Laser-Induced Exothermic Bonding of SiCp/Al Composites with Nanostructured Al/Ni Energetic Interlayer
Abstract
:1. Introduction
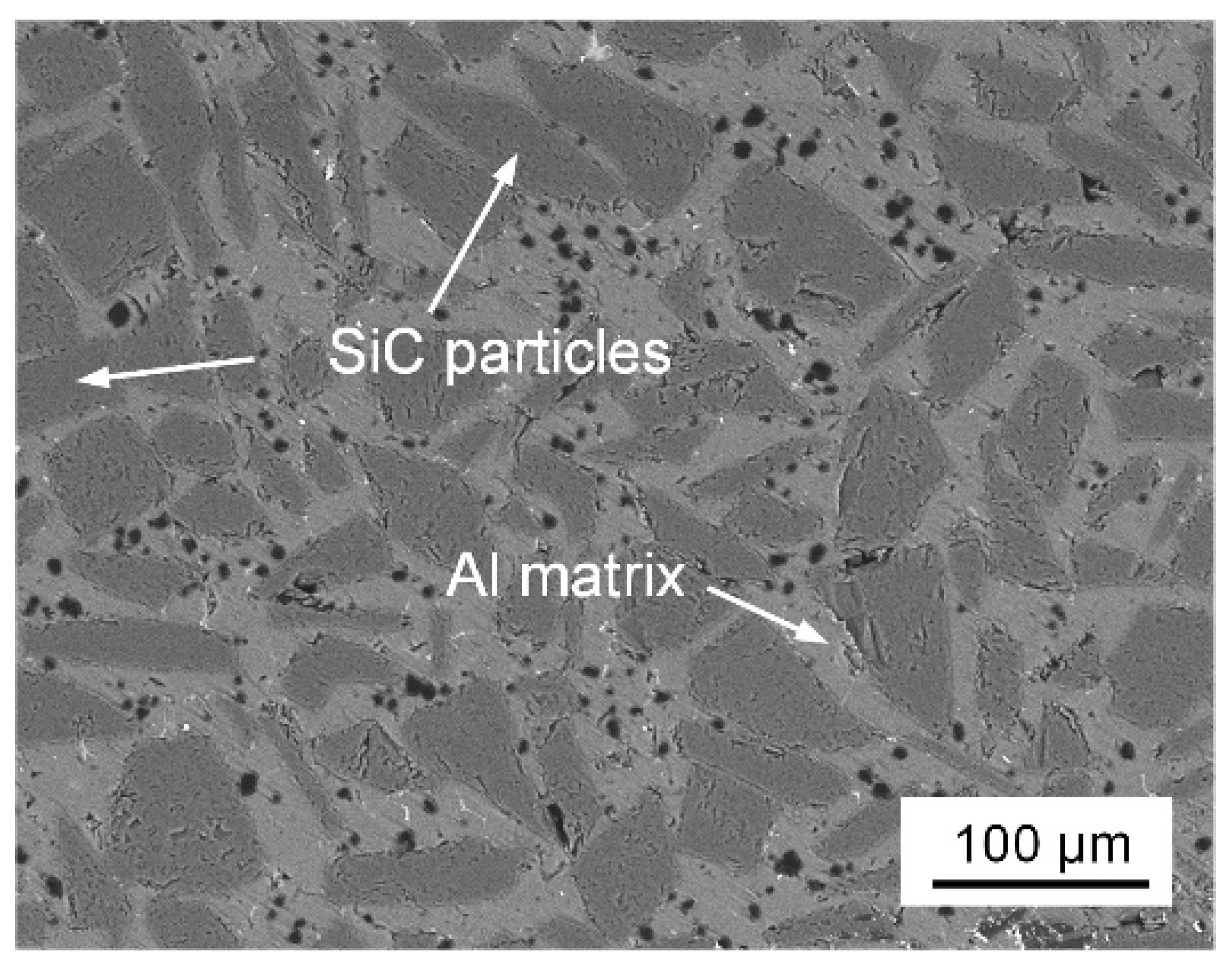
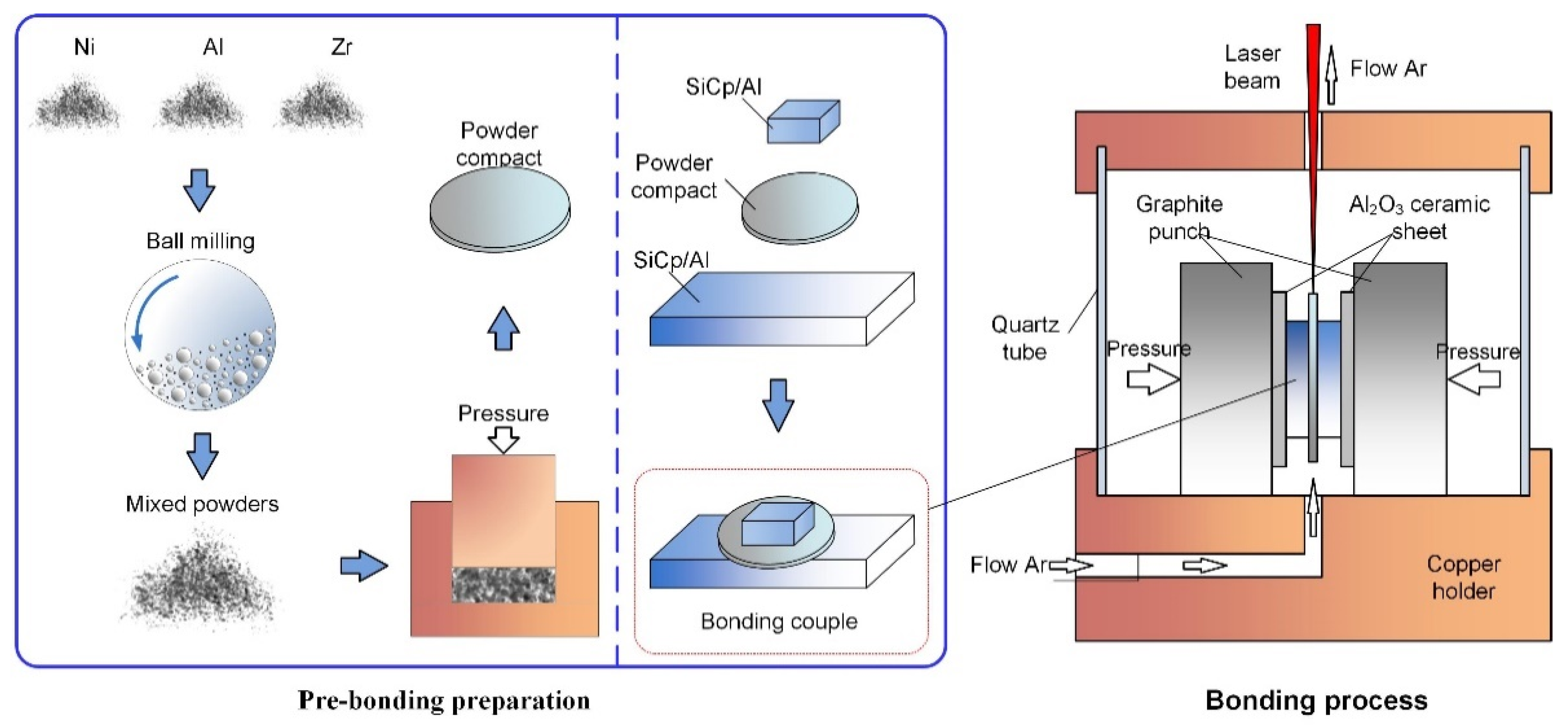
