Sodium, Silver and Lithium-Ion Conducting β″-Alumina + YSZ Composites, Ionic Conductivity and Stability
Abstract
:1. Introduction
2. Materials and Methods
3. Results
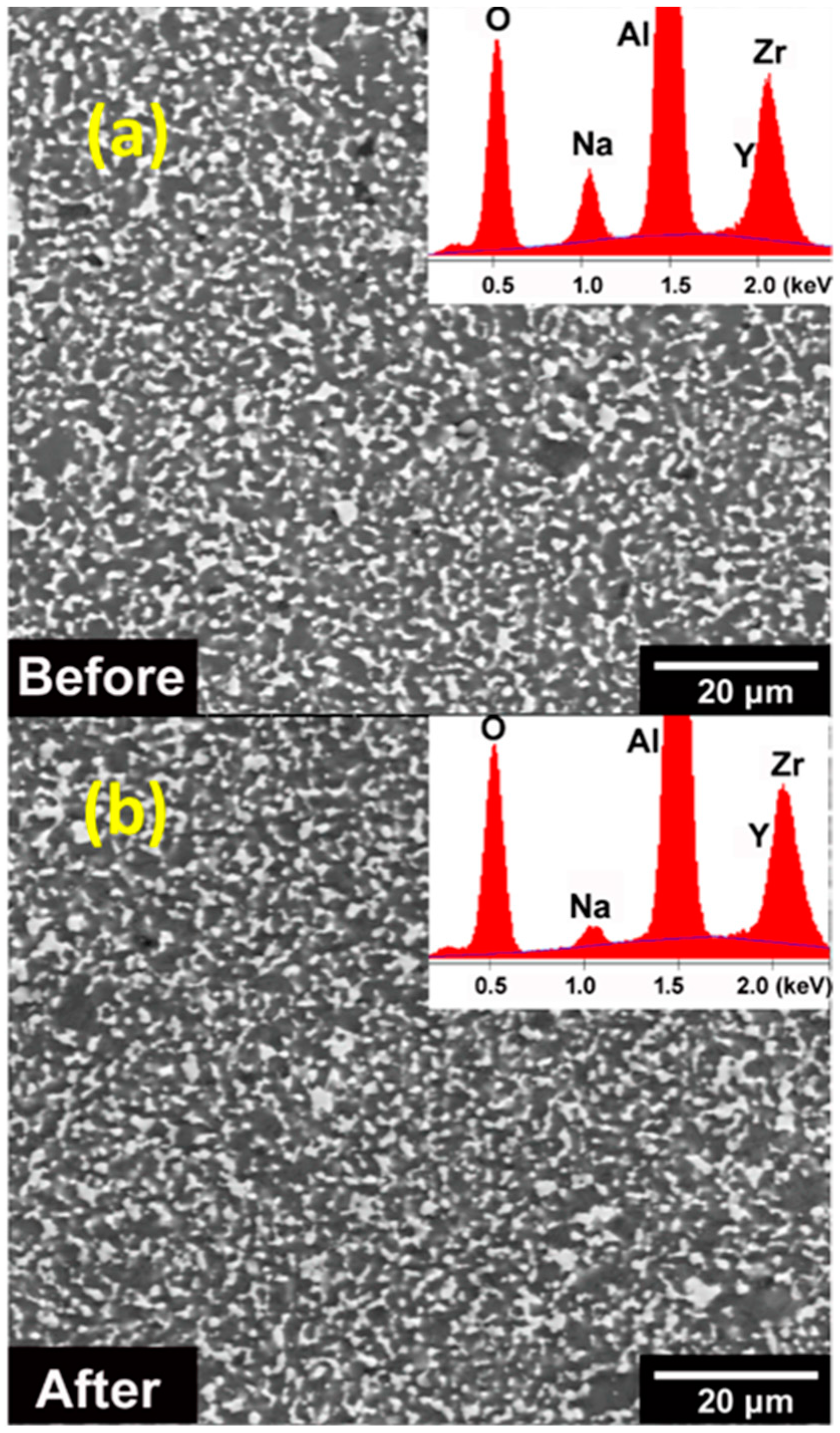
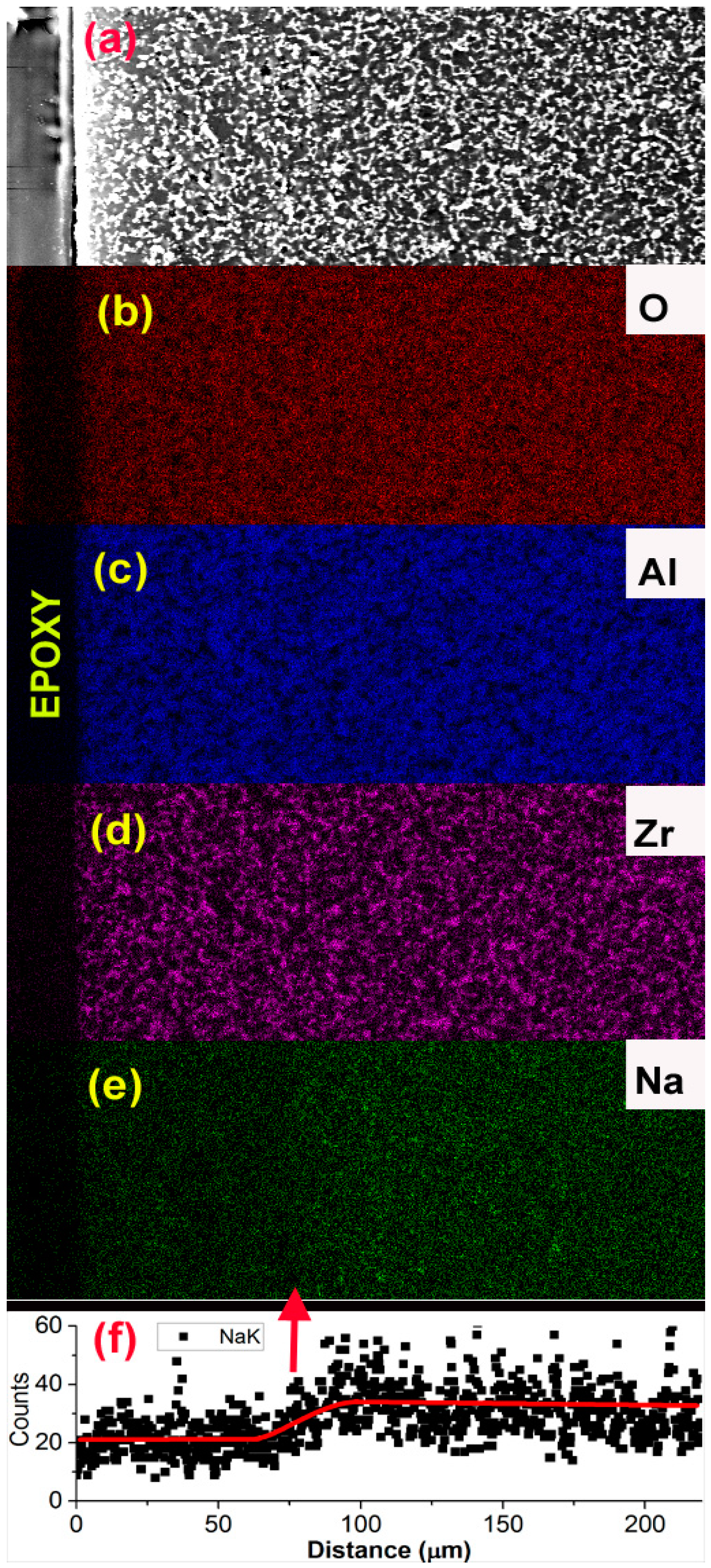
