Synthesis, X-ray Diffraction, NMR and Thermolysis Studies of Cadmium Tin Sulfido Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses of [{(tmeda)Cd}3(SnPh)2S6] (1) and [{(tmeda)Cd}2(SnPh2)2S4] · DME (2)
2.2. Synthesis of [{(iPr3P)Cd}3Cd4(SnPh2)6S13] (3)
2.3. X-ray Crystal Structure Analyses
3. Results & Discussion
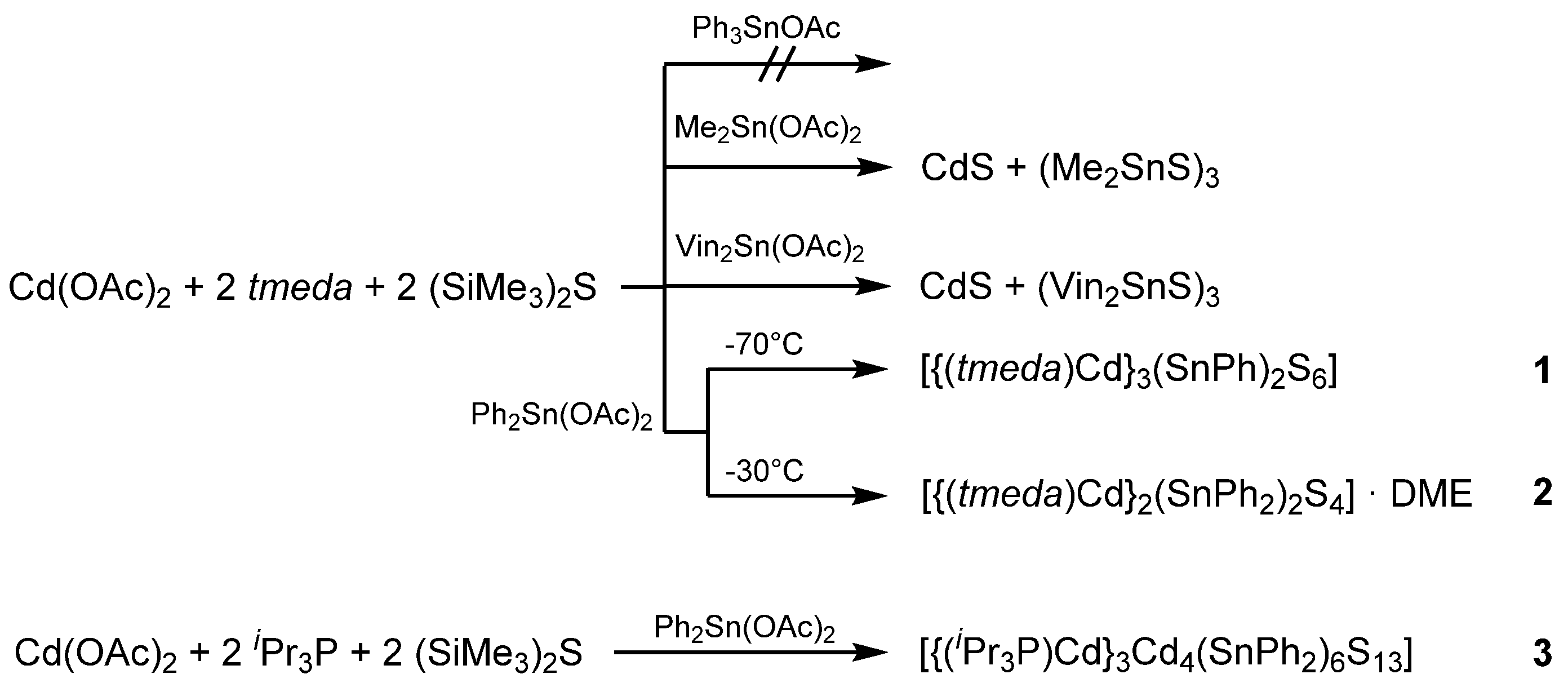
3.1. Syntheses
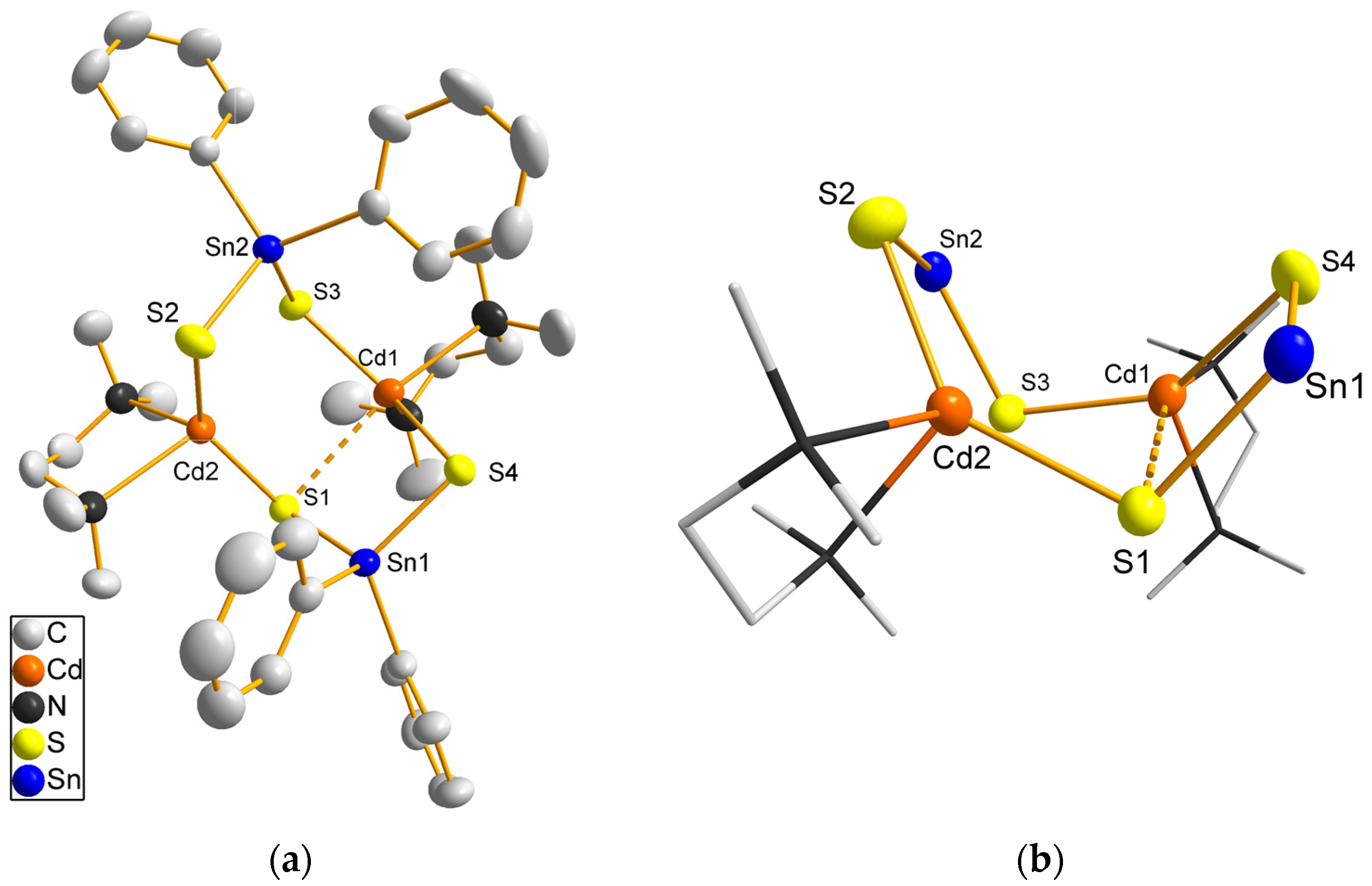
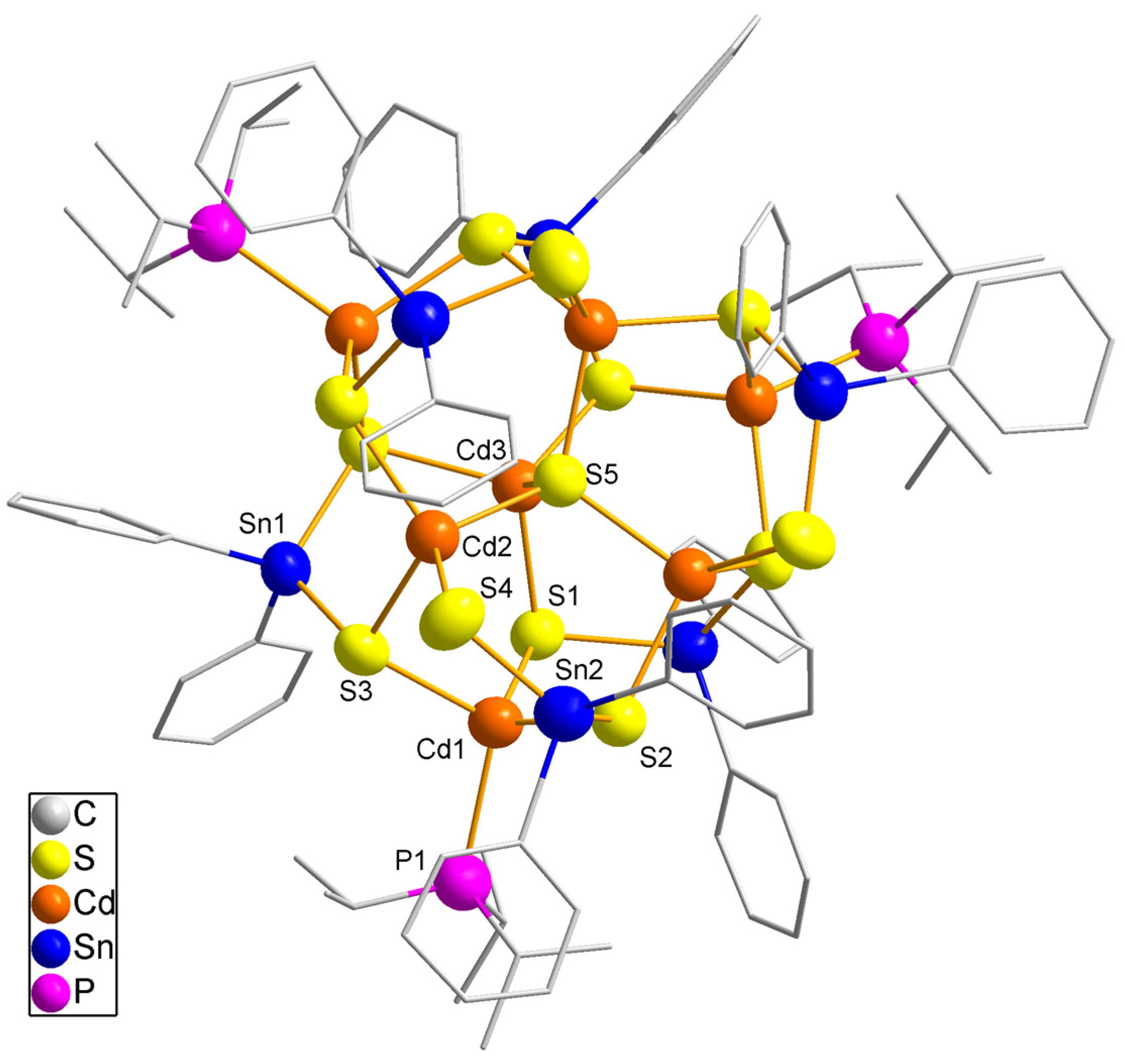
3.2. Single-Crystal Structure Analyses
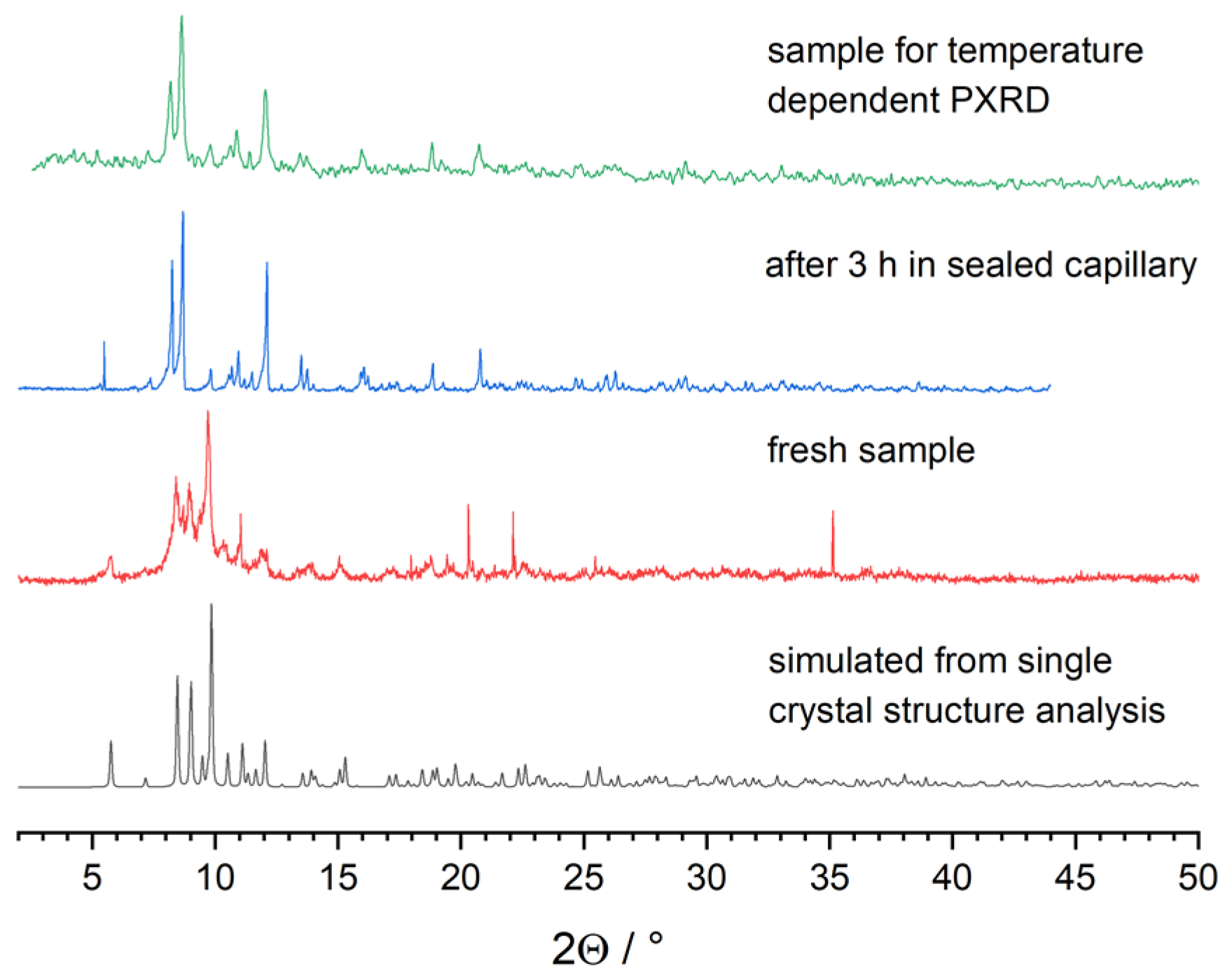
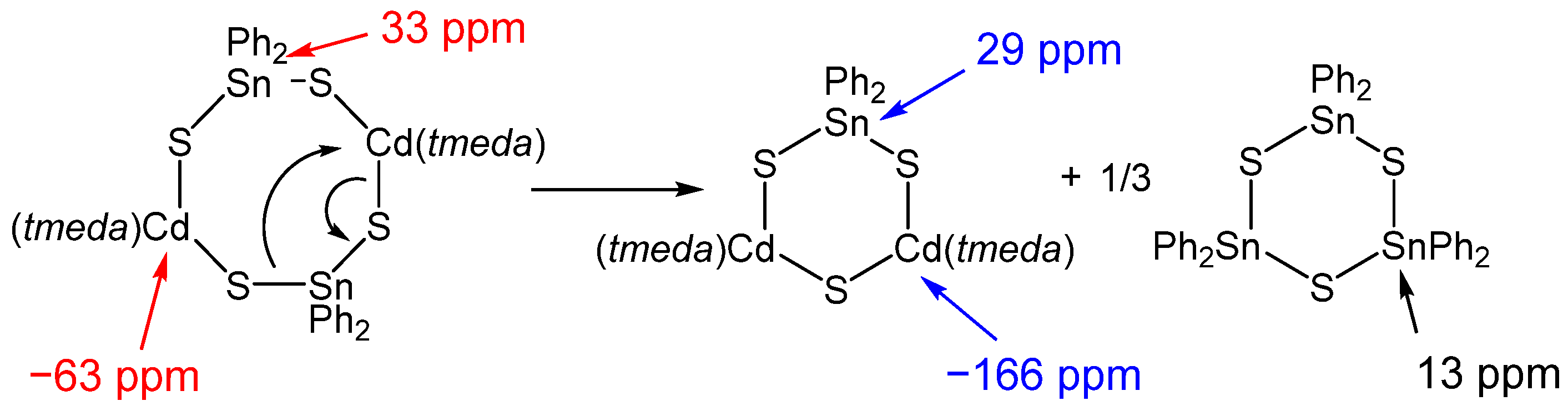
3.3. Characterization of the Complexes by PXRD and NMR Spectroscopy
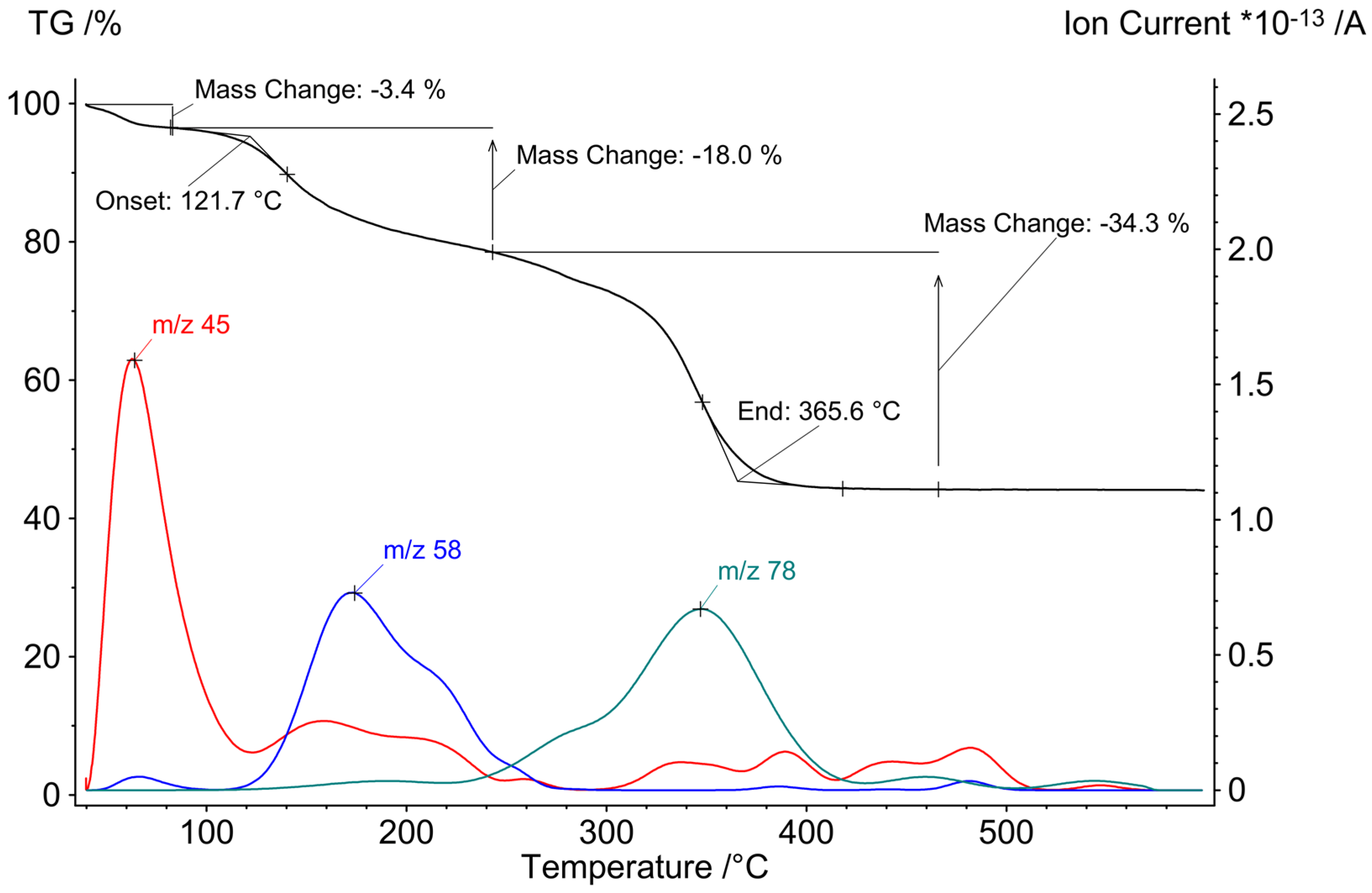
3.4. Thermal Behavior and Thermolysis Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palchik, O.; Iyer, R.G.; Canlas, C.G.; Weliky, D.P.; Kanatzidis, M.G. K10M4M′4S17 (M = Mn, Fe, Co, Zn; M′ = Sn, Ge) and Cs10Cd4Sn4S17: Compounds with a Discrete Supertetrahedral Cluster. Z. Anorg. Allg. Chem. 2004, 630, 2237–2247. [Google Scholar] [CrossRef]
- Bochkarev, M.N.; Andreevichev, V.S.; Vyazankin, N.S. Bis[tri(pentafluorophenyl)germyl]cadmium complexing reactions. Izv. Akad. Nauk. Ser. Khim. 1973, 1973, 702–703. [Google Scholar] [CrossRef]
- Eichhöfer, A.; Buth, G. Synthesis and Structure of the Group 12 Pyrimidinethiolate Complexes ∞3[Zn(S-2-N2C4H3)2], ∞2[Cd(S-2-N2C4H3)2], [Hg(S-2-N2C4H3)2] and [Cd(S-2-N2C4H3)2(tmeda)]. Eur. J. Inorg. Chem. 2005, 2005, 4160–4167. [Google Scholar] [CrossRef]
- Edwards, A.J.; Fallaize, A.; Raithby, P.R.; Rennie, M.-A.; Steiner, A.; Verhorevoort, K.L.; Wright, D.S. A halidefree route to Groups 12 and 13 organometallic and metalloorganic complexes. J. Chem. Soc. Dalton Trans. 1996, 1996, 133–137. [Google Scholar] [CrossRef]
- Yosef, M.; Schaper, A.K.; Fröba, M.; Schlecht, S. Stabilization of the thermodynamically favored polymorph of cadmium chalcogenide nanoparticles CdX (X = S, Se, Te) in the polar mesopores of SBA-15 silica. Inorg. Chem. 2005, 44, 5890–5896. [Google Scholar] [CrossRef]