2. Materials and Methods
3. Results and Discussion
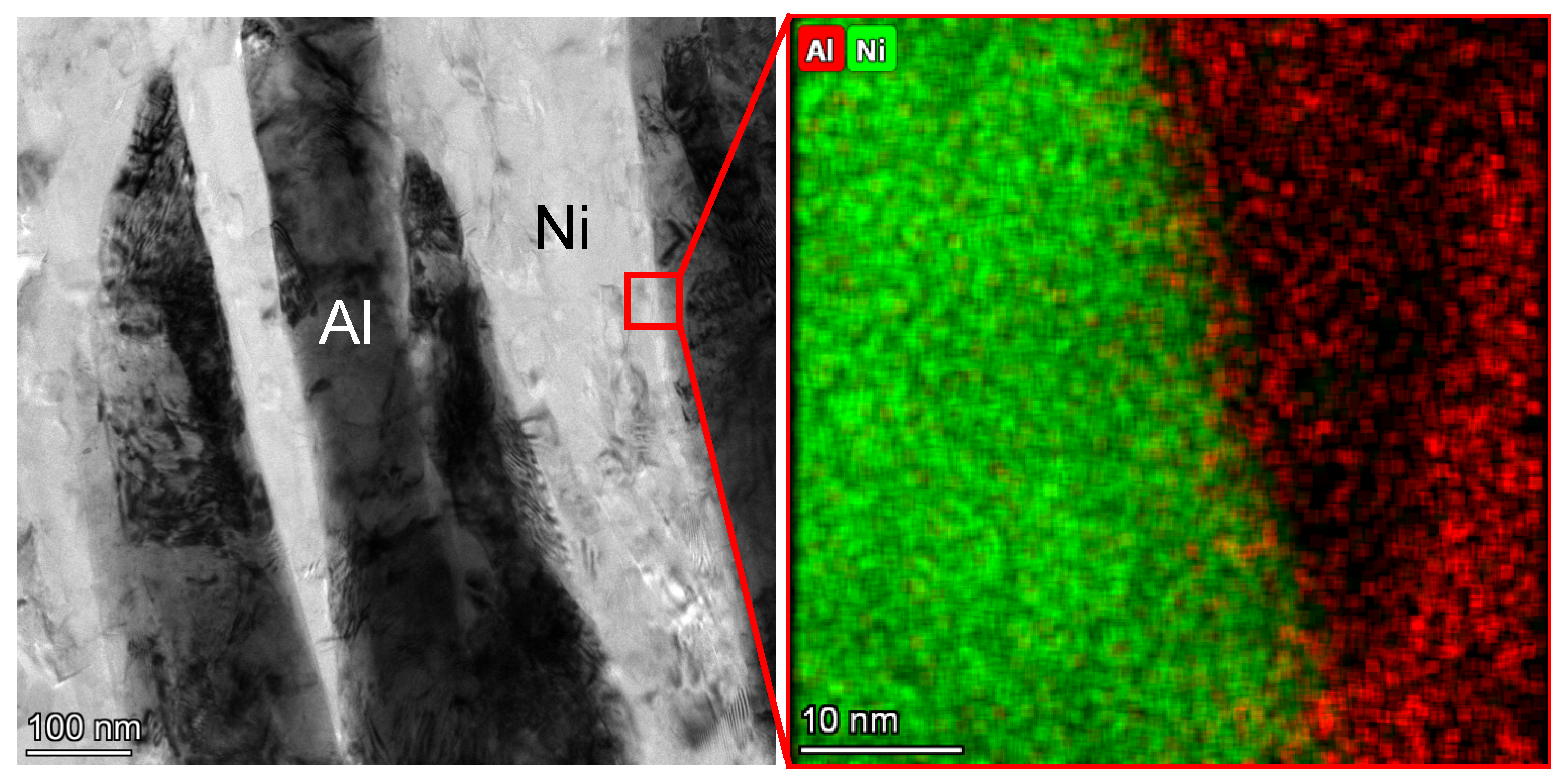
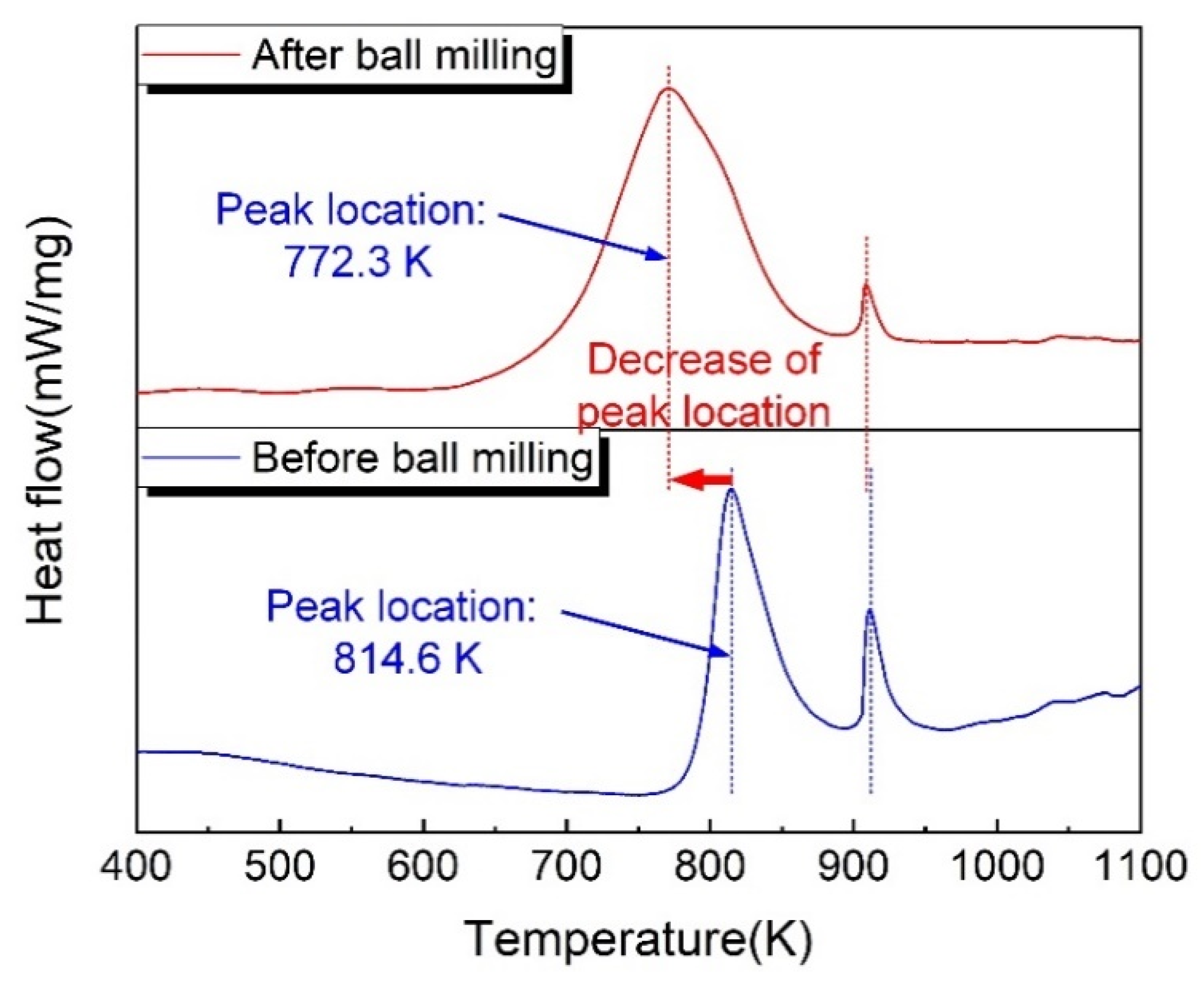
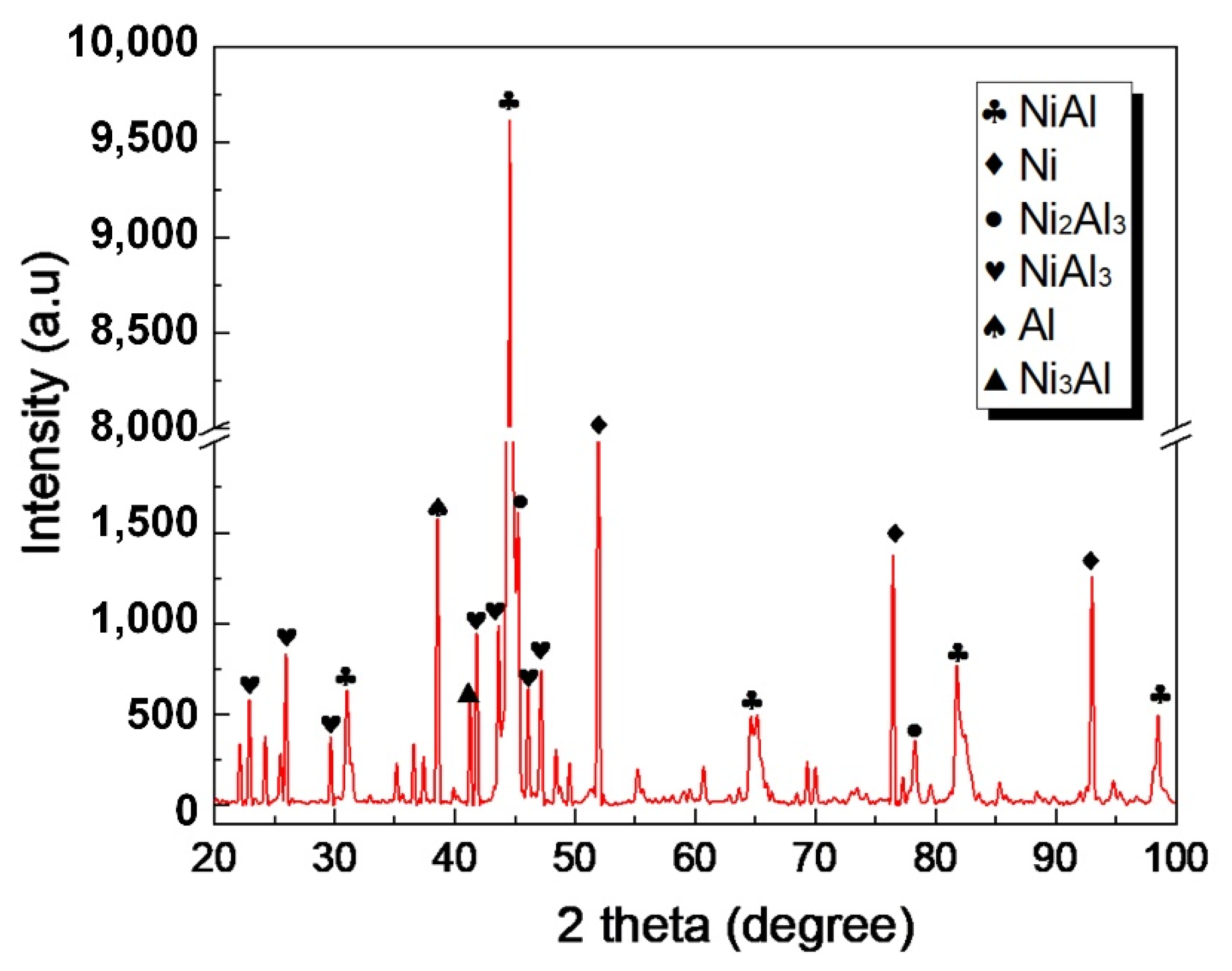
3.1. Nanostructured Al/Ni Energetic Interlayer
