Study of the Structural-Phase State of Hydroxyapatite Coatings Obtained by Detonation Spraying at Different O2/C2H2 Ratios
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- -
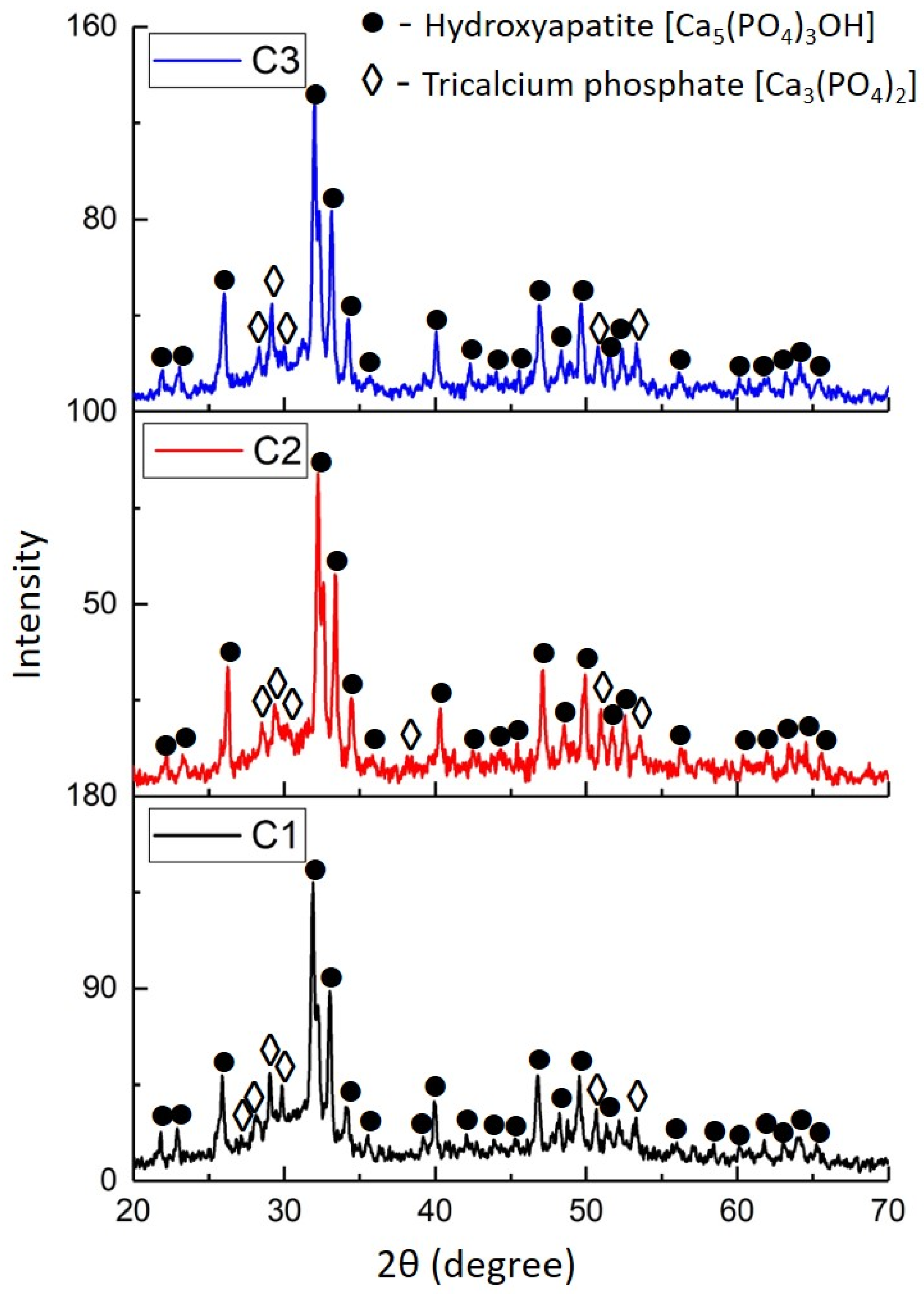
- When varying the molar ratio of O2/C2H2 components from 2.61 to 3.35 and the explosive charge in the range of 73–77%, coatings containing hydroxyapatite (HA, predominant phase), tricalcium phosphate (α-TCP) and amorphous phases were formed. Variation in the above parameters of detonation spraying did not reveal any change in the phase composition of the coatings;
- -
- During the detonation spraying of HA coatings, the formation of amorphous phases occurs due to the rapid cooling of particles heated to the melting point;
- -
- The study of coating surface morphology showed that partial melting of the coating surface was observed in all the studied regimes of detonation spraying;
- -