- Denny, J.A.; Foley, W.S.; Almaraz, E.; Reibenspies, J.H.; Bhuvanesh, N.; Darensbourg, M.Y. Comparisons of zinc with cadmium in N2S2 coordination and as S-bonded adducts to tungsten carbonyls. Dalton Trans. 2012, 41, 143–148. [Google Scholar] [CrossRef]
- Jill Black, S.; Einstein, F.W.B.; Hayes, P.C.; Kumar, R.; Tuck, D.G. Reactions of cadmium thiolate (Cd(SR)2, R = n-butyl, phenyl) and the molecular structure of the 2,2’-bipyridine adduct of bis(n-butyl thioxanthato)cadmium(II). Inorg. Chem. 1986, 25, 4181–4184. [Google Scholar] [CrossRef]
- Philip, A.W.D.; Payne, N.C.; Vittal, J.J.; Yu, Y. Synthesis and NMR spectra (phosphorus-31, cadmium-111/113, selenium-77) of adamantane-like phosphine complexes with the (µ-ER)6Cd4 core and the crystal and molecular structure of [(µ-SPri)6(CdPPh3)2(CdOClO3)2] · EtOH. Inorg. Chem. 1993, 32, 4632–4639. [Google Scholar] [CrossRef]
- Dean, P.A.W.; Vittal, J.J. SYNTHESIS AND STRUCTURE OF cis-syn-[Cd(μ-SEt)(S2CSEt)(P{c-C6H11}3)]2. Main Group Met. Chem. 2002, 25, 695–696. [Google Scholar] [CrossRef]
- Gruber, F. Synthesis and Structure Determination of Two Neutral Cadmium Thiophenolate Clusters. Z. Anorg. Allg. Chem. 2012, 638, 2467–2469. [Google Scholar] [CrossRef]
- DeGroot, M.W.; Taylor, N.J.; Corrigan, J.F. Controlled Synthesis of Ternary II−II′−VI Nanoclusters and the Effects of Metal Ion Distribution on Their Spectral Properties. Inorg. Chem. 2005, 44, 5447–5458. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nakazawa, T. Electrical and Optical Properties of Stannite-Type Quarternary Semiconductor Thin Films. Jpn. J. Appl. Phys. Part 1 1988, 27, 2094–2097. [Google Scholar] [CrossRef]
- Matsushita, H.; Ichikawa, T.; Katsui, A. Structural, thermodynamical and optical properties of Cu2-II-IV-VI4 quaternary compounds. J. Mater. Sci. 2005, 40, 2003–2005. [Google Scholar] [CrossRef]
- Hadke, S.; Levcenko, S.; Sai Gautam, G.; Hages, C.J.; Márquez, J.A.; Izquierdo-Roca, V.; Carter, E.A.; Unold, T.; Wong, L.H. Suppressed Deep Traps and Bandgap Fluctuations in Cu2CdSnS4 Solar Cells with ≈8% Efficiency. Adv. Energy Mater. 2019, 9, 1902509. [Google Scholar] [CrossRef]