3.2. Joint Microstructure
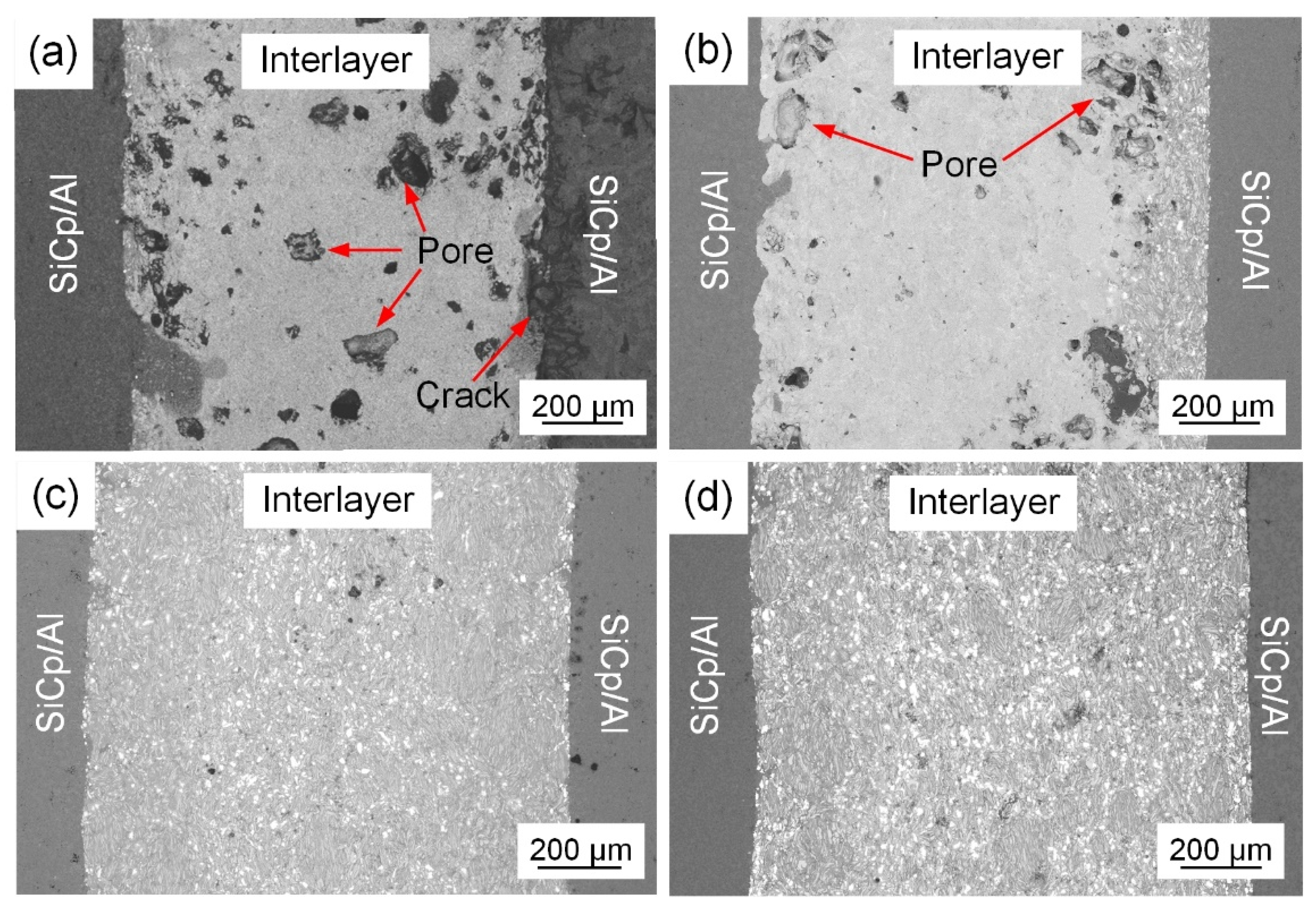
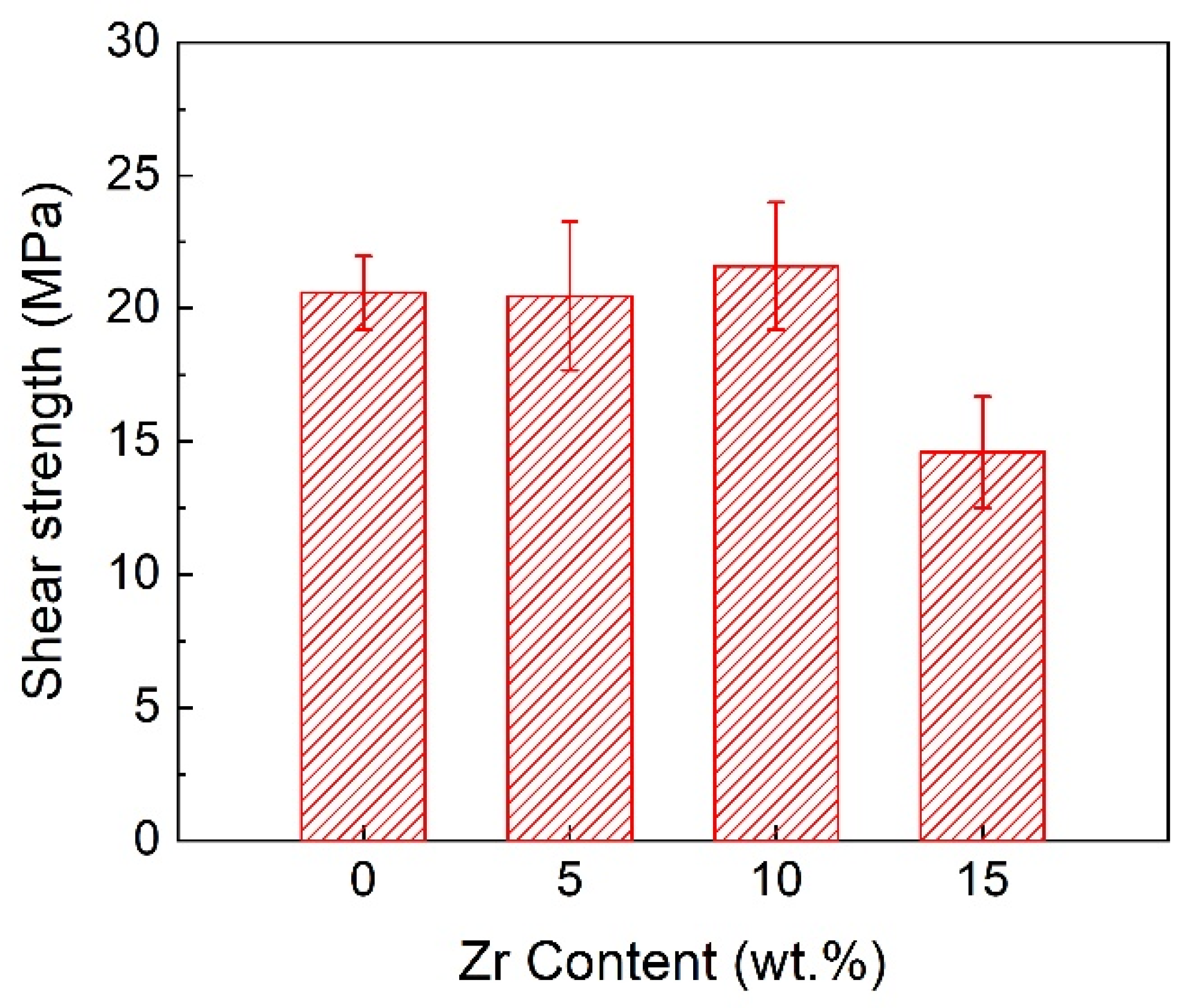
3.3. Effect of Zr Content on Joint Microstructure and Shear Strength
4. Conclusions
- (1)
- During the ball-milling process, the Al and Ni particles underwent strong plastic deformations and were welded to each other, forming the nanostructured Al/Ni energetic materials with a lamellar structure. The thicknesses of the Ni and Al layers ranged from 50 nm to 200 nm. Compared with the original powders, the location of the exothermic peak decreased by 42 K, and its exothermic performance was significantly improved.