- An increase in the O2/C2H2 ratio from 2.61 to 3.35 leads to an increase in the roughness of the coating structure and with an increase in the explosive charge of 77 %, transverse cracks appear in the coating;
- -
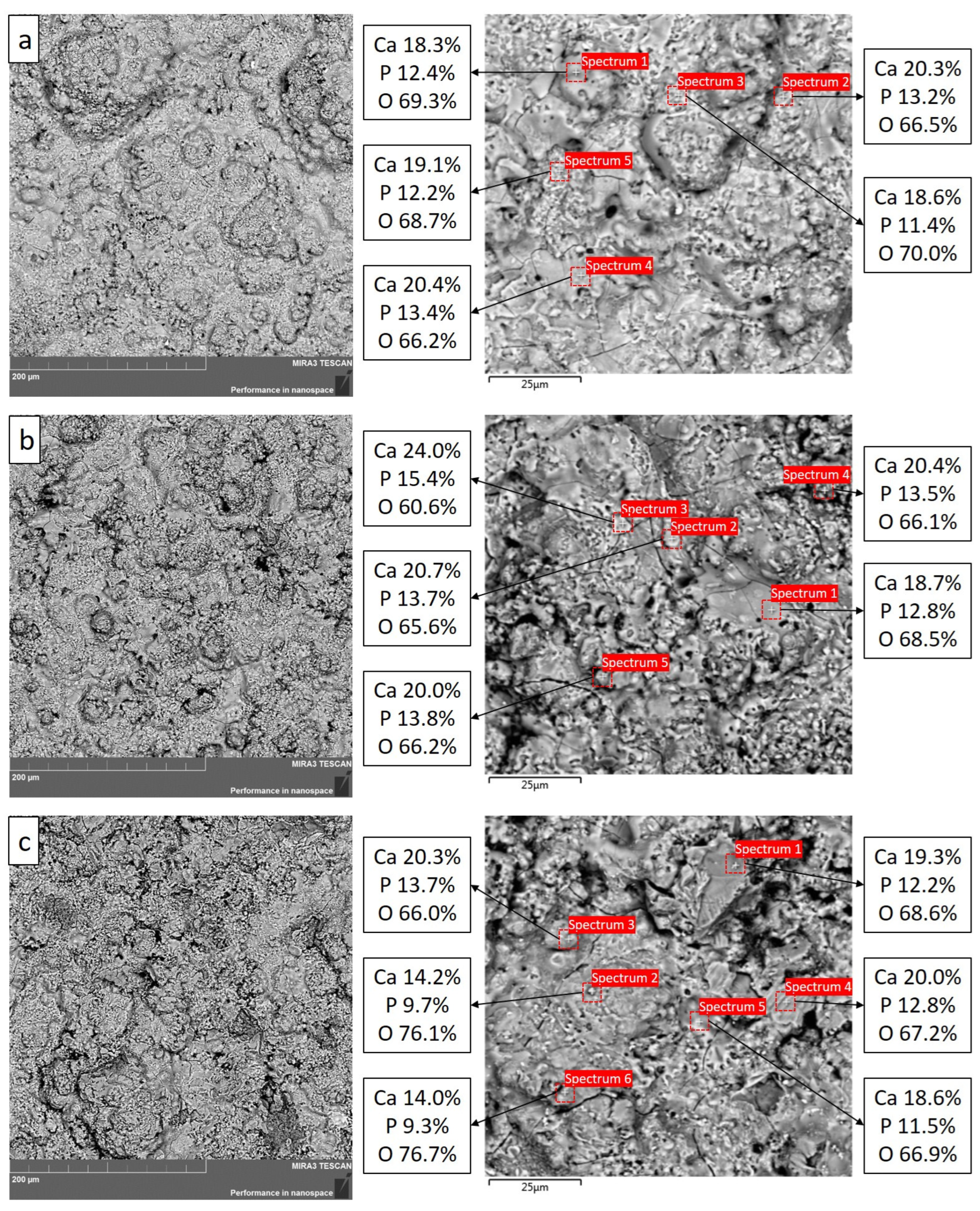
- The results of the EDS analysis of coatings showed the presence of major constituent elements such as Ca, P and O in different atomic percentages. The calculated Ca/P ratio mainly corresponds to Ca/P = 1.5 (α-TCP) and Ca/P = 1.67 (HA), which is in agreement with the XRD results;
- -
- The average hardness of the HA coatings obtained by C1 and C2 regimes has a single value and is about 2.5–2.4 GPa, and the elastic modulus is 51.5–44.1 GPa. Bone tissue has the following characteristics: H = 2–4 GPa, E = 7–26 GPa. In further development of the research topic, special attention will be paid to the possibility of obtaining single-phase HA coatings by detonation spraying with a close value of mechanical properties to bone tissue.