- Fan, P.; Lin, J.; Hu, J.; Yu, Z.; Zhao, Y.; Chen, S.; Zheng, Z.; Luo, J.; Liang, G.; Su, Z. Over 10% Efficient Cu2CdSnS4 Solar Cells Fabricated from Optimized Sulfurization. Adv. Funct. Mater. 2022, 32, 2207470. [Google Scholar] [CrossRef]
- Gao, F.; Yamazoe, S.; Maeda, T.; Nakanishi, K.; Wada, T. Structural and Optical Properties of In-Free Cu2ZnSn(S,Se)4 Solar Cell Materials. Jpn. J. Appl. Phys. 2012, 51, 10NC29. [Google Scholar] [CrossRef]
- Nitsche, R.; Sargent, D.F.; Wild, P. Crystal growth of quaternary 122464 chalcogenides by iodine vapor transport. J. Cryst. Growth 1967, 1, 52–53. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Crystal and electronic band structure of Cu2ZnSnX4 (X=S and Se) photovoltaic absorbers: First-principles insights. Appl. Phys. Lett. 2009, 94, 41903. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Electronic structure and stability of quaternary chalcogenide semiconductors derived from cation cross-substitution of II-VI and I-III-VI2 compounds. Phys. Rev. B 2009, 79, 165211. [Google Scholar] [CrossRef]
- Persson, C. Electronic and optical properties of Cu2ZnSnS4 and Cu2ZnSnSe4. J. Appl. Phys. 2010, 107, 53710. [Google Scholar] [CrossRef]
- Paier, J.; Asahi, R.; Nagoya, A.; Kresse, G. Cu2ZnSnS4 as a potential photovoltaic material: A hybrid Hartree-Fock density functional theory study. Phys. Rev. B 2009, 79, 115126. [Google Scholar] [CrossRef]
- Nakamura, S.; Maeda, T.; Wada, T. Phase Stability and Electronic Structure of In-Free Photovoltaic Materials: Cu2ZnSiSe4, Cu2ZnGeSe4 and Cu2ZnSnSe4. Jpn. J. Appl. Phys. 2010, 49, 121203. [Google Scholar] [CrossRef]
- Schorr, S. The crystal structure of kesterite type compounds: A neutron and X-ray diffraction study. Sol. Energy Mater. Sol. Cells 2011, 95, 1482–1488. [Google Scholar] [CrossRef]
- Just, J.; Lützenkirchen-Hecht, D.; Frahm, R.; Schorr, S.; Unold, T. Determination of secondary phases in kesterite Cu2ZnSnS4 thin films by X-ray absorption near edge structure analysis. Appl. Phys. Lett. 2011, 99, 262105. [Google Scholar] [CrossRef]