- (2)
- Exothermic reactions occurred in the Al/Ni interlayer, providing the required heat for the bonding process. The NiAl products bonded well with the SiCp/Al composites. Near the bonding interface, the interlayer could not react completely due to the cooling effect of substrates, forming a mixture of residual metal particles and Ni-Al compounds.
- (3)
- The addition of Zr content enhanced the interfacial reactions between the bonding interlayer with the SiCp/Al composites. The interlayer products transformed from NiAl to the eutectic organization of NiAl + Ni-Al-Zr, thus decreasing the pores in the joint and improving the bonding quality. With the increase in the Zr content, the joint shear strength first increased and then decreased. When the Zr content was 10 wt.%, the joint shear strength reached a maximum of 22 MPa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zha, H.; Feng, P.; Zhang, J.; Yu, D.; Wu, Z. Material removal mechanism in rotary ultrasonic machining of high-volume fraction SiCp/Al composites. Int. J. Adv. Manuf. Technol. 2018, 97, 2099–2109. [Google Scholar] [CrossRef]
- Tayyebi, M.; Eghbali, B. Microstructure and mechanical properties of SiC-particle-strengthening tri-metal Al/Cu/Ni composite produced by accumulative roll bonding process. Int. J. Miner. Metall. Mater. 2018, 25, 357–364. [Google Scholar] [CrossRef]
- Huang, J.; Tayyebi, M.; Assari, A.H. Effect of SiC particle size and severe deformation on mechanical properties and thermal conductivity of Cu/Al/Ni/SiC composite fabricated by ARB process. J. Manuf. Process. 2021, 68, 57–68. [Google Scholar] [CrossRef]
- Avazzadeh, M.; Alizadeh, M.; Tayyebi, M. Structural, mechanical and corrosion evaluations of Cu/Zn/Al multilayered composites subjected to CARB process. J. Alloys Compd. 2021, 867, 158973. [Google Scholar] [CrossRef]
- Xiao, J.; Li, S.; Bai, S.; Yan, J.; Xiong, D.; Tang, Y. Compression Brazing of SiCp/Al Composite Using a Semisolid Zn-Al-Cu Filler Metal Based on the Strain-Induced Melt Activation Process. JOM 2019, 71, 4931–4939. [Google Scholar] [CrossRef]
- Moradi, M.M.; Aval, H.J.; Jamaati, R.; Amirkhanlou, S.; Ji, S. Effect of SiC nanoparticles on the microstructure and texture of friction stir welded AA2024/AA6061. Mater. Charact. 2019, 152, 169–179. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, X.X.; Wang, Q.Z.; Xiao, B.L.; Ma, Z.Y. Simulations of deformation and damage processes of SiCp/Al composites during tension. J. Mater. Sci. Technol. 2018, 34, 627–634. [Google Scholar] [CrossRef]
- Kwok, Y. Effects of pulse-impact on the welding of SiCp/Al-6061 aluminium matrix composites. Mater. Sci. Technol. 2017, 33, 2298–2304. [Google Scholar] [CrossRef]
- Wang, P.; Xu, D.; Zhai, Y.; Niu, J. The dissimilar brazing of Kovar alloy to SiCp/Al composites using silver-based filler metal foil. Appl. Phys. A 2017, 123, 569. [Google Scholar] [CrossRef]
- Chen, R.F.; Zhao, Y.H.; Shen, Z.X.; Dai, L.G.; Zhang, X.L.; Zhu, R. Study on the Joint Strength of SiCp/Al Metal Matrix Composite by Magnetron Sputtering Method. Mater. Sci. Forum 2009, 628–629, 569–574. [Google Scholar] [CrossRef]
- Guo, W.; Hou, J.; Lin, T.; He, P. Joining high volume fraction SiC particle reinforced aluminum matrix composites (SiCp/Al) by low melting point stannous oxide–zinc oxide–phosphorus pentoxide glass. Ceram. Int. 2021, 47, 3955–3963. [Google Scholar] [CrossRef]
