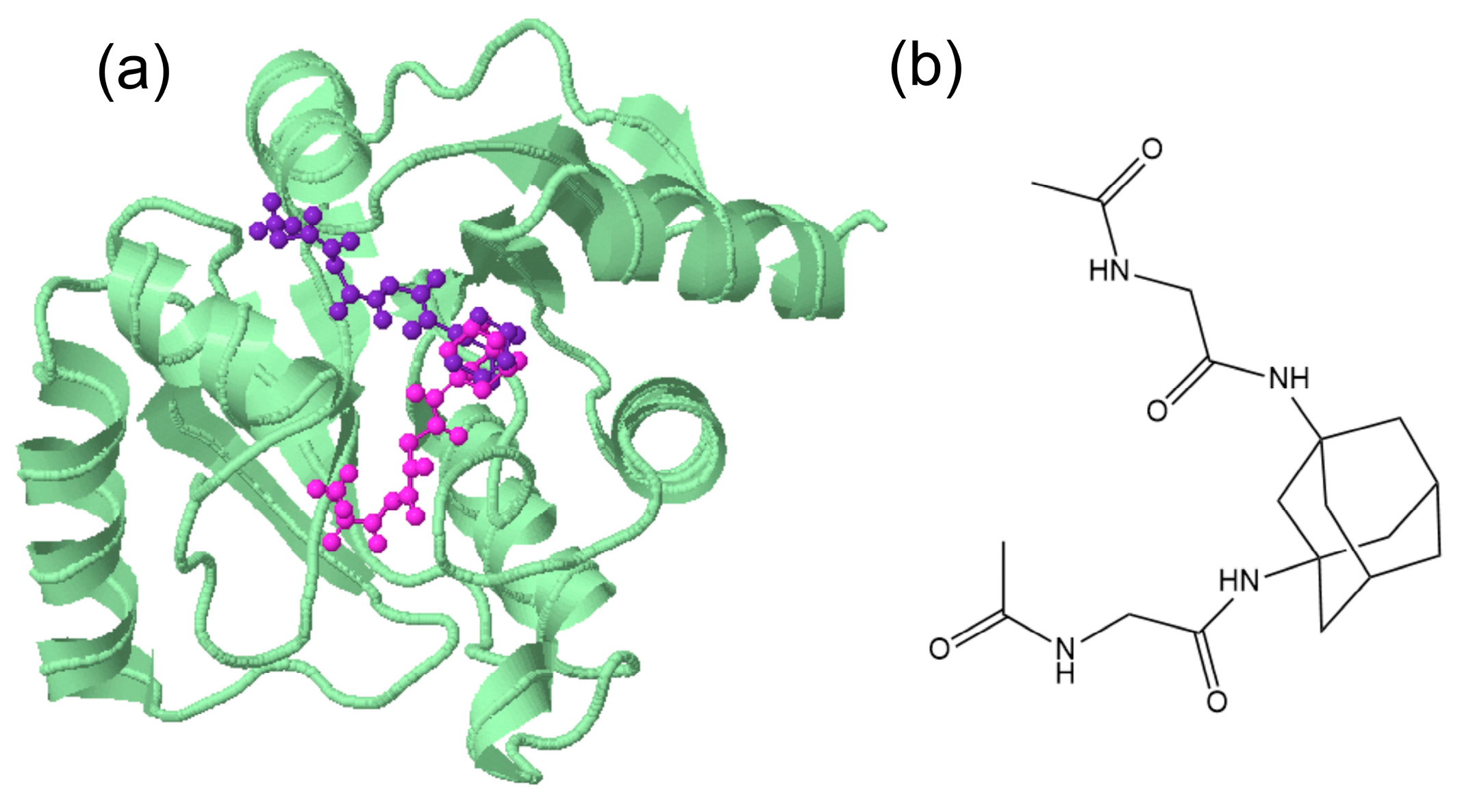
3.1. Single-Crystal Analyses
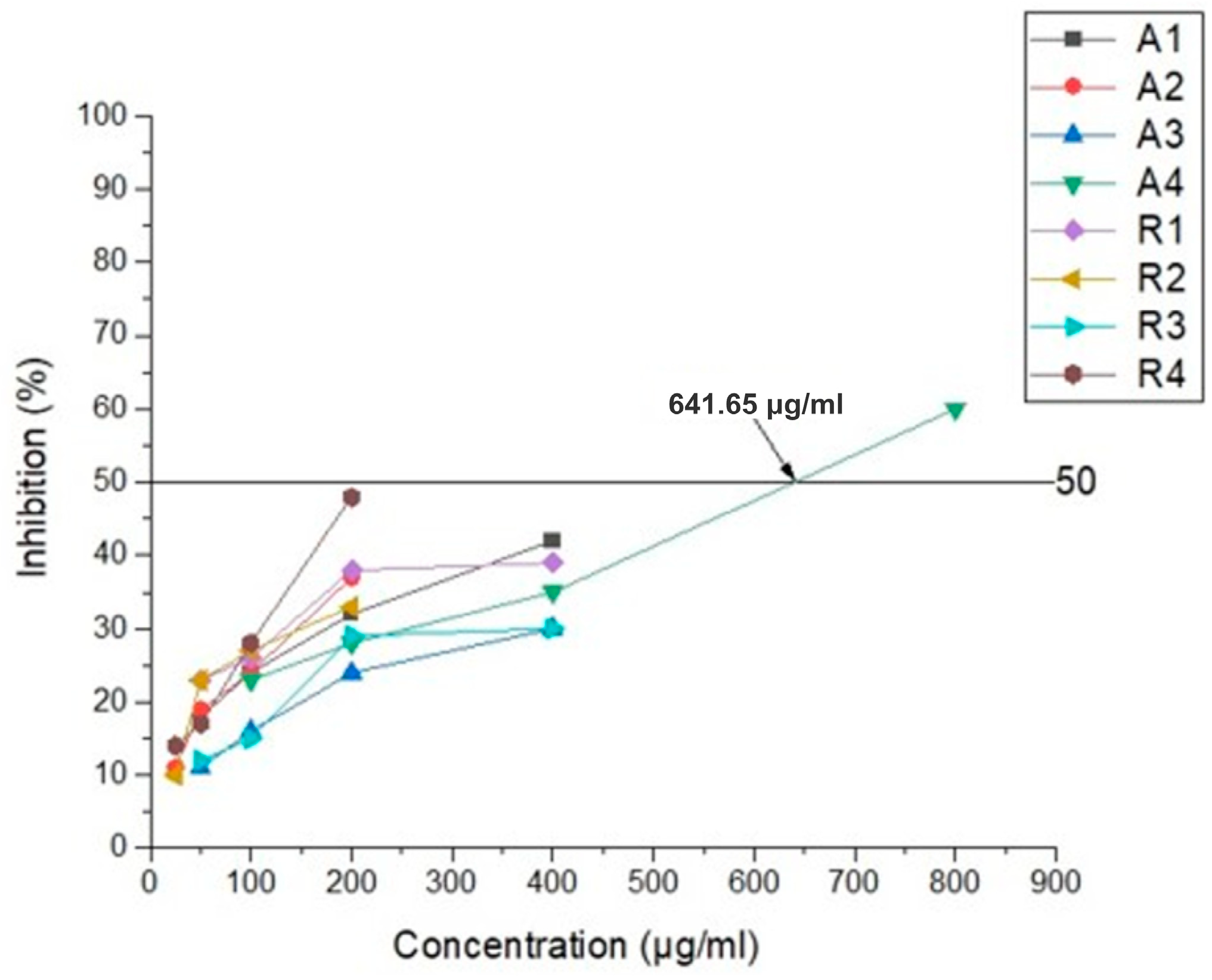
3.2. Antiviral Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.htmL (accessed on 27 February 2023).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gammeltoft, K.A.; Galli, A.; Offersgaard, A.; Fahnøe, U.; Ramirez, S.; Gottwein, J.M. Efficacy of ion-channel inhibitors amantadine, memantine and rimantadine for the treatment of SARS-CoV-2 In Vitro. Viruses 2021, 13, 2082. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Potential for the Repurposing of Adamantane Antivirals for COVID-19. Drugs RD 2021, 21, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.R.; Butterworth, R.F. Repurposing of Adamantanes with Transmitter Receptor Antagonist Properties for the Prevention/Treatment of COVID-19. J. Pharmaceu Pharmacol. 2020, 8, 4. [Google Scholar] [CrossRef]
- Tanas, A.; Tozlu, Ö.Ö.; Gezmiş, T.; Hacimüftüoğlu, A.; Ceylan, O.; Türkez, H. In Vitro and In Vivo Neuroprotective Effects of Sarcosine. BioMed Res. Int. 2022, 2022, 5467498. [Google Scholar] [CrossRef]
- Zheng, Y.; Jin, Y.; Zhang, N.; Wang, D.; Yang, Y.; Zhang, M.; Qu, W. Recovery of N, N-dimethylglycine (DMG) from dimethylglycine hydrochloride by bipolar membrane electrodialysis. Chem. Eng. Process.-Process Intensif. 2022, 176, 108943. [Google Scholar] [CrossRef]
- Chayrov, R.; Kalfin, R.; Lazarova, M.; Tancheva, L.; Sbirkova-Dimitrova, H.; Shivachev, B.; Stankova, I. Development of N, N-Dimethylglycine-Amantadine for Adjunctive Dopaminergic Application: Synthesis, Structure and Biological Activity. Crystals 2022, 12, 1227. [Google Scholar] [CrossRef]
