In Vitro and Electrochemical Characterization of Laser-Cladded Ti-Nb-Ta Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
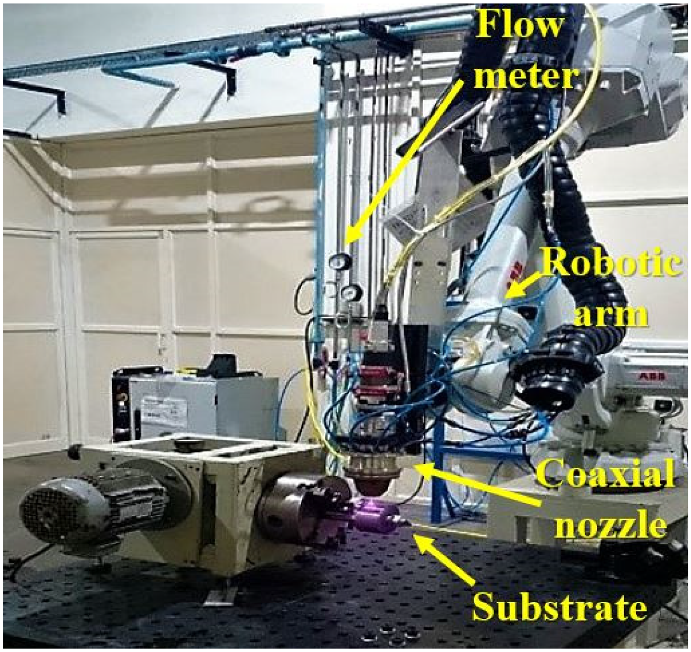
2.1. Laser Cladding
2.2. In Vitro Characterization
MTT Assay
2.3. Electrochemical Characterization
3. Results
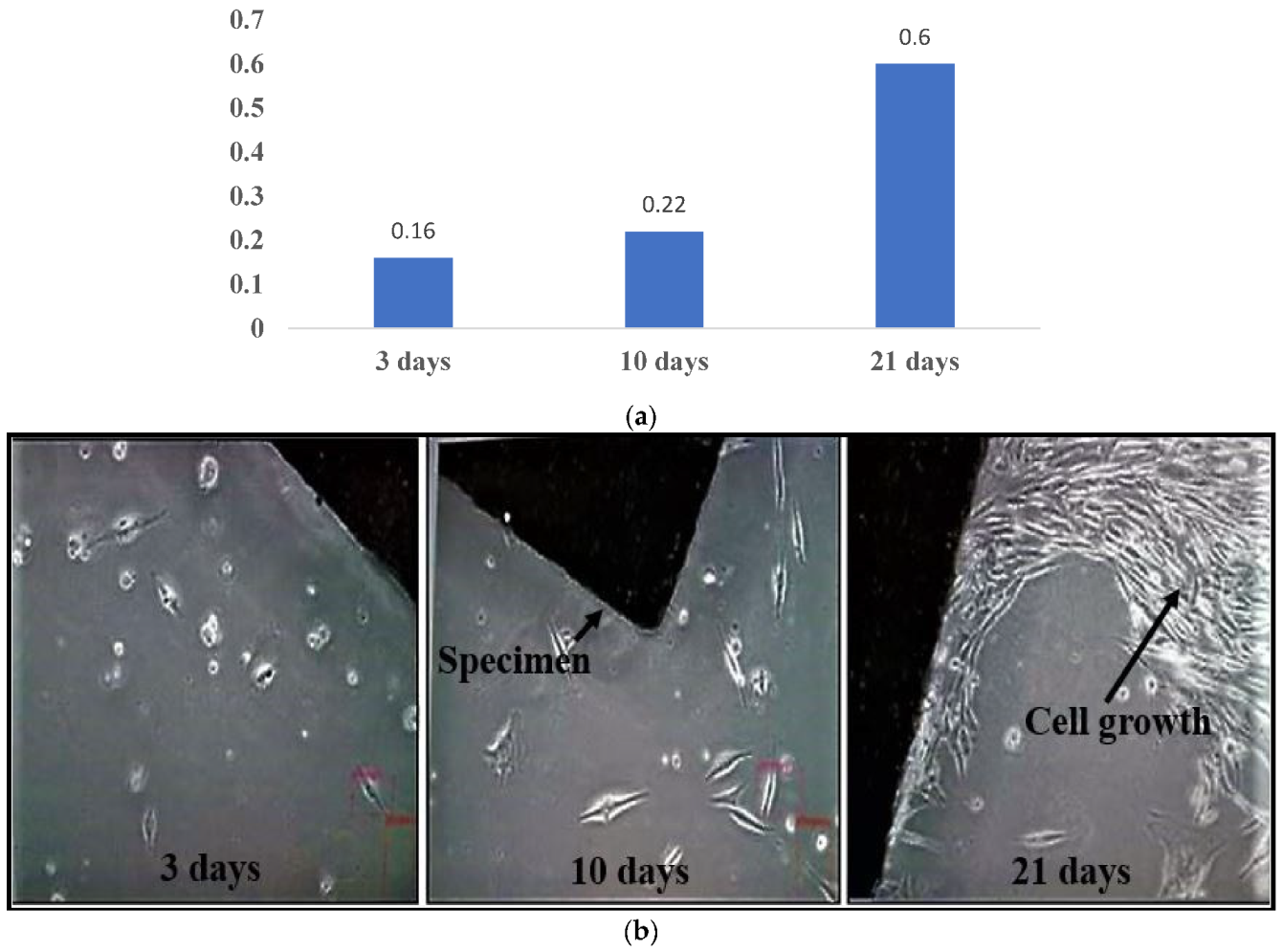
3.1. In Vitro Characterization
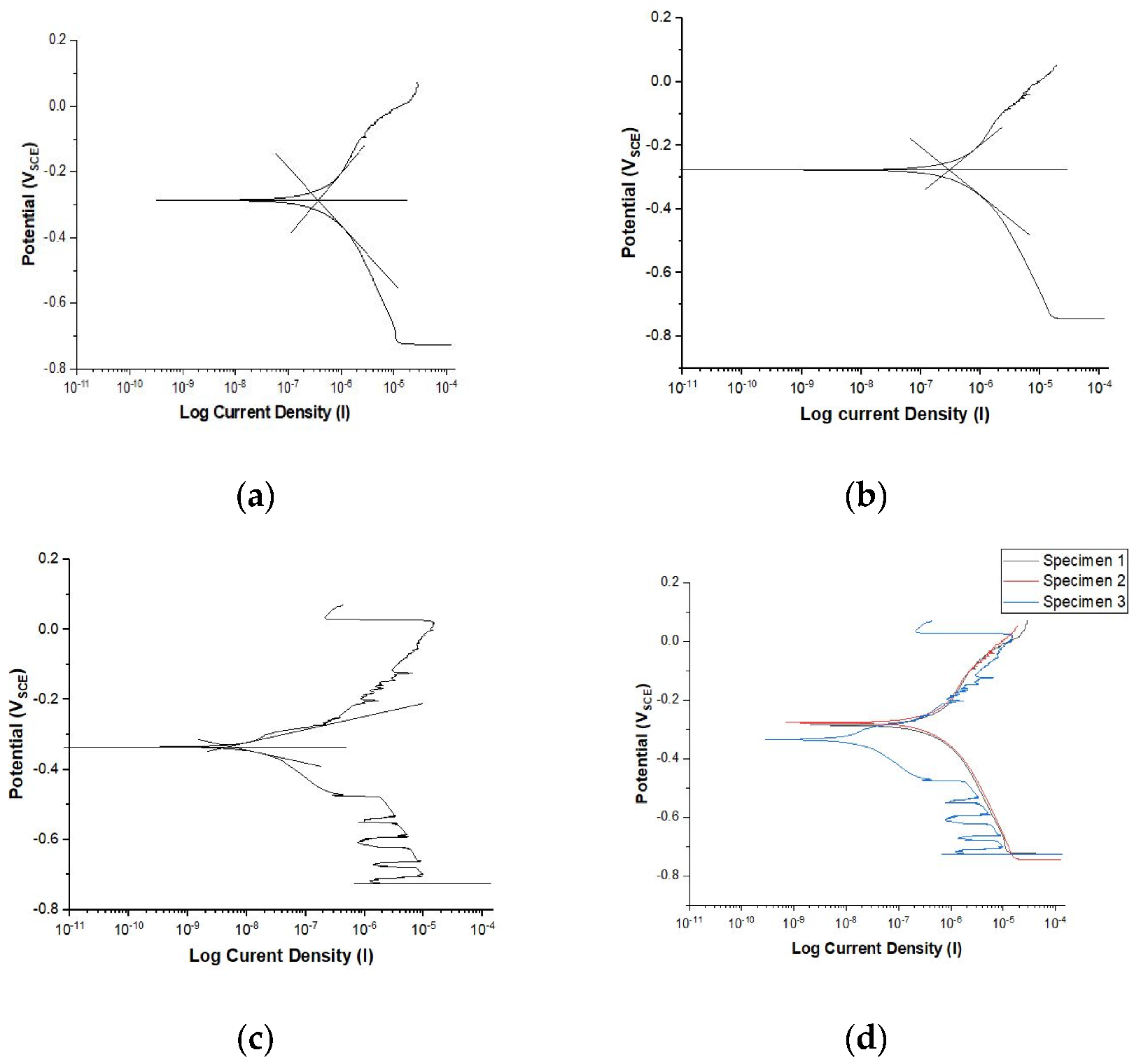
3.2. Electrochemical Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giner, M.; Chicardi, E.; Costa, A.D.; Santana, L.; Vázquez-Gámez, M.Á.; García-Garrido, C.; Colmenero, M.A.; Olmo-Montes, F.J.; Torres, Y.; Montoya-García, M.J. Biocompatibility and Cellular Behavior of TiNbTa Alloy with Adapted Rigidity for the Replacement of Bone Tissue. Metals 2021, 11, 130. [Google Scholar] [CrossRef]
- Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications (UNS R56401). Available online: https://www.astm.org/f0136-13r21e01.html. (accessed on 16 January 2022).
