Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of ZnO Bulk Powder
2.2. Chemical Synthesis of ZnO Nanoparticles
2.3. Biological Synthesis of ZnO Nanoparticles
2.4. Inoculum Preparation
2.5. Antibacterial Bioassay
3. Results and Discussion
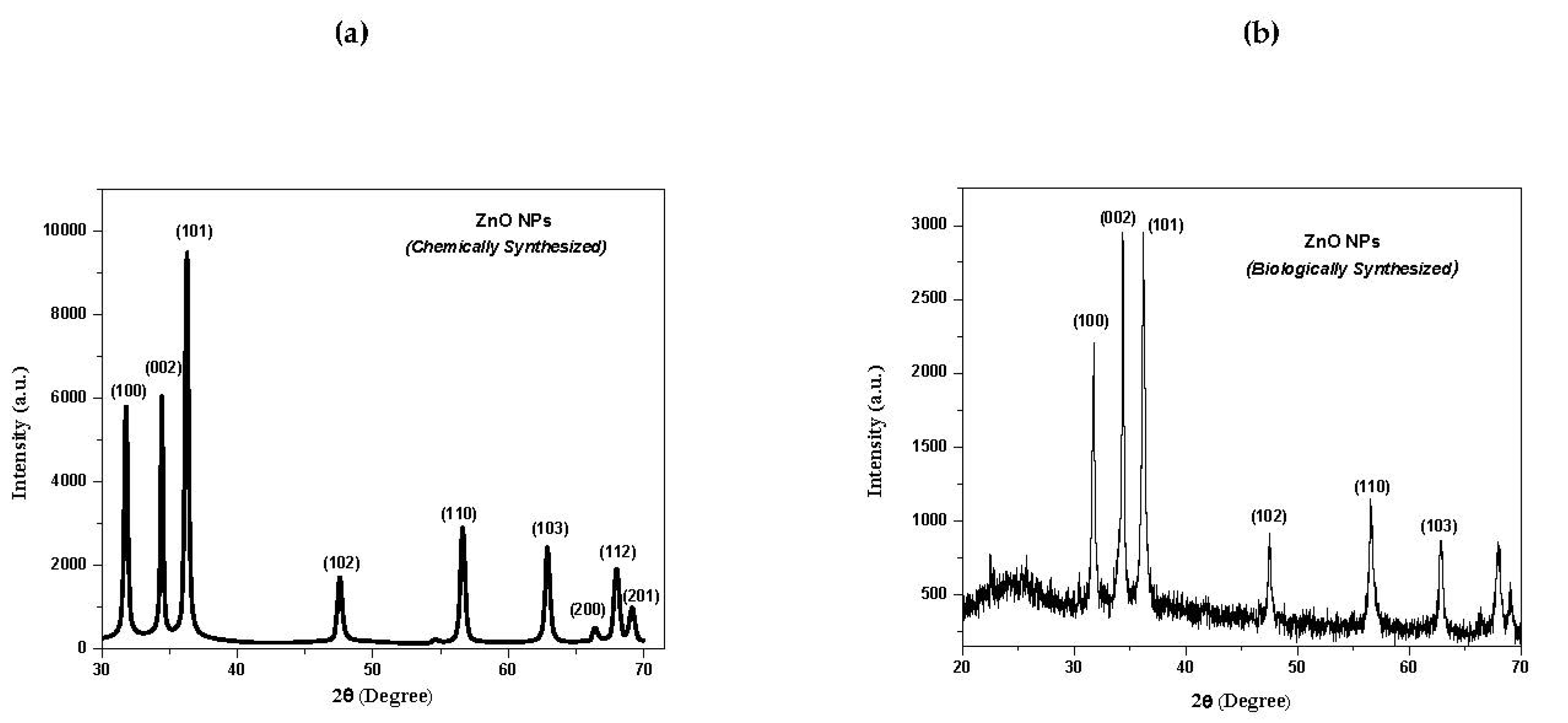
3.1. X-ray Diffraction (XRD) Analysis of ZnO NPs
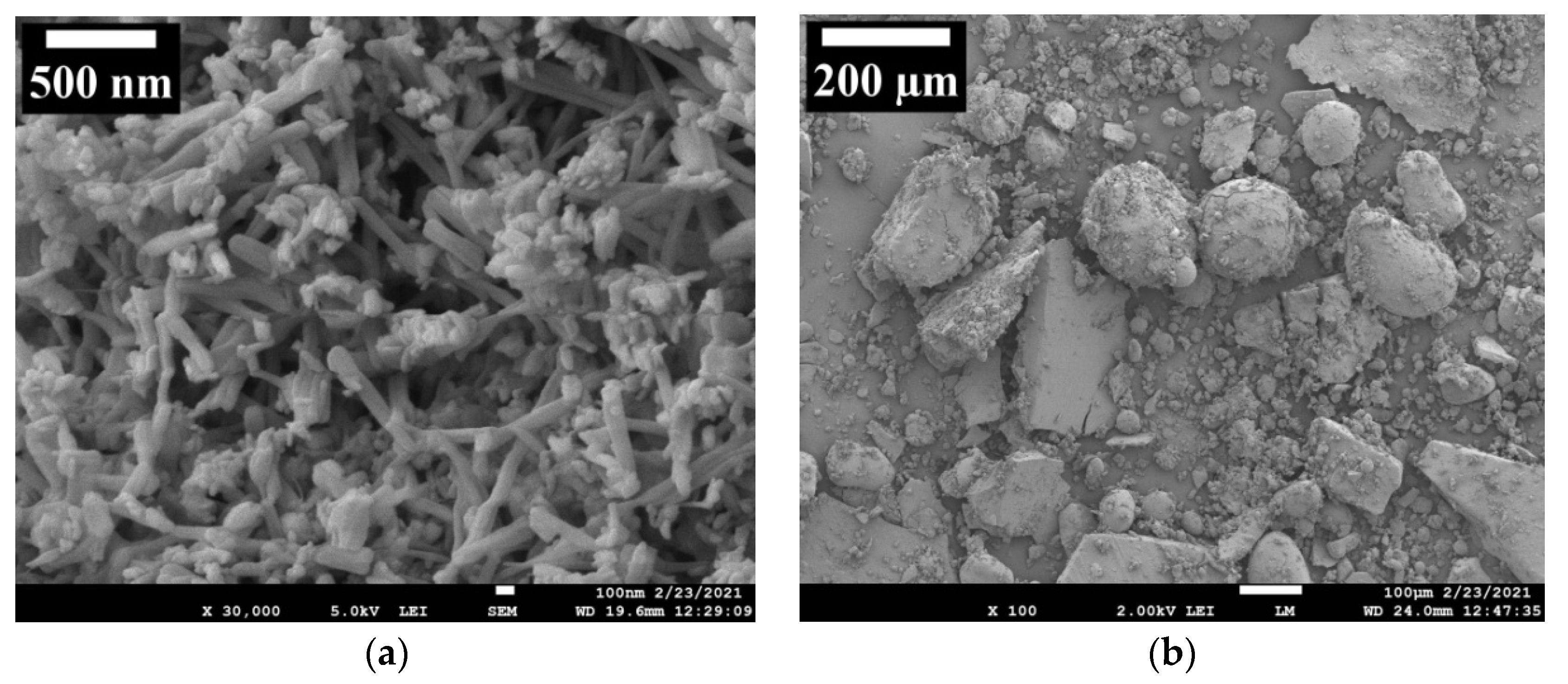
3.2. Field Emission Scanning Electron Microscopy (FE-SEM) Analysis of ZnO NPs
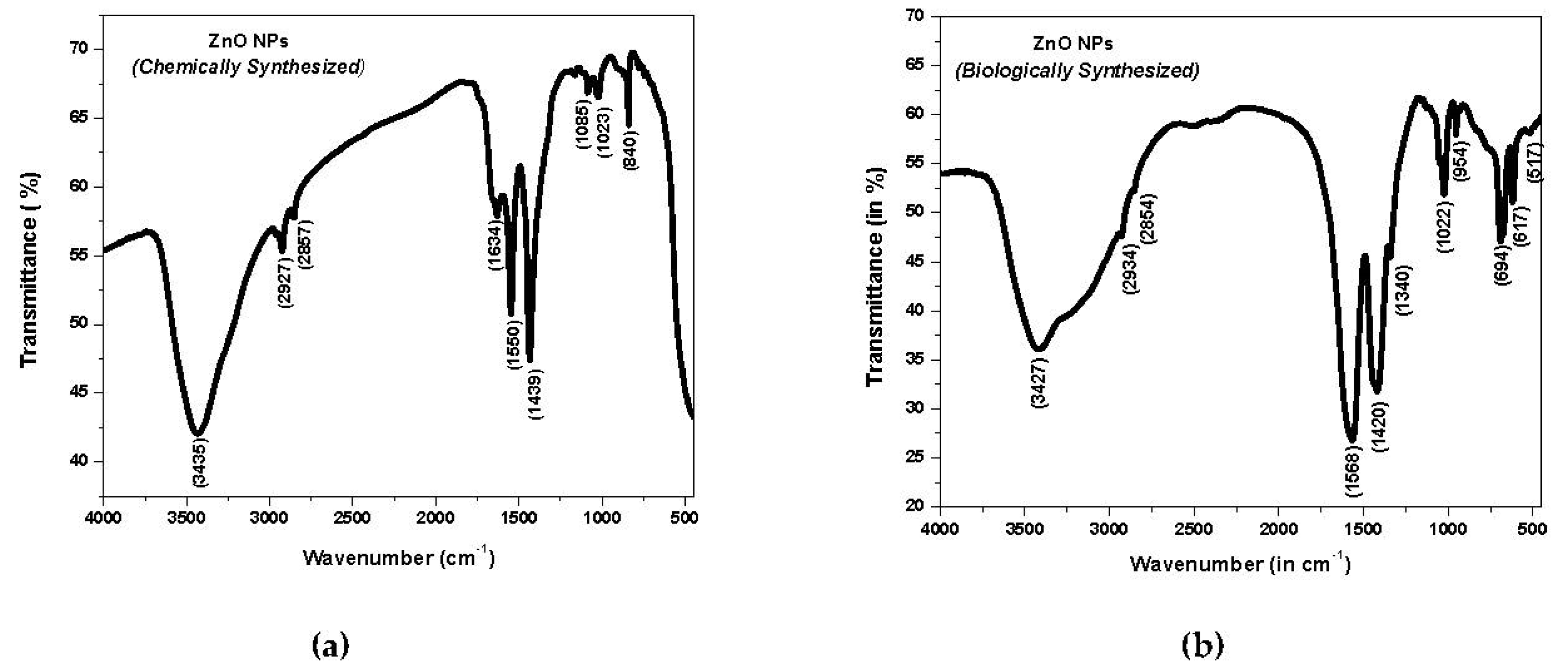
3.3. Fourier Transform Infrared (FTIR) Spectroscopy Analysis of ZnO NPs
3.4. UV–Visible Spectroscopy Analysis of ZnO NPs
3.5. Antibacterial Bioassay of Synthesized ZnO NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Aviv, M.; Berdicevsky, I.; Zilberman, M. Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. J. Biomed. Mater. Res. A 2007, 83, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bonzonga, J.C.J.; Kopelevich, D.; Bitton, G. Assessment of the Environmental Impacts of Nanotechnology on Organisms and Ecosystem; Progress Report; United States Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle characterization: State of the art, challenges, and emerging technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Saafan, A.; Zaazou, M.H. Assessment of Photodynamic Therapy and Nanoparticles Effects on Caries Models. Maced. J. Med. Sci. 2018, 6, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Zhang, Y. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag+ ions based on photo-curable core-shell AgBr/cationic polymer nanocomposites. J. Mater. Sci. Mater. Med. 2017, 28, 103. [Google Scholar] [CrossRef]