- -
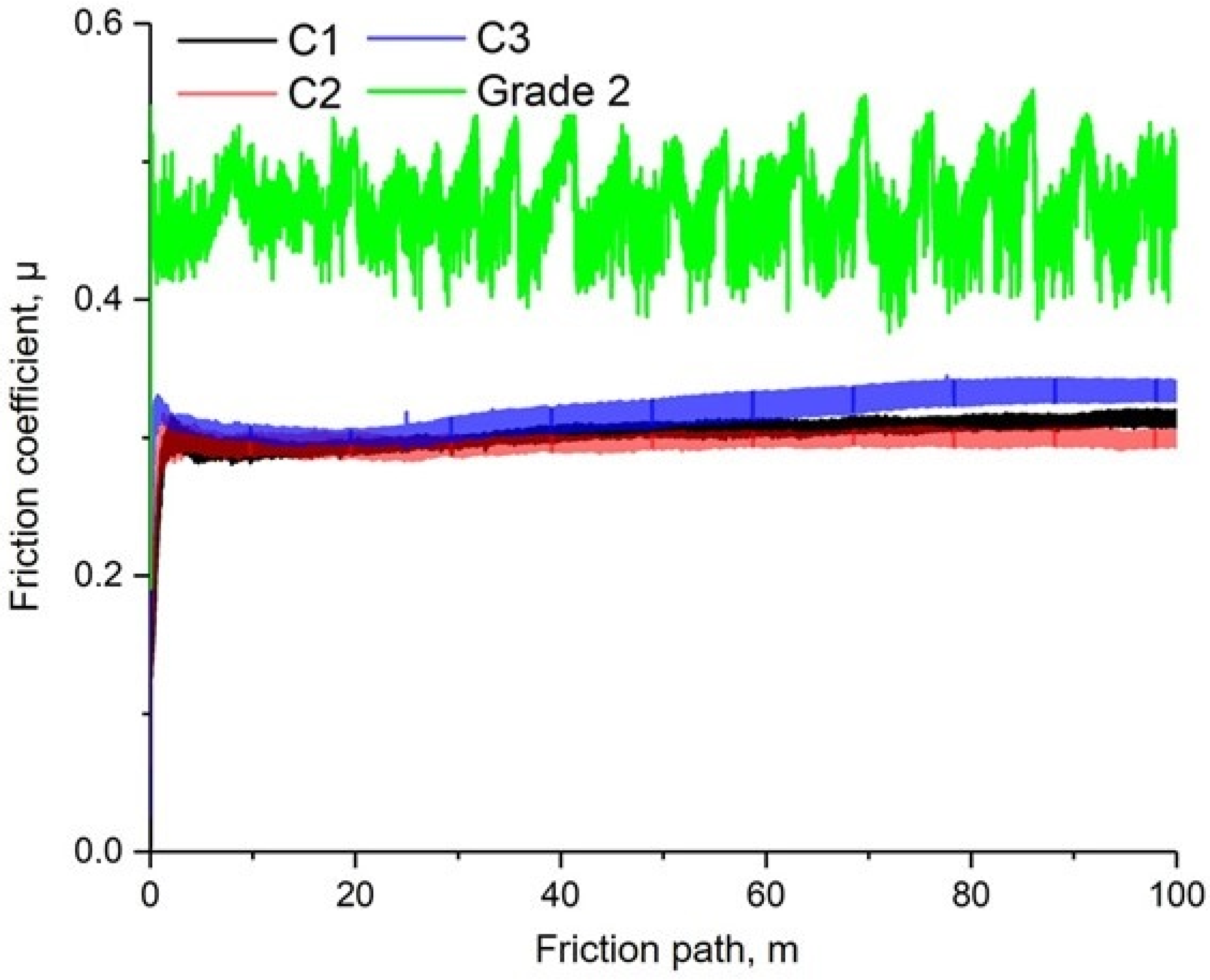
- The application of hydroxyaptite coatings on titanium surfaces reduces the friction coefficient in Ringer’s solution about 1.5 times.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. Vitro and in vivo osteogenic activity of titanium implants coated by pulsed laser deposition with a thin film of fluoridated hydroxyapatite. Int. J. Mol. Sci. 2018, 19, 1127. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Gebert, A.; Schumacher, M.; Hoffmann, V.; Voss, A.; Pilz, S.; Uhlemann, M.; Lode, A.; Gelinsky, M. Electrodeposition of Sr-substituted hydroxyapatite on low modulus beta-type Ti-45Nb and effect on in vitro Sr release and cell response. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110425. [Google Scholar] [CrossRef] [PubMed]
- Pichugin, V.F.; Surmenev, R.A.; Shesterikov, E.V.; Ryabtseva, M.A.; Eshenko, E.V.; Tverdokhlebov, S.I.; Prymak, O.; Epple, M. The preparation of calcium phosphate coatings on titanium and nickel–titanium by RF-magnetron-sputtered deposition: Composition, structure and micromechanical properties. Surf. Coat. Technol. 2008, 202, 3913–3920. [Google Scholar] [CrossRef]
- Moore, B.; Asadi, E.; Lewis, G. Deposition methods for microstructured and nanostructured coatings on metallic bone implants: A review. Adv. Mater. Sci. Eng. 2017, 2017, 5812907. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Małachowska, A.; Kozerski, S. Review of functionally graded thermal sprayed coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Gan, J.A.; Berndt, C.C. Nanocomposite coatings: Thermal spray processing, microstructure and performance. Int. Mater. Rev. 2015, 60, 195–244. [Google Scholar] [CrossRef]
- Ohki, M.; Takahashi, S.; Jinnai, R.; Hoshina, T. Interfacial strength of plasma-sprayed hydroxyapatite coatings. J. Therm. Spray Technol. 2020, 29, 1119–1133. [Google Scholar] [CrossRef]