- Wang, S.G.; Ji, X.H.; Zhao, X.Q.; Dong, N.N. Interfacial characteristics of electron beam welding joints of SiCp/Al composites. Mater. Sci. Technol. 2014, 27, 60–64. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, H.; Feng, J.; Ji, F.; Niu, J.; Brnic, J. Flux-Free Diffusion Joining of SiCp/6063 Al Matrix Composites Using Liquid Gallium with Nano-Copper Particles in Atmosphere Environment. Nanomaterials 2020, 10, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.Y.; Feng, A.H.; Chen, D.L.; Shen, J. Recent Advances in Friction Stir Welding/Processing of Aluminum Alloys: Microstructural Evolution and Mechanical Properties. Crit. Rev. Solid State 2018, 43, 269–333. [Google Scholar] [CrossRef]
- Shteinberg, A.S.; Lin, Y.; Son, S.F.; Mukasyan, A.S. Kinetics of High Temperature Reaction in Ni-Al System: Influence of Mechanical Activation. J. Phys. Chem. A 2010, 114, 6111–6116. [Google Scholar] [CrossRef]
- Yang, Z.; Ning, X.; Yu, X.; Tan, C.; Zhao, H.; Zhang, T.; Li, L.; Nie, Z.; Liu, Y. Energy Release Characteristics of Ni–Al–CuO Ternary Energetic Structural Material Processed by Cold Spraying. J. Therm. Spray Technol. 2020, 29, 1070–1081. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Mukasyan, A.S. Combustion of heterogeneous nanostructural systems (Review). Combust. Explo. Shock Waves 2010, 46, 243–266. [Google Scholar] [CrossRef]
- Fiedler, T.; Belova, I.V.; Broxtermann, S.; Murch, G.E. A thermal analysis on self-propagating high temperature synthesis in joining technology. Comp. Mater. Sci. 2012, 53, 251–257. [Google Scholar] [CrossRef]
- Shi, J.M.; Feng, J.C.; Liu, H.; Tian, X.Y.; Zhang, L.X. Vacuum brazing of the Gr/2024Al composite and TC4 alloy using AgCuTi filler alloy with Ni-Al interlayer as auxiliary heat source. J. Alloys Compd. 2017, 694, 672–681. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, Q.C. Reaction behaviour, microstructure and mechanical properties of TiC-TiB2/Ni composite fabricated by pressure assisted self-propagating high-temperature synthesis in air and vacuum. Mater. Des. 2013, 49, 123–129. [Google Scholar] [CrossRef]
- Jimenez, C.; Mergia, K.; Lagos, M.; Yialouris, P.; Agote, I.; Liedtke, V.; Messoloras, S.; Panayiotatos, Y.; Padovano, E.; Badini, C.; et al. Joining of ceramic matrix composites to high temperature ceramics for thermal protection systems. J. Eur. Ceram. Soc. 2016, 36, 443–449. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Zhou, Z.; Wang, Y. Joining of Cf/Al composites and TiAl intermetallics by laser-induced self-propagating high-temperature synthesis using the Ni-Al-Zr interlayer. Mater. Des. 2016, 110, 130–137. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Jacob, R.J.; Yang, Y.; Wang, Y.; Zhou, Z.; Sekulic, D.P.; Zachariah, M.R. Laser-induced exothermic bonding of carbon fiber/Al composites and TiAl alloys. Mater. Des. 2017, 126, 197–206. [Google Scholar] [CrossRef]
- Umbrajkar, S.M.; Seshadri, S.; Schoenitz, M.; Hoffmann, V.K.; Dreizin, E.L. Aluminum-Rich Al-MoO3 Nanocomposite Powders Prepared by Arrested Reactive Milling. J. Propul. Power 2008, 24, 192–198. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Yang, L.P.; Hu, A.M. Microstructures and Reaction Properties of Ti/Ni, Ti/Al and Ni/Al Multilayer Films. J. Nano Res. 2018, 54, 22–34. [Google Scholar] [CrossRef]