- Magalhaes, A.P.; Moreira, F.C. Silver nanoparticles in resin luting cements: Antibacterial and Physiochemicalproperties. J. Clin. Exp. Dent. 2016, 8, 415–422. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.O.; Ali, A.; Ahmad, I.; Zhao, T.K.; Botha, S.; Maaza, M.; Ezema, F.I. Biosynthesis of iron oxide nanoparticles via a composite of Psidiumguavaja-Moringaoleifera and their antibacterial and photocatalytic study. J. Photochem. Photobiol. B Biol. 2019, 119, 111601. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restore. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, G.L.; Delbem, A.C.B. Nanosynthesis of Silver-Calcium Glycerophosphate: Promising Associationagainst Oral Pathogens. Antibiotics 2018, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Chen, J.H.; Xu, X.L.; Yang, P.H.; Hildebrand, H.F. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int. J. Antimicrob. Agents 2006, 27, 513–517. [Google Scholar] [CrossRef]
- Burguera-Pascu, M.; Rodríguez-Archilla, A.; Baca, P. Substantivity of zinc salts used as rinsing solutions and their effect on the inhibition of Streptococcus mutans. J. Trace Elem. Med. Biol. 2007, 21, 92–101. [Google Scholar] [CrossRef]
- Sevinc, B.A.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. 2011, 94, 22–31. [Google Scholar]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Tokumoto, M.S.; Pulcinelli, S.H.; Santilli, C.V.; Briois, V. Catalysis and temperature dependence on the formation of ZnO nanoparticles and of zinc acetate derivatives prepared by the sol-gel route. J. Phys. Chem. B 2003, 107, 568–574. [Google Scholar] [CrossRef]