- Yang, S.; Man, H.C.; Xing, W.; Zheng, X. Adhesion strength of plasma-sprayed hydroxyapatite coatings on laser gas-nitrided pure titanium. Surf. Coat. Technol. 2009, 203, 3116–3122. [Google Scholar] [CrossRef]
- Kashin, A.D.; Sedelnikova, M.B.; Uvarkin, P.V.; Ugodchikova, A.V.; Luginin, N.A.; Sharkeev, Y.P.; Khimich, M.A.; Bakina, O.V. Functionalizing Diatomite-Based Micro-Arc Coatings for Orthopedic Implants: Influence of TiO2 Addition. Biomimetics 2023, 8, 280. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Kashin, A.D.; Uvarkin, P.V.; Tolmachev, A.I.; Sharkeev, Y.P.; Ugodchikova, A.V.; Luginin, N.A.; Bakina, O.V. Porous Biocoatings Based on Diatomite with Incorporated ZrO2 Particles for Biodegradable Magnesium Implants. J. Funct. Biomater. 2023, 14, 241. [Google Scholar] [CrossRef]
- Prezas, P.R.; Soares, M.J.; Borges, J.P.; Silva, J.C.; Oliveira, F.J.; Graça, M.P.F. Bioactivity Enhancement of Plasma-Sprayed Hydroxyapatite Coatings through Non-Contact Corona Electrical Charging. Nanomaterials 2023, 13, 1058. [Google Scholar] [CrossRef] [PubMed]
- Alontseva, D.; Azamatov, B.; Safarova, Y.; Voinarovych, S.; Nazenova, G. A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility. Coatings 2023, 13, 1175. [Google Scholar] [CrossRef]
- Paterlini, A.; Alexis, J.; Balcaen, Y.; Bertrand, G. Cold Spraying of Thick Biomimetic and Stoichiometric Apatite Coatings for Orthopaedic Implants. Coatings 2022, 12, 722. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Baizhan, D. Creation of Bioceramic Coatings on the Surface of Ti–6Al–4V Alloy by Plasma Electrolytic Oxidation Followed by Gas Detonation Spraying. Coatings 2021, 11, 1433. [Google Scholar] [CrossRef]