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Kumari, R.; Scharnweber, T.; Pfleging, W.; Besser, H.; Majumdar, J.D. Laser Surface Textured Titanium Alloy (Ti-6Al-4V)-Part II-Studies on Bio-compatibity. Appl. Surf. Sci. 2015, 357, 750. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material. Mater. Sci. Eng. C. 2017, 71, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, N.; Noritake, K.; Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, M. Experiment study on fracture fixation with low rigidity titanium alloy. J. Mater. Sci. Mater. Med. 2008, 19, 1581–1586. [Google Scholar] [CrossRef]
- Choe, H.C. Nanotubular surface and morphology of Ti-binary and Ti-ternary alloys for biocompatibility. Thin Solid Films 2011, 519, 4652–4657. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.Y.; Tardelli, J.D.; Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and -Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Park, S.Y.; Jo, C.I.; Choe, H.C.; Brantley, W.A. Hydroxyapatite deposition on micropore-formed Ti-Ta-Nb alloys by plasma electrolytic oxidation for dental applications. Surf. Coat. Technol. 2016, 294, 1152–1157. [Google Scholar] [CrossRef]
- Zhukova, Y.S.; Pustov, Y.A.; Konopatsky, A.S.; Filonov, M.R. Characterization of electrochemical behavior and surface oxide films on superelastic biomedical Ti–Nb–Ta alloy in simulated physiological solutions. J. Alloys Compd. 2014, 586, S535. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Krishna, B.V.; Xue, W.; Bose, S. Application of Laser Engineered Net Shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants. J. Mater. Sci. Mater. Med. 2009, 20, 29–34. [Google Scholar] [CrossRef]
- Bhardwaj, T.; Shukla, M.; Paul, C.P.; Bindra, K.S. Direct Energy Deposition—Laser Additive Manufacturing of Titanium-Molybdenum alloy: Parametric studies, microstructure and mechanical properties. J. Alloys Compd. 2019, 787, 1238–1248. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Shi, C.; Mao, D. Microscopic Analysis and Electrochemical Behavior of Fe-Based Coating Produced by Laser Cladding. Metals 2017, 7, 435. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Amado, J.M.; Rodríguez, A.; Montero, J.N.; Tobar MJYáñez, A. A comparison of laser deposition of commercially pure titanium using gas atomized or Ti sponge powders. Surf. Coat. Technol. 2019, 374, 253–263. [Google Scholar] [CrossRef]
- Weng, F.; Chen, C.; Yu, H. Research status of laser cladding on titanium and its alloys: A review. Mater. Mater. Des. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Okazaki, Y. Effect of friction on anodic polarization properties of metallic biomaterials. Biomaterials 2002, 23, 2071–2077. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Chang, L.; Yang, H.; Ruan, W. Porous Nb-Ti-Ta alloy scaffolds for bone tissue engineering: Fabrication, mechanical properties and in vitro/vivo biocompatibility. Mater. Sci. Eng. C 2017, 78, 503–512. [Google Scholar] [CrossRef]
- Dobbelstein, H.; Thiele, M.; Gurevich, E.L.; George, E.P.; Ostendorf, A. Direct metal deposition of refractory high entropy alloy MoNbTaW. Phys. Procedia 2016, 83, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Xiang, K.; Chen, L.Y.; Chai, L.; Guo, N.; Wang, H. Microstructural characteristics and properties of CoCrFeNiNbx high-entropy alloy coatings on pure titanium substrate by pulsed laser cladding. Appl. Surf. Sci. 2020, 517, 146214. [Google Scholar] [CrossRef]