- Singhal, M.; Chhabra, V.; Kang, P.; Shah, D.O. Synthesis of ZnO nanoparticles for varistor application using Znsubstituted aerosol OT microemulsion. Mater. Res. Bull. 1997, 32, 239–247. [Google Scholar] [CrossRef]
- Rataboul, F.; Nayral, C.; Casanove, M.J.; Maisonnat, A.; Chaudret, B. Synthesis and characterization of monodisperse zinc and zinc oxide nanoparticles from the organometallic precursor [Zn(C6H11)2]. J. Organomet. Chem. 2002, 643, 307–312. [Google Scholar] [CrossRef]
- Okuyama, K.; Lenggoro, W.W. Preparation of nanoparticles via spray route. Chem. Eng. Sci. 2003, 58, 537–547. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Nazari, T.; Badraghi, T.J.; Kazemzad, M. Synthesis of ZnO nanoparticles and electrodeposition of poly-pyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 2009, 4, 247–257. [Google Scholar]
- Wei, Y.L.; Chang, P.C. Characteristics of nano zinc oxide synthesized under ultrasonic conditions. J. Phys. Chem. Solids 2008, 69, 688–692. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhu, Y.J.; Wang, S.W. Sono-chemical and microwave-assisted synthesis of linked single-crystalline ZnO rods. Mater. Chem. Phys. 2004, 88, 421–426. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Zhai, H.J.; Wu, W.H.; Lu, F.; Wang, H.S.; Wang, C. Effects of ammonia and cetyl-trimethyl-ammonium bromide (CTAB) on morphologies of ZnO nano- and micro-materials under solvothermal process. Mater. Chem. Phys. 2008, 112, 1024–1028. [Google Scholar] [CrossRef]
- Bitenc, M.; Marinsek, M.; Crnjak, O.Z. Preparation and characterization of zinc hydroxide carbonate and porous zinc oxide particles. J. Eur. Ceram. Soc. 2008, 28, 2915–2921. [Google Scholar] [CrossRef]