- Gledhill, H. In vitro fatigue behaviour of vacuum plasma and detonation gun sprayed hydroxyapatite coatings. Biomaterials 2001, 22, 1233–1240. [Google Scholar] [CrossRef]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. Direct morphological comparison of vacuum plasma sprayed and detonation gun sprayed hydroxyapatite coatings for orthopaedic applications. Biomaterials 1999, 20, 315–322. [Google Scholar] [CrossRef]
- Kowalski, S.; Gonciarz, W.; Belka, R.; Góral, A.; Chmiela, M.; Lechowicz, Ł.; Kaca, W.; Żórawski, W. Plasma-Sprayed Hydroxyapatite Coatings and Their Biological Properties. Coatings 2022, 12, 1317. [Google Scholar] [CrossRef]
- Melero, H.C.; Sakai, R.T.; Vignatti, C.A.; Benedetti, A.V.; Fernández, J.; Guilemany, J.M.; Suegama, P.H. Corrosion resistance evaluation of HVOF produced hydroxyapatite and TiO2-hydroxyapatite coatings in Hanks’ solution. Mater. Res. 2018, 21, 1–11. [Google Scholar] [CrossRef]
- Kantay, N.; Rakhadilov, B.; Kurbanbekov, S.; Yeskermessov, D.; Yerbolatova, G.; Apsezhanova, A. Influence of Detonation-Spraying Parameters on the Phase Composition and Tribological Properties of Al2O3 Coatings. Coatings 2021, 11, 793. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Tyurin, Y.; Kakimzhanov, D.; Baizhan, D.; Kolisnichenko, O.; Zhurerova, L. Deposition of duplex Cr3C2-NiCr coatings on steel using a combined technique of gas detonation spraying and pulse-plasma treatment. High Temp. Mater. Process. 2021, 25, 25–37. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Baizhan, D.R.; Sagdoldina, Z.B.; Buitkenov, D.B.; Maulet, M. Phase composition and structure of composite Ti/HA coatings synthesized by detonation spraying. AIP Conf. Proc. 2020, 2297, 020022. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Kakimzhanov, D.; Baizhan, D.; Muslimanova, G.; Pazylbek, S.; Zhurerova, L. Comparative Study of Structures and Properties of Detonation Coatings with α-Al2O3 and γ-Al2O3 Main Phases. Coatings 2021, 11, 1566. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Maulet, M.; Kakimzhanov, D.N.; Stepanova, O.A.; Botabaeva, G.B. Comparative study of the structure and properties of homogeneous and gradient Ni-Cr-Al. Eurasian J. Phys. Funct. Mater. 2022, 6, 47–55. [Google Scholar]