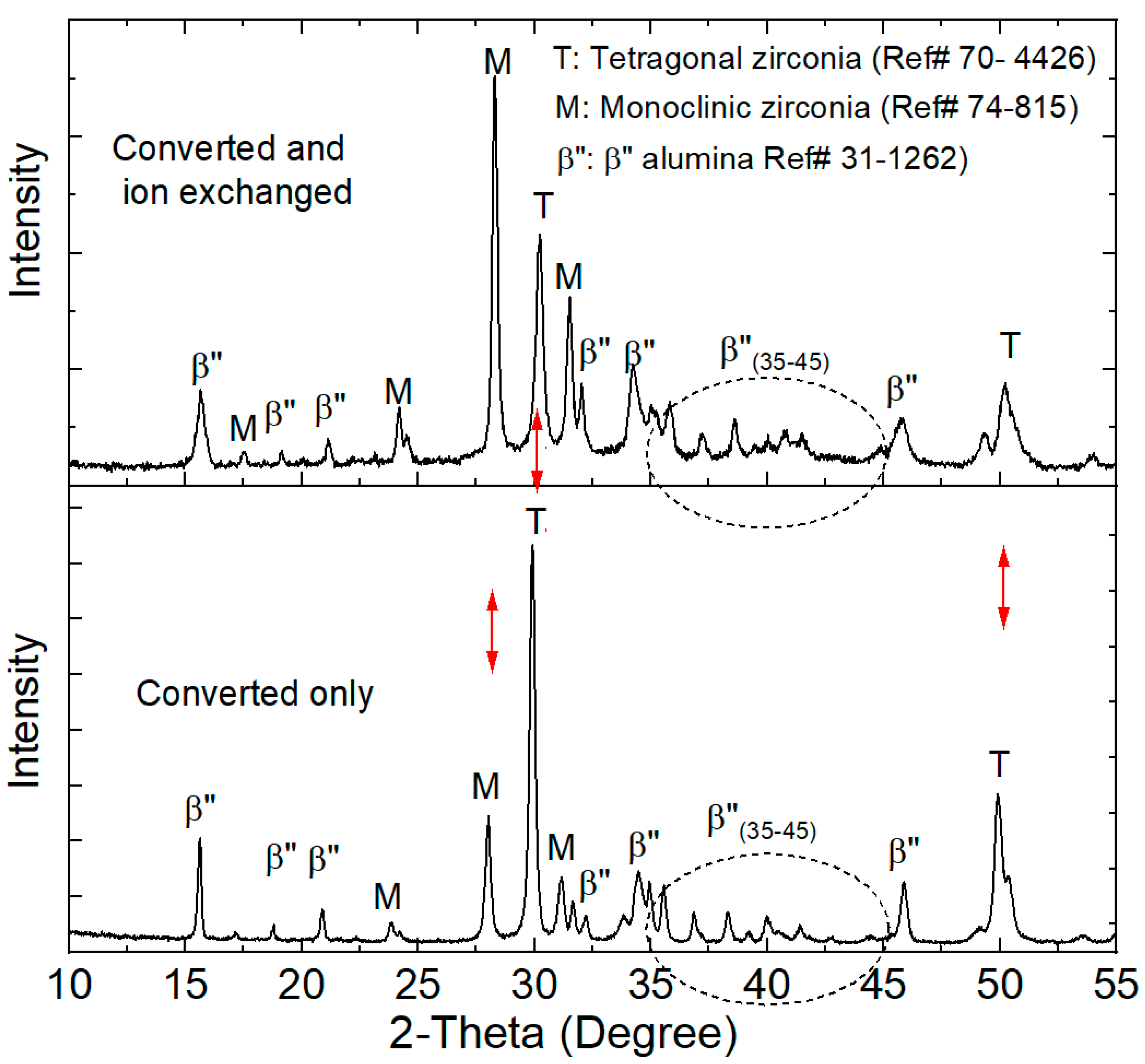
3.1. Microstructure, Elemental Distribution, and Crystal Structure