- Ajmal, H.M.S.; Khan, F.; Nam, K.; Kim, H.Y.; Kim, S.D. Ultraviolet Photodetection Based on High-Performance Co-Plus-Ni Doped ZnO Nanorods Grown by Hydrothermal Method on Transparent Plastic Substrate. Nanomaterials 2020, 10, 1225. [Google Scholar] [CrossRef]
- Ajmal, H.M.S.; Khan, F.; Huda, N.U.; Lee, S.; Nam, K.; Kim, H.Y.; Eom, T.-H.; Kim, S.D. High-Performance Flexible Ultraviolet Photodetectors with Ni/Cu-Codoped ZnO Nanorods Grown on PET Substrates. Nanomaterials 2019, 9, 1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermark, K.; Rensmo, H.; Siegbahn, H.; Keis, K.; Hagfeldt, A.; Ojamäe, L.; Persson, P. PES studies of Ru (dcbpyH2) 2 (NCS) 2 adsorption on nanostructured ZnO for solar cell applications. J. Phys. Chem. B 2002, 106, 10102–10107. [Google Scholar] [CrossRef]
- Luna, I.Z.; Hilary, L.N.; Chowdhury, A.M.S.; Gafur, M.A.; Khan, N.; Khan, R.A. Preparation and Characterization of Copper Oxide Nanoparticles Synthesized via Chemical Precipitation Method. Open Access Libr. J. 2015, 2, 1409. [Google Scholar] [CrossRef]
- Jha, S.; Singh, R.; Pandey, A.; Bhardwaj, M.; Tripathi, S.K.; Mishra, R.K.; Dikshit, A. Bacterial toxicological assay of calcium oxide nanoparticles against some plant growth-promoting rhizobacteria. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 460–466. [Google Scholar]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticle using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, antibacterial activity, and antidiabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically Approved Standard, 8th ed.; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Novy, P.; Kloucek, P.; Rondevaldova, J.; Havlik, J.; LenkaKourimska, L.; Kokoska, L. Thymoquinone vapor significantly affects the results of Staphylococcus aureus sensitivity tests using the standard broth microdilution method. Fitoterapia 2014, 94, 102–107. [Google Scholar] [CrossRef]