- Chayrov, R.; Volkova, T.; Perlovich, G.; Zeng, L.; Li, Z.; Štícha, M.; Liu, R.; Stankova, I. Synthesis, Neuroprotective Effect and Physicochemical Studies of Novel Peptide and Nootropic Analogues of Alzheimer Disease Drug. Pharmaceuticals 2022, 15, 1108. [Google Scholar] [CrossRef]
- Barberis, E.; Amede, E.; Tavecchia, M.; Marengo, E.; Cittone, M.G.; Rizzi, E.; Sainaghi, P.P. Understanding protection from SARS-CoV-2 using metabolomics. Sci. Rep. 2021, 11, 13796. [Google Scholar] [CrossRef]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- APEX4 Data Collection Software, Version 2021.4-0; Bruker AXS Inc.: Madison, WI, USA, 2021.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXL-97. Program for Crystal-Structure Refinement; ScienceOpen, Inc.: Burlington, MA, USA, 1997. [Google Scholar]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sudo, K.; Konno, K.; Yokota, T.; Shigeta, S. A sensitive assay system screening antiviral compounds against herpes simplex virus type 1 and type 2. J. Virol. Methods 1994, 49, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti–COVID-19 drug design. Sci. Adv. 2020, 6, eabd4596. [Google Scholar] [CrossRef]
- Jia, Z.; Yan, L.; Ren, Z.; Wu, L.; Wang, J.; Guo, J.; Zheng, L.; Ming, Z.; Zhang, L.; Lou, Z.; et al. Delicate structural coordination of the Severe Acute Respiratory Syndrome coronavirus Nsp13 upon ATP hydrolysis. Nucleic Acids Res. 2019, 47, 6538–6550. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jayachandran, U.; Bonneau, F.; Fiorini, F.; Basquin, C.; Domcke, S.; Le Hir, H.; Conti, E. Molecular Mechanisms for the RNA-Dependent ATPase Activity of Upf1 and Its Regulation by Upf2. Mol. Cell 2011, 41, 693–703. [Google Scholar] [CrossRef]