- Mendis, B.G.; Shannon, M.D.; Goodman, M.C.J.; Major, J.D.; Claridge, R.; Halliday, D.P.; Durose, K. Direct observation of Cu, Zn cation disorder in Cu2ZnSnS4 solar cell absorber material using aberration corrected scanning transmission electron microscopy. Prog. Photovolt: Res. Appl. 2014, 22, 24–34. [Google Scholar] [CrossRef]
- Tombak, A.; Kilicoglu, T.; Ocak, Y.S. Solar cells fabricated by spray pyrolysis deposited Cu2CdSnS4 thin films. Renew. Energy 2020, 146, 1465–1470. [Google Scholar] [CrossRef]
- Nakayama, N.; Ito, K. Sprayed films of stannite Cu2ZnSnS4. Appl. Surf. Sci. 1996, 92, 171–175. [Google Scholar] [CrossRef]
- Kamoun, N.; Bouzouita, H.; Rezig, B. Fabrication and characterization of Cu2ZnSnS4 thin films deposited by spray pyrolysis technique. Thin Solid Films 2007, 515, 5949–5952. [Google Scholar] [CrossRef]
- Madarásza, J.; Bombiczb, P.; Okuyaa, M.; Kanekoa, S. Thermal decomposition of thiourea complexes of Cu(I), Zn(II), and Sn(II) chlorides as precursors for the spray pyrolysis deposition of sulfide thin films. Solid State Ionics 2001, 141–142, 439–446. [Google Scholar] [CrossRef]
- Kociok-Köhn, G.; Molloy, K.C.; Sudlow, A.L. Molecular routes to Cu2ZnSnS4: A comparison of approaches to bulk and thin-film materials. Can. J. Chem. 2014, 92, 514–524. [Google Scholar] [CrossRef]
- Ramasamy, K.; Malik, M.A.; O’Brien, P. The chemical vapor deposition of Cu2ZnSnS4 thin films. Chem. Sci. 2011, 2, 1170–1172. [Google Scholar] [CrossRef]
- Fuhrmann, D.; Dietrich, S.; Krautscheid, H. Zinc Tin Chalcogenide Complexes and Their Evaluation as Molecular Precursors for Cu2ZnSnS4 (CZTS) and Cu2ZnSnSe4 (CZTSe). Inorg. Chem. 2017, 56, 13123–13131. [Google Scholar] [CrossRef] [PubMed]
- DeGroot, M.W.; Corrigan, J.F. Coordination Complexes of Zinc with Reactive ESiMe3 (E = S, Se, Te) Ligands. Organometallics 2005, 24, 3378–3385. [Google Scholar] [CrossRef]
- Babcock, J.R.; Zehner, R.W.; Sita, L.R.; Babcock, J.R.; Zehner, R.W.; Sita, L.R. A Heterocumulene Metathesis Route to Cd[ESiMe3]2 and Passivated CdE (E = S and Se) Nanocrystals // A Heterocumulene Metathesis Route to Cd[ESiMe3]2 and Passivated CdE (E = S and Se) Nanocrystals. Chem. Mater. 1998, 10, 2027–2029. [Google Scholar] [CrossRef]