- Singh, R.; Jha, S.; Pandey, A.; Dikshit, A.; Mishra, R.K. Comparison of Antibacterial Activities of essential oils of JuniperuscommunisL., Pinusroxburghii Sarg. and Taxodiumdistichum L. against Klebsiellapneumoniea. J. Emerg. Technol. Innov. Res. 2018, 5, 661–669. [Google Scholar]
- Tripathi, S.K.; Singh, R.; Jha, S.; Pandey, A.; Dikshit, A. Comparison of antibacterial activities leaf extracts of Eclipta alba against Klebsiellapneumoniae. J. Emerg. Technol. Innov. Res. 2018, 5, 349–352. [Google Scholar]
- Khoshhesab, Z.M.; Sarfaraz, M.; Asadabad, M.A. Preparation of ZnO nanostructures by chemical precipitation method. Synth. React. Inorg. Met. Nano-Metal Chem. 2011, 41, 814–819. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnol. 2012, 2012, 372505. [Google Scholar] [CrossRef] [Green Version]
- Jayarambabu, N.; Kumari, S.; Rao, K.V.; Prabhu, Y.T. Germination and Growth Characteristics of Mungbean Seeds (Vignaradiata L.) affected by Synthesized Zinc Oxide Nanoparticles. Int. J. Curr. Eng. Technol. 2014, 4, 3411. [Google Scholar]
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Shionoya, S.; Yen, W.M. (Eds.) Phosphor Handbook; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Berger, L.I. Semiconductor Materials; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Fan, X.M.; Lian, J.S.; Zhao, L.; Liu, Y.H. Single violet luminescence emitted from ZnO films obtained by oxidation of Zn film on quartz glass. Appl. Surf. Sci. 2005, 252, 420–424. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Phan, T.N.; Buckner, T.; Sheng, J.; Baldeck, J.D.; Marquis, R.E. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol. Immunol. 2004, 19, 31–38. [Google Scholar] [CrossRef]
- Giertsen, E. Effects of mouth rinses with triclosan, zinc ions, copolymer, and sodium lauryl sulphate combined with fluoride on acid formation by dental plaque in vivo. Caries Res. 2004, 38, 430–435. [Google Scholar] [CrossRef]
- Turner, R.D.; Mesnage, S.; Hobbs, J.K.; Foster, S.J. Molecular imaging of glycan chains couples cell-wall polysaccharide architecture to bacterial cell morphology. Nat. Commun. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Villegas, N.A.; Compagnucci, M.J.S.; Aja, M.S.; Rocca, D.M.; Becerra, M.C.; Molina, G.F.; Palma, S.D. Novel antibacterial resin-based filling material containing nanoparticles for the potential one-step treatment of caries. J. Healthc. Eng. 2019, 2019, 6367919. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Fang, M.; Jiao, K.; Tang, L.H.; Xiao, Y.H.; Shen, L.J.; Chen, J.H. Tetrapod-like zinc oxide whisker enhancement of resin composite. J. Dent. Res. 2010, 89, 746–750. [Google Scholar] [CrossRef]
- Hojati, S.T.; Alaghemand, H.; Hamze, F.; Babaki, F.A.; Rajab-Nia, R.; Rezvani, M.B.; Kaviani, M.; Atai, M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent. Mater. 2013, 29, 495–505. [Google Scholar] [CrossRef]
- Mahendra, R.; Ranjita, S. Metal Nanoparticles in Pharma, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behavior of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2006, 9, 479–489. [Google Scholar] [CrossRef]