- Michalska, K.; Kim, Y.; Jedrzejczak, R.; Maltseva, N.I.; Stols, L.; Endres, M.; Joachimiak, A. Crystal structures of SARS-CoV-2 ADP-ribose phosphatase: From the apo form to ligand complexes. IUCrJ 2020, 7, 814–824. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.-D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, L.; Shaw, N.; Gao, Y.; Wang, J.; Sun, Y.; Lou, Z.; Yan, L.; Zhang, R.; Rao, Z. Structural basis and functional analysis of the SARS coronavirus nsp14–nsp10 complex. Proc. Natl. Acad. Sci. USA 2015, 112, 9436–9441. [Google Scholar] [CrossRef]
- Dinesh, D.C.; Chalupska, D.; Silhan, J.; Koutna, E.; Nencka, R.; Veverka, V.; Boura, E. Structural basis of RNA recognition by the SARS-CoV-2 nucleocapsid phosphoprotein. PLoS Pathog. 2020, 16, e1009100. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Lemus, M.; Minasov, G.; Shuvalova, L.; Inniss, N.L.; Kiryukhina, O.; Brunzelle, J.; Satchell, K.J. High-resolution structures of the SARS-CoV-2 2′-O-methyltransferase reveal strategies for structure-based inhibitor design. Sci. Signal. 2020, 13, eabe1202. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Yang, G.; Xue, R.; Liu, M.; Wang, F.; Hu, J.; Guo, X.; Chang, S. COVID-19 Docking Server: A meta server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics 2020, 36, 5109–5111. [Google Scholar] [CrossRef]
- Moriarty, N.W.; Grosse-Kunstleve, R.W.; Adams, P.D. electronic Ligand Builder and Optimization Workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr. Sect. D 2009, 65, 1074–1080. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Chayrov, R.; Parisis, N.A.; Chatziathanasiadou, M.V.; Vrontaki, E.; Moschovou, K.; Melagraki, G.; Sbirkova-Dimitrova, H.; Shivachev, B.; Schmidtke, M.; Mitrev, Y.; et al. Synthetic Analogues of Aminoadamantane as Influenza Viral Inhibitors—In Vitro, In Silico and QSAR Studies. Molecules 2020, 25, 3989. [Google Scholar] [CrossRef]