- Foody, M.J.; Weimer, M.S.; Bhandari, H.; Hock, A.S. Comparison of Ligand Architecture on Vapor Deposition Precursors: Synthesis, Characterization, and Reactivity of Volatile Cadmium Bis-Amidinate Complexes. Inorg. Chem. 2021, 60, 6191–6200. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.C. Tertiary phosphines containing secondary alkyl radicals // 247. Tertiary phosphines containing secondary alkyl radicals. J. Chem. Soc. 1933, 1933, 1043–1044. [Google Scholar] [CrossRef]
- Maeda, Y.; Okawara, R. Studies on dialkyltin diacetate derivatives. J. Organomet. Chem. 1967, 10, 247–256. [Google Scholar] [CrossRef]
- Dietzel, S.; Jurkschat, K.; Tzschach, A.; Zschunke, A. Synthese und spektroskopische Untersuchungen von Di-t-butylzinn(IV)-dicarboxylaten. Z. Anorg. Allg. Chem. 1986, 537, 163–168. [Google Scholar] [CrossRef]
- Mokal, V.B.; Jain, V.K. Steric effects on the formation of isolable products in the reactions of dibutyltin oxides with carboxylic acids. J. Organomet. Chem. 1992, 441, 215–226. [Google Scholar] [CrossRef]
- Lockhart, T.P.; Manders, W.F.; Holt, E.M. Solution and Solid-state Molecular Structures of Me2Sn(OAc)2 (I) and Its Hydrolyzate, ([Me2Sn(OAc)]2O)2 (II), by Solution and Solid-state 13C NMR. X-ray Diffraction Study of II. J. Am. Chem. Soc. 1986, 108, 6611–6616. [Google Scholar] [CrossRef]
- Seyferth, D.; Stone, F.G.A. Vinyl Derivatives of the Metals. I. Synthesis of Vinyltin Compounds. J. Am. Chem. Soc. 1957, 79, 515–517. [Google Scholar] [CrossRef]
- Brown, M.P.; Okawara, R.; Rochow, E.G. Infrared spectra of some methyl derivatives of germanium and tin. Spectrochim. Acta 1960, 16, 595–601. [Google Scholar] [CrossRef]
- Puff, H.; Schuh, W.; Sievers, R.; Wald, W.; Zimmer, R. Niedermolekulare Diorganozinn-Sauerstoff-Verbindungen: Di-t-butyl- und Di-t-amylzinnoxid. J. Organomet. Chem. 1984, 260, 271–280. [Google Scholar] [CrossRef]
- Anderson, H.H. Volatile n-Butyltin and Phenyltin Tricarboxylates. Organotin Oxymonocarboxylates. Inorg. Chem. 1964, 3, 912–914. [Google Scholar] [CrossRef]
- Brauer, G. Handbuch der Präparativen Anorganischen Chemie; Ferdinand Enke Verlag: Stuttgart, Germany, 1978. [Google Scholar]