- Hamad, A.M.; Atiyea, Q.M. In vitro study of the effect of zinc oxide nanoparticles on Streptococcus mutans isolated from human dental caries. J. Phys. Conf. Ser. 2021, 1879, 022041. [Google Scholar] [CrossRef]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Intern 2020, 46, 1494–1502. [Google Scholar] [CrossRef]


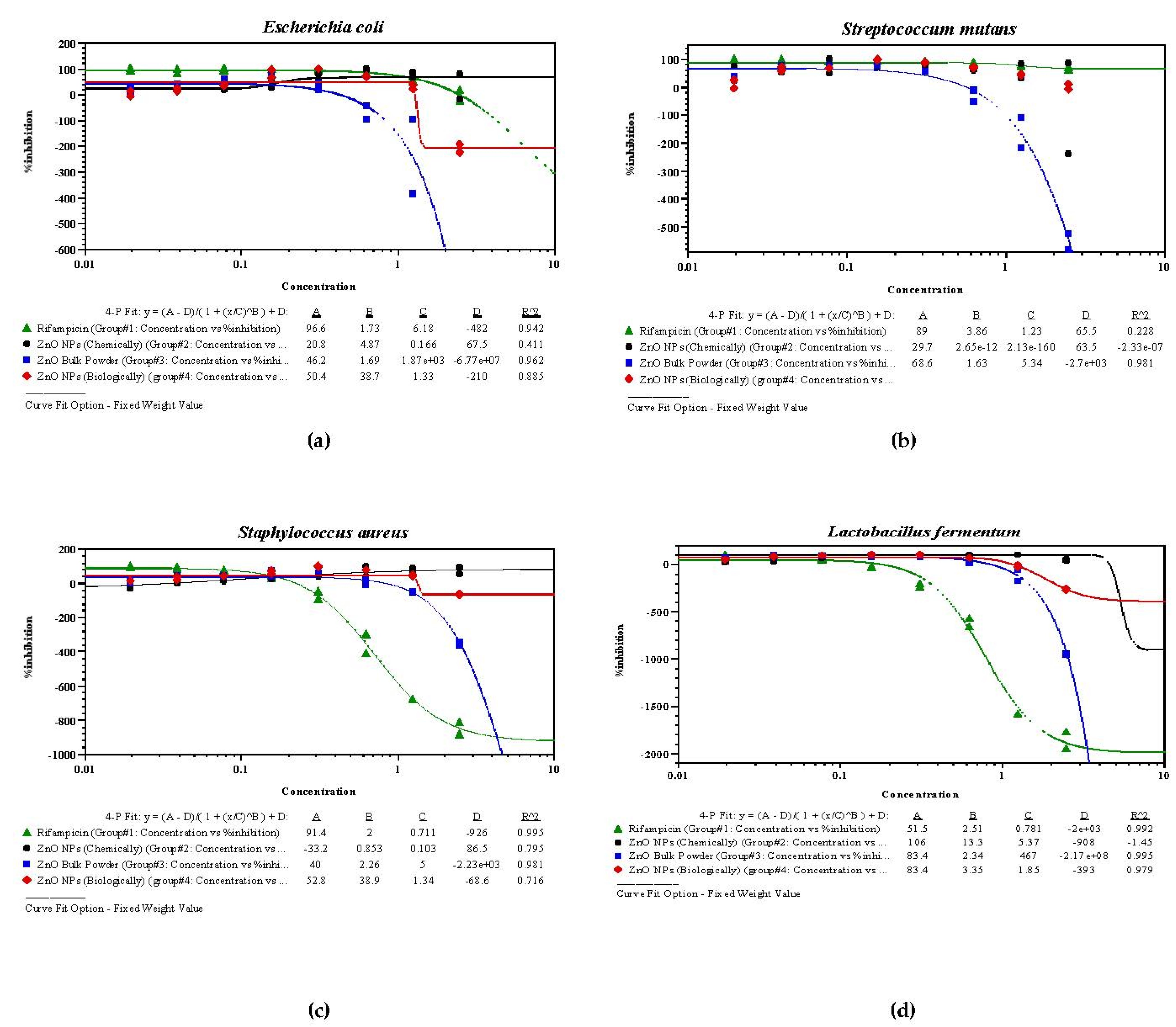
| S. No. | Drugs/Samples | E. coli | S. mutans | S. aureus | L. fermentum | ||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | ||
| 1 | Rifampicin | 0.152 | 0.081 | 0.081 | 0.108 | 0.146 | 0.075 | 0.044 | 0.075 |
| 2 | ZnO NPs (chemically) | 0.184 | 0.193 | 0.332 | 0.308 | 0.272 | 0.296 | 0.433 | 0.407 |
| 3 | ZnO bulk powder | 0.689 | 0.648 | 0.248 | 0.482 | 0.572 | 0.628 | 0.576 | 0.516 |
| 4 | ZnO NPs (biologically) | 0.257 | 0.261 | 0.279 | 0.293 | 0.122 | 0.134 | 0.457 | 0.426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.K.; Jha, S.; Singh, A.K.; Mishra, S.K.; Pathak, A.K.; Ojha, R.P.; Yadav, R.S.; Dikshit, A. Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry. Crystals 2022, 12, 1063. https://doi.org/10.3390/cryst12081063
Tiwari AK, Jha S, Singh AK, Mishra SK, Pathak AK, Ojha RP, Yadav RS, Dikshit A. Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry. Crystals. 2022; 12(8):1063. https://doi.org/10.3390/cryst12081063
Chicago/Turabian StyleTiwari, Ajay Kumar, Saket Jha, Abhimanyu Kumar Singh, Sheo Kumar Mishra, Ashok Kumar Pathak, Rudra Prakash Ojha, Raghvendra Singh Yadav, and Anupam Dikshit. 2022. "Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry" Crystals 12, no. 8: 1063. https://doi.org/10.3390/cryst12081063