- Kapon, M.; Reisner, G.M. Space-group changes—Revised structures of seven compounds. Acta Crystallogr. Sect. C 1990, 46, 349–350. [Google Scholar] [CrossRef]
- Kuznetsov, N.Y.; Tikhov, R.M.; Godovikov, I.A.; Medvedev, M.G.; Lyssenko, K.A.; Burtseva, E.I.; Kirillova, E.S.; Bubnov, Y.N. Stereoselective synthesis of novel adamantane derivatives with high potency against rimantadine-resistant influenza A virus strains. Org. Biomol. Chem. 2017, 15, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Hamodrakas, S.J.; Perrakis, A.; Antoniadou-Vyzas, E. A de novo Designed Possible 5-Lipoxygenase Inhibitor: [alpha]-Acetoxy-N-[1-(1-tricyclo [3.3.1.13,7]dec-1-yl)ethyl]benzeneacetamide. Acta Crystallogr. Sect. C 1996, 52, 677–679. [Google Scholar] [CrossRef]
- Ponath, S.; Menger, M.; Grothues, L.; Weber, M.; Lentz, D.; Strohmann, C.; Christmann, M. Mechanistic Studies on the Organocatalytic α-Chlorination of Aldehydes: The Role and Nature of Off-Cycle Intermediates. Angew. Chem. 2018, 130, 11857–11861. [Google Scholar] [CrossRef]
- Kukushkin, M.E.; Skvortsov, D.A.; Kalinina, M.A.; Tafeenko, V.A.; Burmistrov, V.V.; Butov, G.M.; Zyk, N.V.; Majouga, A.G.; Beloglazkina, E.K. Synthesis and cytotoxicity of oxindoles dispiro derivatives with thiohydantoin and adamantane fragments. Phosphorus Sulfur. Silicon Relat. Elem. 2020, 195, 544–555. [Google Scholar] [CrossRef]
- Dado, G.P.; Desper, J.M.; Holmgren, S.K.; Rito, C.J.; Gellman, S.H. Effects of covalent modifications on the solid-state folding and packing of N-malonylglycine derivatives. J. Am. Chem. Soc. 1992, 114, 4834–4843. [Google Scholar] [CrossRef]