- Skakov, M.; Bayandinova, M.; Ocheredko, I.; Tuyakbayev, B.; Nurizinova, M.; Gradoboev, A. Influence of Diabase Filler on the Structure and Tribological Properties of Coatings Based on Ultrahigh Molecular Weight Polyethylene. Polymers 2023, 15, 3465. [Google Scholar] [CrossRef] [PubMed]
- Yeskermessov, D.; Rakhadilov, B.; Zhurerova, L.; Apsezhanova, A.; Aringozhina, Z.; Booth, M.; Tabiyeva, Y. Surface modification of coatings based on Ni-Cr-Al by pulsed plasma treatment. AIMS Mater. Sci. 2023, 10, 755–766. [Google Scholar] [CrossRef]
- Skakov, M.; Ocheredko, I.; Tuyakbayev, B.; Bayandinova, M.; Nurizinova, M. Development and Studying of the Technology for Thermal Spraying of Coatings Made from Ultra-High-Molecular-Weight Polyethylene. Coatings 2023, 13, 698. [Google Scholar] [CrossRef]
- Liga, C.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. Infrared Spectrosc.-Mater. Sci. Eng. Technol. 2012, 12, 124–147. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, G.; Wang, Z.; Guo, X. Effect of post-heat treatment on the microstructure of micro-plasma sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2019, 367, 225–230. [Google Scholar] [CrossRef]
- Fomin, A.; Fomina, M.; Koshuro, V.; Rodionov, I.; Zakharevich, A.; Skaptsov, A. Structure and mechanical properties of hydroxyapatite coatings produced on titanium using plasma spraying with induction preheating. Ceram. Int. 2017, 43, 11189–11196. [Google Scholar] [CrossRef]
- Klyui, N.I.; Chornyi, V.S.; Zatovsky, I.V.; Tsabiy, L.I.; Buryanov, A.A.; Protsenko, V.V.; Temchenko, V.P.; Skryshevsky, V.A.; Glasmacher, B.; Gryshkov, O. Properties of gas detonation ceramic coatings and their effect on the osseointegration of titanium implants for bone defect replacement. Ceram. Int. 2021, 47, 25425–25439. [Google Scholar] [CrossRef]