- Schmidt, M.; Ruf, H. Über die Umsetzung von Organohalogensilanen mit Natriumselenid. Z. Anorg. Allg. Chem. 1963, 321, 270–273. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Grange, P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- STOE & Cie GmbH. X-Area Version 1.70; STOE & Cie GmbH: Darmstadt, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond Version 3.2k; CRYSTAL IMPACT: Bonn, Germany, 2014. [Google Scholar]
- Bruker AXS. TOPAS Version 5; Bruker AXS: Karlsruhe, Germany, 2014. [Google Scholar]
- Ulrich, F.; Zachariasen, W. XIV. Über die Kristallstruktur des α- und ß-CdS, sowie des Wurtzits. Z. Kristallogr. 1925, 62, 260–273. [Google Scholar] [CrossRef]
- Wiedemeier, H.; von Schnering, H.G. Refinement of the structures of GeS, GeSe, SnS and SnSe. Z. Kristallogr. 1978, 148, 295–303. [Google Scholar] [CrossRef]
- Menzebach, B.; Bleckmann, P. Die Kristallstruktur von Hexamethylcyclotristannathian. J. Organomet. Chem. 1975, 91, 291–294. [Google Scholar] [CrossRef]
- Fuhrmann, D. Synthese, Charakterisierung und Thermolyse von potentiellen molekularen Vorläufern für Cu2ZnSnE4 und Ag2ZnSnE4 (E = S, Se). Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2017. [Google Scholar]
- Dakternieks, D.; Jurkschat, K.; Schollmeyer, D.; Wu, H. Synthesis, NMR studies and molecular structures of [(PhSSn)2(CH2)3]2 and [PhSn(S2CNEt2)]2(S)(CH2)3. J. Organomet. Chem. 1995, 492, 145–150. [Google Scholar] [CrossRef]
- Feng, M.-L.; Ye, D.; Huang, X.-Y. Quaternary tin(IV) antimony(III) sulfide decorated with lanthanum(III) ethylenediamine complexes: [La(en)4SbSnS5]2 x 0.5 H2O. Inorg. Chem. 2009, 48, 8060–8062. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Merzweiler, K. [(PhSnS3)2(CuPPhMe2)6], ein sechskerniger Kupfer(I)-Komplex mit PhSnS3-Liganden. Z. Anorg. Allg. Chem. 2002, 628, 905–906. [Google Scholar] [CrossRef]
- Eußner, J.P.; Dehnen, S. Bronze, silver and gold: Functionalized group 11 organotin sulfide clusters. Chem. Commun. 2014, 50, 11385–11388. [Google Scholar] [CrossRef]