- Ma, X.; Deng, S.; Liang, J.; Chen, J.; Su, J.; Huang, H.; Song, Q. Unsymmetric monothiooxalamides from S8, bromodifluoro reagents and anilines: Synthesis and applications. Tetrahedron Chem. 2022, 3, 100026. [Google Scholar] [CrossRef]
- Arias-Arias, J.L.; Vega-Aguilar, F.; Picado-Soto, D.; Corrales-Aguilar, E.; Loría, G.D. In Vitro Inhibition of Zika Virus Replication with Amantadine and Rimantadine Hydrochlorides. Microbiol. Res. 2021, 12, 727–738. [Google Scholar] [CrossRef]
- Ballester, P.J. Machine learning scoring functions based on random forest and support vector regression. In Proceedings of the Pattern Recognition in Bioinformatics: 7th IAPR International Conference, PRIB 2012, Tokyo, Japan, 8–10 November 2012; Proceedings 7. pp. 14–25. [Google Scholar]





| Compound | 1R | 2A | 2R |
|---|---|---|---|
| Empirical formula | C16H29ClN2O | C26H50Cl2N4O5 | C15H27.67ClN2O1.33 |
| Formula weight | 300.86 | 569.60 | 292.85 |
| Temperature | 290.0 | 290.0 | 290.0 |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P21/c | C2 | Iba2 |
| a/Å | 15.869 (4) | 11.369 (4) | 10.6014 (6) |
| b/Å | 9.922 (3) | 6.3354 (14) | 32.429 (2) |
| c/Å | 11.158 (3) | 20.894 (5) | 9.9143 (6) |
| α/° | 90 | 90 | 90 |
| β/° | 98.260 (9) | 100.645 (15) | 90 |
| γ/° | 90 | 90 | 90 |
| Volume/Å3 | 1738.5 (8) | 1479.1 (7) | 3408.5 (4) |
| Z | 4 | 2 | 8 |
| ρcalc g/cm3 | 1.149 | 1.279 | 1.141 |
| μ/mm−1 | 0.219 | 0.261 | 0.223 |
| F(000) | 656.0 | 616.0 | 1275.0 |
| Crystal size/mm3 | 0.15 × 0.12 × 0.12 | 0.2 × 0.15 × 0.15 | 0.25 × 0.21 × 0.2 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 4.856 to 50.686 | 3.968 to 52.536 | 4.042 to 54.286 |