- Khvostov, M.V.; Bulina, N.V.; Zhukova, N.A.; Morenkova, E.G.; Rybin, D.K.; Makarova, S.V.; Leonov, S.V.; Gorodov, V.S.; Ulianitsky, V.Y.; Tolstikova, T.G. A study on biological properties of titanium implants coated with multisubstituted hydroxyapatite. Ceram. Int. 2022, 48, 34780–34792. [Google Scholar] [CrossRef]
- Batraev, I.S.; Ulianitsky, V.Y.; Dina, D.V. Detonation spraying of copper: Theoretical analysis and experimental studies. Mater. Today Proc. 2017, 4, 11346–11350. [Google Scholar] [CrossRef]
- Kuchumova, I.D.; Batraev, I.S.; Cherkasova, N.Y.; Rybin, D.K.; Ukhina, A.V.; Botta, W.J.; Koga, G.Y.; Jorge, A.M. The influence of the O2/C2H2 ratio on the structure and properties of Fe66Cr10Nb5B19 detonation coatings. Mater. Today Proc. 2020, 25, 384–386. [Google Scholar] [CrossRef]
- D’Haese, R.; Pawlowski, L.; Bigan, M.; Jaworski, R.; Martel, M. Phase evolution of hydroxapatite coatings suspension plasma sprayed using variable parameters in simulated body fluid. Surf. Coat. Technol. 2010, 204, 1236–1246. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Sagdoldina, Z.B.; Baizhan, D.R.; Zhurerova, L.G.; Yeskermessov, D.K.; Kalitova, A.A.; Smaiylova, M. Obtaining of Hydroxyapatite Coatings on A Titanium Substrate by Detonation-Gas Spraying. Eurasian Phys. Tech. J. 2021, 18, 30–36. [Google Scholar] [CrossRef]
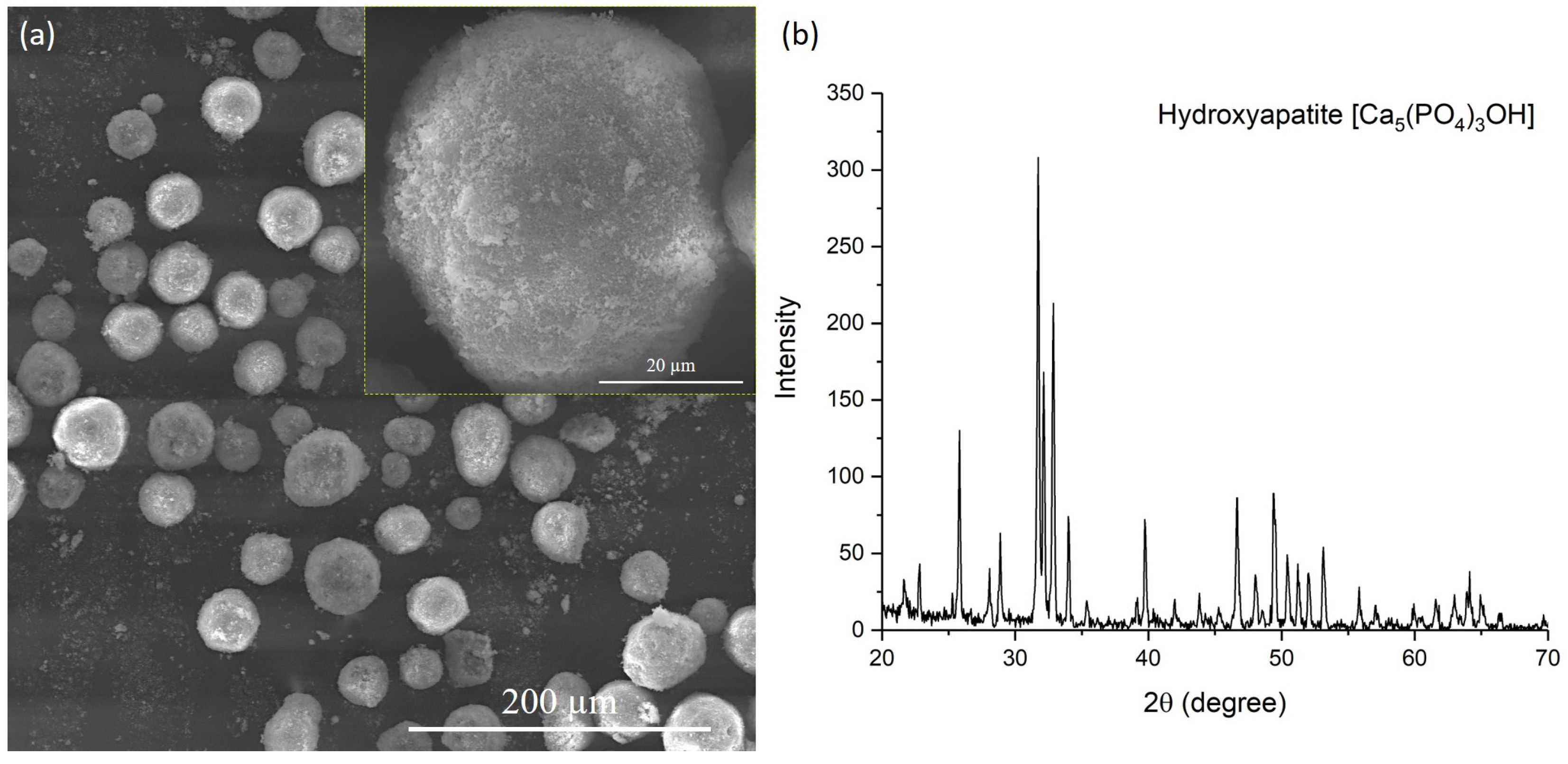

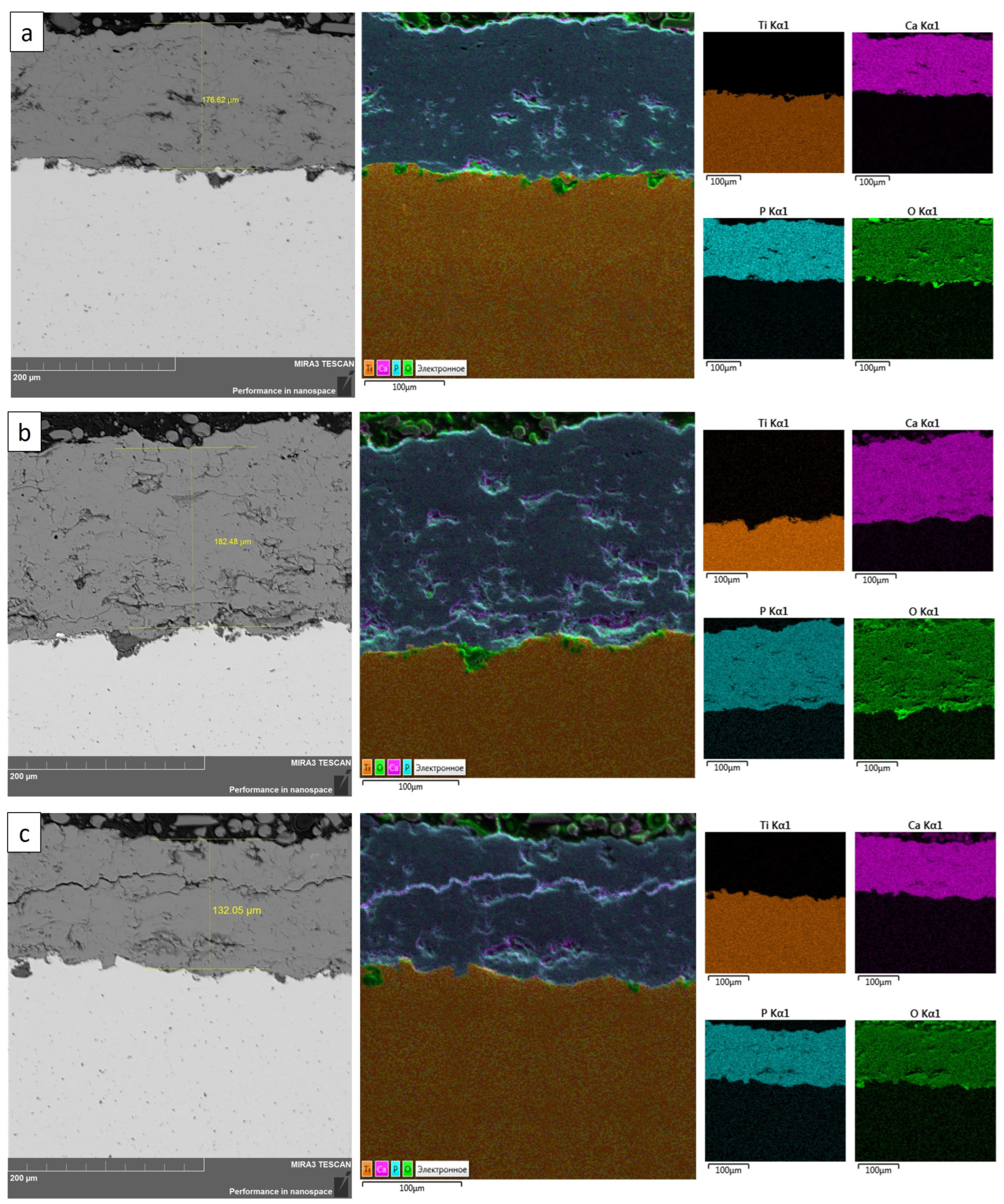

| Coating Type | O2/C2H2 Molar Ratio | Explosive Charge, % (Volume of Barrel Filling with Gas Mixture) | Spraying Distance, mm |
|---|---|---|---|
| C1 | 2.61 | 73 | 100 |
| C2 | 3.03 | 74 | 100 |
| C3 | 3.35 | 77 | 100 |
| Sample | Detected Phases | Phase Content, Mas. % | Lattice Parameters, Ǻ | CSR Size, nm | Δd/d × 10−3 |