- Adams, R.D.; Zhang, B.; Murphy, C.J.; Yeung, L.K. Halide enhancement of the luminescence of Cd10S4 thiolate clusters. Chem. Commun. 1999, 1999, 383–384. [Google Scholar] [CrossRef]
- Wallbank, A.I.; Lebold, T.P.; Borecki, A.; Polgar, A.M.; Waters, B.M.; Workentin, M.S.; Corrigan, J.F. ZnII and CdII Ferrocenechalcogenolate Complexes. Eur. J. Inorg. Chem. 2017, 2017, 372–377. [Google Scholar] [CrossRef]
- Bendova, M.; Puchberger, M.; Schubert, U. Characterization of “Cd10S4(SPh)12”, the Thermal Decomposition Product of (NMe4)4[Cd10S4(SPh)16]: Synthesis of a Neutral Cd54 Sulfide Cluster and of a Polymeric Chain of Thiolate-Bridged Cd17 Sulfide Clusters. Eur. J. Inorg. Chem. 2010, 2010, 3299–3306. [Google Scholar] [CrossRef]
- Ka-Luo, T.; Xiang-Lin, J.; Shao-Juan, J.; You-Qi, T. Cadmium Thiolates obtained from Cambridge Structural Database. Jiegou Huaxue 1995, 14, 399. [Google Scholar]
- Weber, A.; Krauth, H.; Perlt, S.; Schubert, B.; Kötschau, I.; Schorr, S.; Schock, H.W. Multi-stage evaporation of Cu2ZnSnS4 thin films. Thin Solid Film. 2009, 517, 2524–2526. [Google Scholar] [CrossRef]



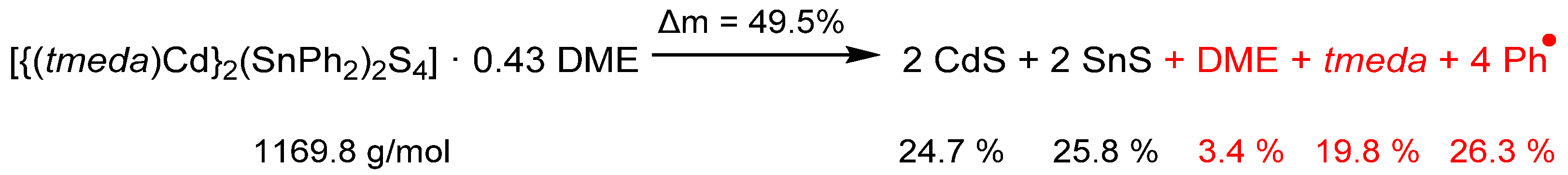
| 1 | 2 | 3 | |
|---|---|---|---|
| Formula | C30H58Cd3N6S6Sn2 | C40H62Cd2N4O2S4Sn2 | C99H123Cd7P3S13Sn6 |
| molar mass M/g mol−1 | 1269.84 | 1221.35 | 3321.6 |
| temperature/K | 180(2) | 200(2) | 180(2) |
| Wavelength | 154.186 | 71.073 | 154.186 pm |
| crystal color and shape | colorless block | colorless block | colorless cube |
| crystal size/mm | 0.01·0.02·0.02 | 0.17·0.24·0.35 | 0.02·0.02·0.02 |
| crystal system | triclinic | monoclinic | cubic |
| space group | P | P21/c | Pa |
| a/pm | 1279.0(4) | 1029.73(5) | 2889.9(2) |
| b/pm | 1473.1(4) | 2470.59(8) | 2889.9(2) |
| c/pm | 1647.3(4) | 1995.41(9) | 2889.9(2) |
| α/° | 114.98(3) | 90 | 90 |
| β/° | 92.85(3) | 99.724(4) | 90 |
| γ/° | 111.21(3) | 90 | 90 |
| V/106 pm3 | 2545.7(2) | 5003.5(4) | 24,134(5) |
| Z | 2 | 4 | 8 |
| calc. density/g cm−3 | 1.8 | 1.62 | 1.83 |
| absorption coefficient µ/mm−1 | 20.31 | 2.03 | 22.12 |
| θ range/° | 2.6–26.7 | 3.4–40 | |
| measured reflections | 29,186 | 9608 | |