| Reflections collected/independent | 35,156 | 33,678 | 85,704 |
| Rint/Rsigma | 0.2312/0.1089 | 0.1393/0.0605 | 0.0958/0.0246 |
| Data/restraints/parameters | 3181/111/224 | 2971/1/173 | 3781/1/194 |
| Goodness-of-fit on F2 | 1.098 | 1.179 | 1.049 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.1323, wR2 = 0.3260 | R1 = 0.1085, wR2 = 0.2445 | R1 = 0.0464, wR2 = 0.1132 |
| Final R indexes [all data] | R1 = 0.1991, wR2 = 0.3635 | R1 = 0.1265, wR2 = 0.2538 | R1 = 0.0621, wR2 = 0.1256 |
| Largest diff. peak/hole/e Å−3 | 0.82/−0.41 | 0.38/−0.45 | 0.51/−0.36 |
| Flack parameter | n.a | 0.16 (6) | 0.39 (3) |
| CCDC number | 2263306 | 2263307 | 2263308 |
| D | H | A | d (D-H)/Å | d (H-A)/Å | d (D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| N2 | H2 | Cl1 1 | 0.96 | 2.26 | 3.209 (6) | 170.2 |
| N6A | H6A | Cl1 | 1.01 | 2.16 | 3.130 (18) | 159.1 |
| C18A | H18C | Cl1 2 | 0.96 | 2.92 | 3.803 (13) | 153.0 |
| C5A | H5AA | Cl1 1 | 0.97 | 2.81 | 3.587 (9) | 137.1 |
| C5A | H5AB | Cl1 2 | 0.97 | 2.88 | 3.804 (11) | 160.5 |
| C7A | H7AA | O4 1 | 0.96 | 2.48 | 3.367 (13) | 153.1 |
| C7A | H7AB | Cl1 3 | 0.96 | 2.77 | 3.553 (16) | 139.7 |
| C5B | H5BA | Cl1 3 | 0.97 | 2.80 | 3.76 (3) | 171.5 |
| C5B | H5BB | Cl1 1 | 0.97 | 2.89 | 3.69 (3) | 140.9 |
| C7B | H7BB | Cl1 | 0.96 | 2.80 | 3.34 (5) | 116.6 |
| C18B | H18F | Cl1 2 | 0.96 | 2.55 | 3.03 (4) | 111.3 |
| N6B | H6B | Cl1 | 0.98 | 2.14 | 3.10 (7) | 168.8 |
| D | H | A | d (D-H)/Å | d (H-A)/Å | d (D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| N6 | H6A | Cl1 | 0.78 (5) | 2.36 (5) | 3.102 (4) | 162 (4) |
| N6 | H6B | Cl1 1 | 0.92 (5) | 2.22 (5) | 3.121 (4) | 165 (4) |
| C5 | H5A | Cl1 2 | 0.97 | 2.71 | 3.647 (4) | 161.7 |