- Xiang, K.; Chai, L.; Zhang, C.; Guan, H.; Wang, Y.; Ma, Y.; Li, Y. Investigation of microstructure and wear resistance of laser-clad CoCrNiTi and CrFeNiTi medium-entropy alloy coatings on Ti sheet. Opt. Laser Technol. 2022, 145, 107518. [Google Scholar] [CrossRef]
- Kim, S.E.; Jeong, H.W.; Hyun, Y.T.; Lee, Y.T.; Jung, C.H.; Kim, S.K.; Lee, J.H. Elastic Modulus and In Vitro Biocompatibility of Ti-xNb and Ti-xTa Alloys Met. Mater. Int. 2007, 13, 145–149. [Google Scholar] [CrossRef]
- Assis SLd Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815. [Google Scholar] [CrossRef]
- Karayan, A.I.; Park, S.W.; Lee, K.M. Corrosion behavior of Ti–Ta–Nb alloys in simulated physiological media. Mater. Lett. 2008, 62, 1843–1845. [Google Scholar] [CrossRef]
- Balla, V.K.; Banerjee, S.; Bose, S.; Bandyopadhyay, A. Direct Laser Processing of Tantalum Coating on Titanium for Bone Replacement Structures. Acta Biomater. 2010, 6, 2329–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, A.H.; Gepreel MA, H.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti–Nb–Ta base alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 574. [Google Scholar] [CrossRef]
- Giatmana, D.D.; Affi, J.; Fonna, S.; Niinomi, M.; Nakai, M. Corrosion resistance of new beta type titanium alloy, Ti-29Nb-13Ta-4.6Zr in artificial saliva solution. IOP Conf. Ser. Mater. Sci. Eng. 2018, 352, 012008. [Google Scholar]
- du Plooy, R.; Akinlabi, E.T. Analysis of laser cladding of Titanium alloy. Mater. Today Proc. 2018, 5, 19594–19603. [Google Scholar] [CrossRef]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Electrochemical Behavior of Titanium and Its Alloys as Dental Implants in Normal Saline. Adv. Phys. Chem. 2009, 2009, 574359. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chuang, W.S.; Huang, J.C.; Wang, X.; Chou, H.S.; Lai, Y.J.; Lin, P.H. On the bio-corrosion and biocompatibility of TiTaNb medium entropy alloy films. Appl. Surf. Sci. 2020, 508, 145307. [Google Scholar] [CrossRef]
- Kobayashi, E.; Wang, T.J.; Doi, H.; Hamanaka, H. Mechanical properties and corrosion resistance of Ti–6Al–7Nb alloy dental castings. J. Mater. Sci. Mater. Med. 1998, 9, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameters | Values |
|---|---|
| Power | 1200 watt |
| Cladding powder flow | 12 g/min |
| Cladding travel speed | 30 mm/s |
| Bed temp (preheat) | 200 °C |
| Powder carrier gas | 15 lpm |
| Nozzle shielding gas | 15 lpm |
| Focusing lens | 300 mm |
| Spot size (diameter) | 3 mm |
| Pass layer distance | around 1.5 mm |
| 1 | 2 | 3 | Avg | |
|---|---|---|---|---|
| Icorr (µA/cm2) | 3.75 × 10−1 | 2.99 × 10−1 | 5.18 × 10−3 | 2.26 × 10−1 |
| Ecorr (VSCE) | −2.867 × 10−1 | −2.771 × 10−1 | −3.348 × 10−1 | −3.00 × 10−1 |
| CR (mmpy) | 3.30 × 10−3 | 2.64 × 10−3 | 4.57 × 10−5 | 2.00 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, R.; Pande, S.; Salunkhe, S.; Natu, H.; Abouel Nasr, E.; Shanmugam, R.; Hussein, H.M.A.M. In Vitro and Electrochemical Characterization of Laser-Cladded Ti-Nb-Ta Alloy for Biomedical Applications. Crystals 2022, 12, 954. https://doi.org/10.3390/cryst12070954
Soni R, Pande S, Salunkhe S, Natu H, Abouel Nasr E, Shanmugam R, Hussein HMAM. In Vitro and Electrochemical Characterization of Laser-Cladded Ti-Nb-Ta Alloy for Biomedical Applications. Crystals. 2022; 12(7):954. https://doi.org/10.3390/cryst12070954
Chicago/Turabian StyleSoni, Raj, Sarang Pande, Sachin Salunkhe, Harshad Natu, Emad Abouel Nasr, Ragavanantham Shanmugam, and Hussein Mohammed Abdel Moneam Hussein. 2022. "In Vitro and Electrochemical Characterization of Laser-Cladded Ti-Nb-Ta Alloy for Biomedical Applications" Crystals 12, no. 7: 954. https://doi.org/10.3390/cryst12070954