|---|---|---|---|---|---|
| C1 (O2/C2H2—2.61) | Ca5(PO4)3OH | 50 | a = 9.3949 c = 6.8811 | 48 | 1 |
| Ca3(PO4)2 | 19 | a = 12.9870 b = 27.2012 c = 12.9328 | 18 | 0.7 | |
| Amorphous phase | 31 | - | - | - | |
| C2 (O2/C2H2—3.03) | Ca5(PO4)3OH | 53 | a = 9.3949 c = 6.8897 | 59 | 1 |
| Ca3(PO4)2 | 19 | a = 12.9515 b = 27.3361 c = 12.9063 | 25 | 1 | |
| Amorphous phase | 28 | - | - | - | |
| C3 (O2/C2H2—3.35) | Ca5(PO4)3OH | 51 | a = 9.3896 c = 6.8866 | 60 | 0.8 |
| Ca3(PO4)2 | 20 | a = 12.9492 b = 27.3800 c = 12.8901 | 18 | 0.7 | |
| Amorphous phase | 29 | - | - | - |
| Sample | Spectrum 1 | Spectrum 2 | Spectrum 3 | Spectrum 4 | Spectrum 5 |
|---|---|---|---|---|---|
| C1 (O2/C2H2—2.61) | 1.47 | 1.53 | 1.63 | 1.52 | 1.56 |
| C2 (O2/C2H2—3.03) | 1.46 | 1.51 | 1.55 | 1.51 | 1.44 |
| C3 (O2/C2H2—3.35) | 1.58 | 1.46 | 1.48 | 1.56 | 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baizhan, D.; Sagdoldina, Z.; Buitkenov, D.; Kambarov, Y.; Nabioldina, A.; Zhumabekova, V.; Bektasova, G. Study of the Structural-Phase State of Hydroxyapatite Coatings Obtained by Detonation Spraying at Different O2/C2H2 Ratios. Crystals 2023, 13, 1564. https://doi.org/10.3390/cryst13111564
Baizhan D, Sagdoldina Z, Buitkenov D, Kambarov Y, Nabioldina A, Zhumabekova V, Bektasova G. Study of the Structural-Phase State of Hydroxyapatite Coatings Obtained by Detonation Spraying at Different O2/C2H2 Ratios. Crystals. 2023; 13(11):1564. https://doi.org/10.3390/cryst13111564
Chicago/Turabian StyleBaizhan, Daryn, Zhuldyz Sagdoldina, Dastan Buitkenov, Yedilzhan Kambarov, Aiym Nabioldina, Venera Zhumabekova, and Gulsym Bektasova. 2023. "Study of the Structural-Phase State of Hydroxyapatite Coatings Obtained by Detonation Spraying at Different O2/C2H2 Ratios" Crystals 13, no. 11: 1564. https://doi.org/10.3390/cryst13111564