| C5 | H5B | Cl1 3 | 0.97 | 2.88 | 3.761 (4) | 151.0 |
| C7 | H7B | Cl1 3 | 0.96 | 2.90 | 3.767 (6) | 151.3 |
| C7 | H7C | O1 | 0.96 | 2.32 | 3.27 (3) | 174.2 |
| N2 | H2 | O4 3 | 0.84 (5) | 2.10 (5) | 2.943 (3) | 174 (4) |
| D | H | A | d (D-H)/Å | d (H-A)/Å | d (D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| N6 | H6A | Cl1 1 | 0.86 | 2.62 | 3.272 (12) | 133.2 |
| N6 | H6A | O1 2 | 0.86 | 2.52 | 2.969 (15) | 113.8 |
| C15 | H15B | O4 | 0.97 | 2.49 | 3.086 (11) | 119.2 |
| C16 | H16B | O4 | 0.97 | 2.62 | 3.184 (12) | 117.2 |
| O1 | H1A | N2 3 | 0.85 | 2.66 | 3.491 (16) | 166.9 |
| O1 | H1B | N2 4 | 0.90 | 2.69 | 3.521 (14) | 153.2 |
| C7 | H7A | O2 2 | 0.96 | 2.65 | 3.600 (19) | 170.4 |
| N6 | H6B | Cl1 5 | 0.89 (14) | 2.47 (14) | 3.285 (12) | 151 (12) |
| Compound (μg/mL) | Inhibition (%) | Compound (μg/mL) | Inhibition (%) |
|---|---|---|---|
| 1A 400 | 42 | 1R 400 | 39 |
| 1A 200 | 32 | 1R 200 | 38 |
| 1A 100 | 24 | 1R 100 | 26 |
| 1A 50 | 18 | 1R 50 | 23 |
| 2A 200 | 37 | 2R 200 | 33 |
| 2A 100 | 24 | 2R 100 | 27 |
| 2A 50 | 19 | 2R 50 | 23 |
| 2A 25 | 11 | 2R 25 | 10 |
| 3A 400 | 30 | 3R 400 | 30 |
| 3A 200 | 24 | 3R 200 | 29 |
| 3A 100 | 16 | 3R 100 | 15 |
| 3A 50 | 11 | 3R 50 | 12 |
| 4A 800 | 60 | 4R 200 | 48 |
| 4A 400 | 35 | 4R 100 | 28 |
| 4A 200 | 28 | 4R 50 | 17 |
| 4A 100 | 23 | 4R 25 | 14 |
| Protein Target/Compound | 1R | 2A | 2R | 4A | ||||
|---|---|---|---|---|---|---|---|---|
| kcal/mol | RFpKd | kcal/mol | RFpKd | kcal/mol | RFpKd | kcal/mol | RFpKd | |
| Main Protease (Mpro or 3CLpro) 6LU7 | −5.80 | 6.13 | −6.20 | 4.61 | −5.70 | 5.52 | −7.00 | 6.17 |
| Papain-like protease (PLpro) 6WUU | −6.90 | 6.27 | −6.30 | 5.63 | −6.80 | 6.21 | −7.40 | 6.34 |