- Liao, S.; Luo, X.; Tao, J.; Tang, B.; Guo, X.; Ding, Q. Microstructure and reaction properties of Ni/Al micro-nano composites produced by accumulative roll bonding process. Mater. Res. Express 2019, 6, 96503. [Google Scholar] [CrossRef]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Ligachev, A.E.; Potemkin, G.V.; Lepakova, O.K.; Zhidkov, M.V.; Teresov, A.D.; Golobokov, N.N.; Maksimov, Y.M.; Kolobov, Y.R.; Koval, N.N. Ignition of a Ti-Al-C System by an Electron Beam. Combust. Explo. Shock Waves 2018, 54, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Fritz, G.M.; Grzyb, J.A.; Knio, O.M.; Grapes, M.D.; Weihs, T.P. Characterizing solid-state ignition of runaway chemical reactions in Ni-Al nanoscale multilayers under uniform heating. J. Appl. Phys. 2015, 118, 135101. [Google Scholar] [CrossRef]
- Morsi, K. Review: Reaction synthesis processing of Ni-Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Mason, B.A.; Groven, L.J.; Lin, Y.; Cherukara, M.; Son, S.F.; Strachan, A.; Mukasyan, A.S. Tailored Reactivity of Ni+Al Nanocomposites: Microstructural Correlations. J. Phys. Chem. C 2012, 116, 21027–21038. [Google Scholar] [CrossRef]
- Reeves, R.V.; Mukasyan, A.S.; Son, S.F. Thermal and Impact Reaction Initiation in Ni/Al Heterogeneous Reactive Systems. J. Phys. Chem. C 2010, 114, 14772–14780. [Google Scholar] [CrossRef]
- Shuck, C.E.; Mukasyan, A.S. Reactive Ni/Al Nanocomposites: Structural Characteristics and Activation Energy. J. Phys. Chem. A 2017, 121, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hu, Q.; Wang, B.; Zhou, B.; Chen, P.; Liu, R. Fabrication and characterization of the Ni–Al energetic structural material with high energy density and mechanical properties. J. Alloys Compd. 2020, 832, 154894. [Google Scholar] [CrossRef]
- Wang, P.; Xu, D.X.; Cheng, D.F.; Li, Q.; Niu, J.T. Active brazing filler metal on SiC particle reinforced aluminium matrix composites. Sci. Technol. Weld. Join. 2015, 20, 361–370. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Zhou, Z.; Yang, Y.; Sekulic, D.P.; Zachariah, M.R. Microstructure and mechanical properties of Cf/Al-TiAl laser-assisted brazed joint. J. Mater. Process. Technol. 2018, 255, 195–203. [Google Scholar] [CrossRef]


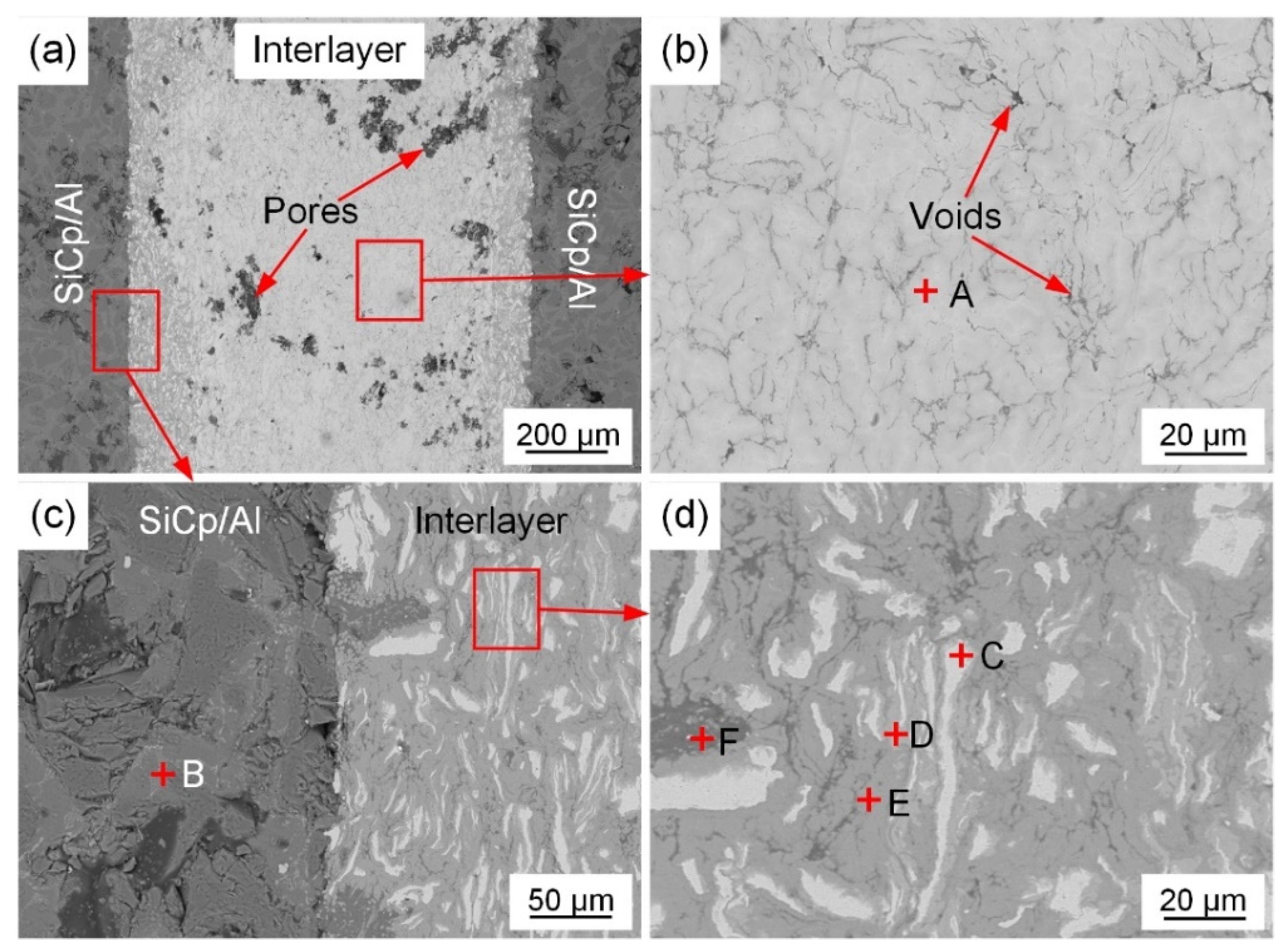


| Position | Al | Ni | Si | Possible Phase |
|---|---|---|---|---|
| A | 44.5 | 55.5 | - | NiAl |
| B | 71.3 | 21.1 | 4.6 | NiAl3 |
| C | 79.1 | 16.0 | 4.9 | Ni-rich Ni3Al |
| D | 65.2 | 34.8 | - | Ni2Al3 |
| E | 72.6 | 23.7 | 3.7 | NiAl3 |
| F | 99.1 | 0.9 | - | Al |
| Position | Al | Ni | Zr | Si | Possible Phase |
|---|---|---|---|---|---|
| A | 17.2 | 6.8 | 76.0 | - | Ni-Al-Zr |
| B | 28.3 | 6.6 | 62.4 | 2.7 | Ni-Al-Zr |
| C | 21.0 | 73.1 | - | 5.9 | Ni3Al |
| D | 74.4 | 9.2 | 14.2 | 2.3 | Ni-Al-Zr |
| E | 40.1 | 43.5 | 16.4 | - | Ni-Al-Zr |
| F | 57.0 | 43.0 | - | - | NiAl |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Hu, B.; Liu, X.; Wei, Y.; Li, Z.; He, P.; Cheng, Z.; Wang, Y.; Deng, D.; Yang, X. Laser-Induced Exothermic Bonding of SiCp/Al Composites with Nanostructured Al/Ni Energetic Interlayer. Crystals 2022, 12, 938. https://doi.org/10.3390/cryst12070938
Feng G, Hu B, Liu X, Wei Y, Li Z, He P, Cheng Z, Wang Y, Deng D, Yang X. Laser-Induced Exothermic Bonding of SiCp/Al Composites with Nanostructured Al/Ni Energetic Interlayer. Crystals. 2022; 12(7):938. https://doi.org/10.3390/cryst12070938
Chicago/Turabian StyleFeng, Guangjie, Bingxu Hu, Xiaojian Liu, Yan Wei, Zhuoran Li, Peng He, Zhiliang Cheng, Yifeng Wang, Dean Deng, and Xiuxia Yang. 2022. "Laser-Induced Exothermic Bonding of SiCp/Al Composites with Nanostructured Al/Ni Energetic Interlayer" Crystals 12, no. 7: 938. https://doi.org/10.3390/cryst12070938