| independent reflections (Rint) | 10,534 (0.0263) | 2369 (0.1115) | |
| observed reflections (I > 2σ(I)) | 7717 | 1229 | |
| Parameters | 494 | 220 | |
| R1 (observed reflections) | 0.0278 | 0.0736 | |
| wR2 (all data) | 0.0634 | 0.1686 | |
| max./min. residual e- density/10−6 pm−3 | 1.1 and −0.6 | 0.3 and −0.8 |
| Bond Lengths | Bond Angles | ||
|---|---|---|---|
| Sn1-S1 | 239.17(9) | S1-Sn1-S4 | 103.55(3) |
| Sn1-S4 | 236.86(9) | S2-Sn2-S3 | 107.79(3) |
| Sn2-S2 | 236.38(9) | S1-Cd1-S3 | 91.23(3) |
| Sn2-S3 | 238.15(9) | S1-Cd1-S4 | 85.39(3) |
| Cd1-S1 | 300.01(9) | S3-Cd1-S4 | 141.72(3) |
| Cd1-S3 | 248.04(9) | S1-Cd2-S2 | 139.06(3) |
| Cd1-S4 | 248.7(1) | N-Cd1-N | 75.9(1) |
| Cd2-S1 | 249.34(8) | N-Cd2-N | 77.9(1) |
| Cd2-S2 | 247.82(9) | ||
| Bond Lengths | Bond Angles | ||
|---|---|---|---|
| Sn1-S1 | 241.5(9) | S1-Sn1-S3 | 113.0(3) |
| Sn1-S3 | 236.8(9) | S2-Sn2-S4 | 122.0(4) |
| Sn2-S4 | 237(1) | S1-Cd1-S2 | 108.6(3) |
| Sn2-S2 | 245.9(9) | S1-Cd1-S3 | 105.1(3) |
| Cd1-S1 | 257.7(9) | S2-Cd1-S3 | 112.7(3) |
| Cd1-S2 | 252.4(9) | S2-Cd2-S3 | 110.7(3) |
| Cd1-S3 | 255.7(9) | S2-Cd2-S4 | 101.8(4) |
| Cd1-P1 | 261.5(9) | S3-Cd2-S4 | 101.7(3) |
| Cd2-S2 | 254.2(9) | S2-Cd2-S5 | 100.3(2) |
| Cd2-S3 | 262.2(9) | S3-Cd2-S5 | 105.8(4) |
| Cd2-S4 | 244(1) | S4-Cd2-S5 | 120.1(4) |
| Cd2-S5 | 251.6(9) | S1-Cd3-S5 | 111.3(2) |
| Cd3-S1 | 252.8(9) | S1-Cd3-S1 | 107.6(2) |
| Cd3-S5 | 247.5(9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuhrmann, D.; Bollfraß, N.H.; Icker, M.; Krautscheid, H. Synthesis, X-ray Diffraction, NMR and Thermolysis Studies of Cadmium Tin Sulfido Complexes. Crystals 2023, 13, 721. https://doi.org/10.3390/cryst13050721
Fuhrmann D, Bollfraß NH, Icker M, Krautscheid H. Synthesis, X-ray Diffraction, NMR and Thermolysis Studies of Cadmium Tin Sulfido Complexes. Crystals. 2023; 13(5):721. https://doi.org/10.3390/cryst13050721
Chicago/Turabian StyleFuhrmann, Daniel, Nick Hermann Bollfraß, Maik Icker, and Harald Krautscheid. 2023. "Synthesis, X-ray Diffraction, NMR and Thermolysis Studies of Cadmium Tin Sulfido Complexes" Crystals 13, no. 5: 721. https://doi.org/10.3390/cryst13050721