| Helicase (Nsp13, NCB site) 6JYT | −6.40 | 5.85 | −5.80 | 4.76 | −6.4 | 5.45 | −6.70 | 632 |
| Helicase (ADP site) 6JYT/2XZL | −5.50 | 5.07 | −5.30 | 4.16 | −5.60 | 4.85 | −6.50 | 5.40 |
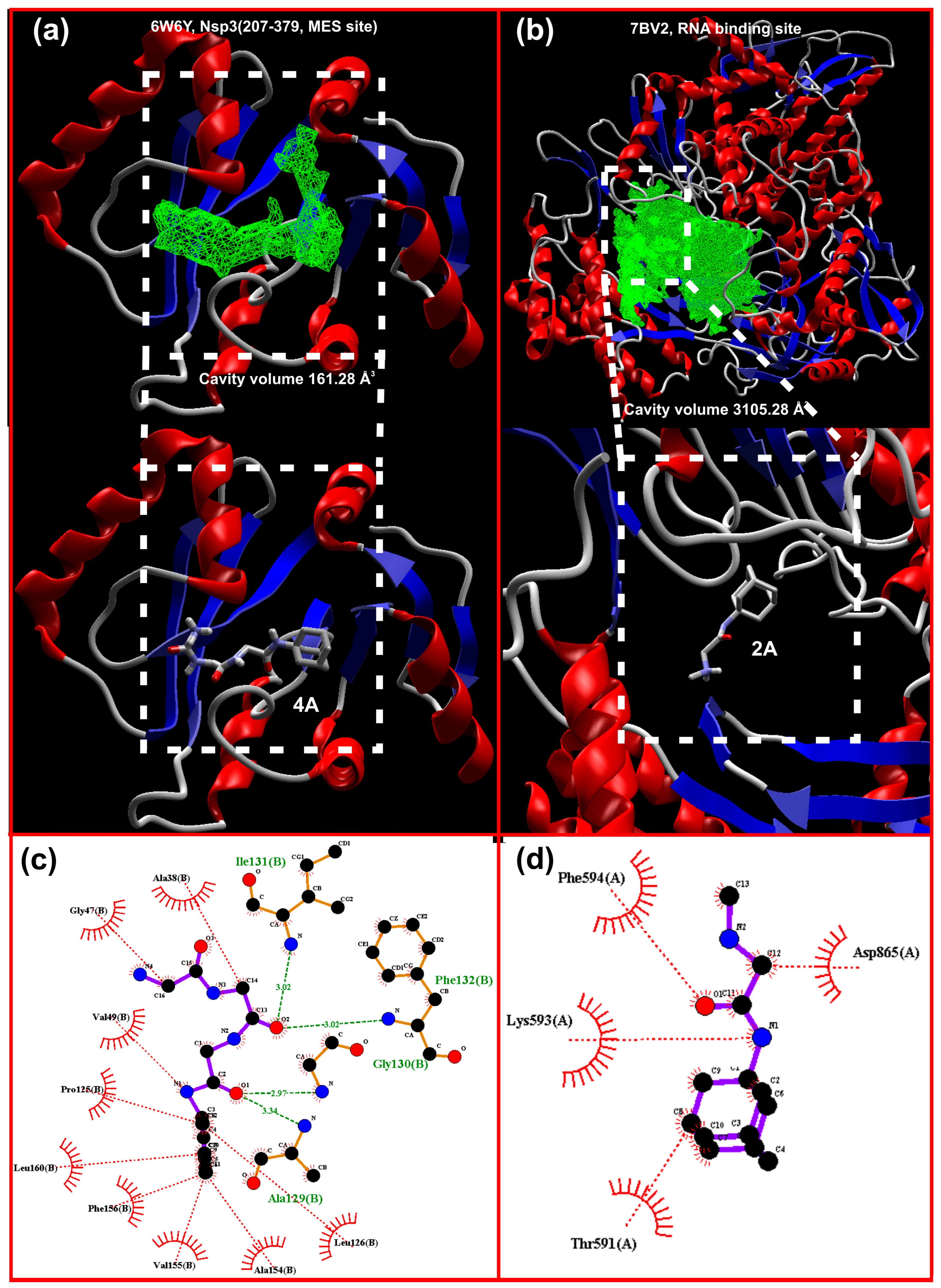
| Nsp3 (207-379, MES site) 6W6Y | −6.80 | 6.07 | −6.30 | 5.39 | −6.5 | 5.28 | −8.00 | 6.64 |
| RdRp (RTP site) RNA-dependent polymerase 7BV2 | −7.90 | 7.14 | −6.00 | 4.35 | −7.9 | 6.81 | −7.60 | 636 |
| RdRp (RNA site) 7BV2 | 6.20 | 5.05 | −8.30 | 6.58 | −6.3 | 4.79 | −7.60 | 6.30 |
| Nsp14 (ExoN) 5C8S | −5.50 | 5.68 | −5.50 | 4.54 | −5.40 | 5.63 | −7.30 | 6.38 |
| N protein (NCB site) 6YI3 | −6.40 | 5.80 | −6.00 | 4.79 | −6.20 | 4.92 | −7.00 | 6.17 |
| Nsp16 (SAM site) 6WVN | −6.20 | 5.86 | −6.00 | 4.64 | −6.30 | 5.88 | −7.40 | 6.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkova, K.; Stoymirska, A.; Chayrov, R.; Shishkov, S.; Sbirkova-Dimitrova, H.; Rusew, R.; Shivachev, B.; Stankova, I. Amantadine and Rimantadine Analogues—Single-Crystal Analysis and Anti-Coronaviral Activity. Crystals 2023, 13, 1374. https://doi.org/10.3390/cryst13091374
Shishkova K, Stoymirska A, Chayrov R, Shishkov S, Sbirkova-Dimitrova H, Rusew R, Shivachev B, Stankova I. Amantadine and Rimantadine Analogues—Single-Crystal Analysis and Anti-Coronaviral Activity. Crystals. 2023; 13(9):1374. https://doi.org/10.3390/cryst13091374
Chicago/Turabian StyleShishkova, Kalina, Antoniya Stoymirska, Radoslav Chayrov, Stoyan Shishkov, Hristina Sbirkova-Dimitrova, Rusi Rusew, Boris Shivachev, and Ivanka Stankova. 2023. "Amantadine and Rimantadine Analogues—Single-Crystal Analysis and Anti-Coronaviral Activity" Crystals 13, no. 9: 1374. https://doi.org/10.3390/cryst13091374