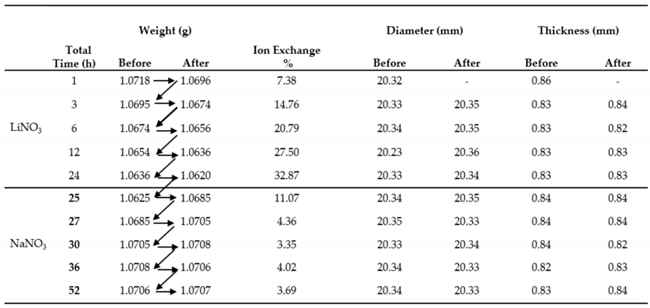
3.2. Ion Exchange Kinetics
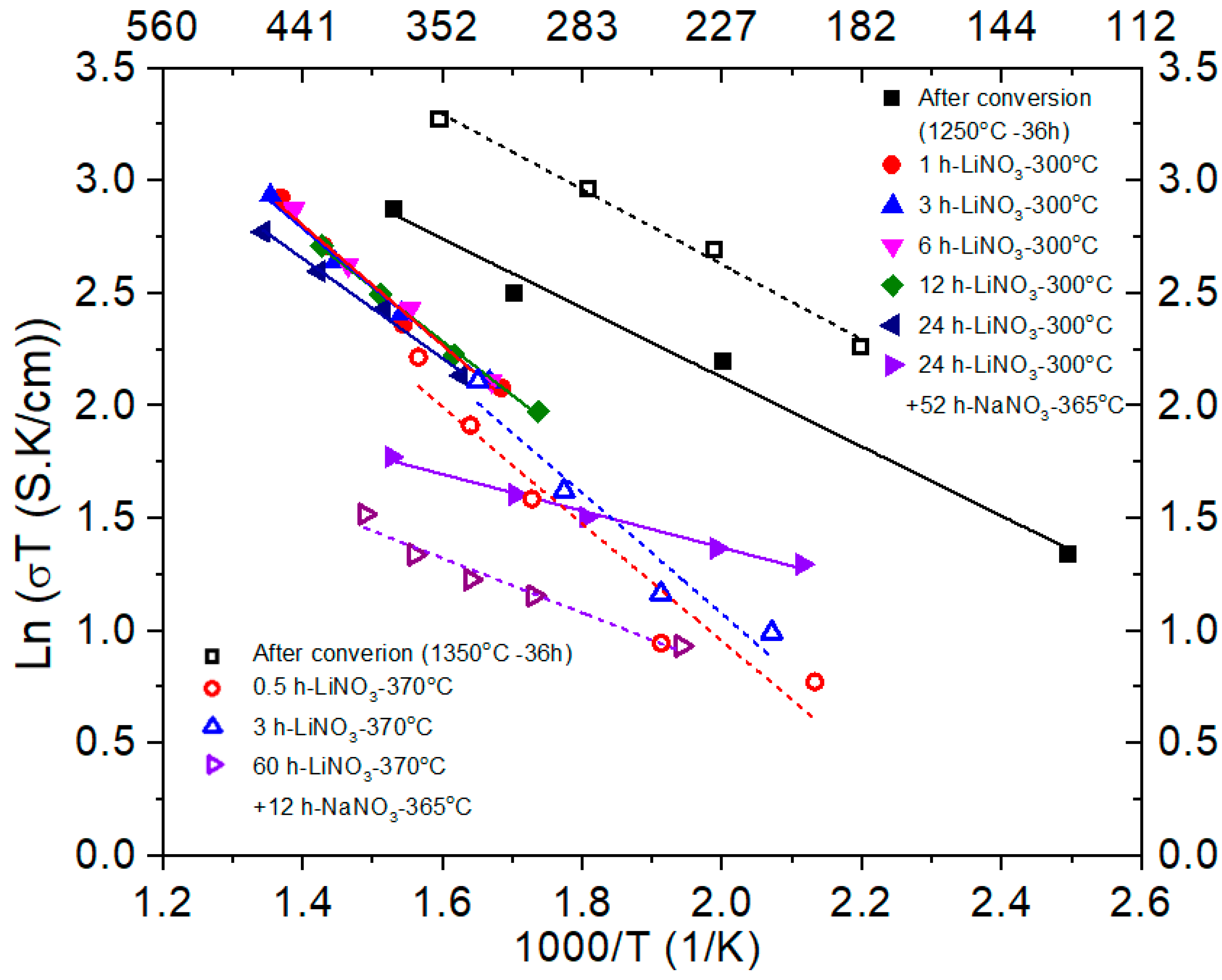
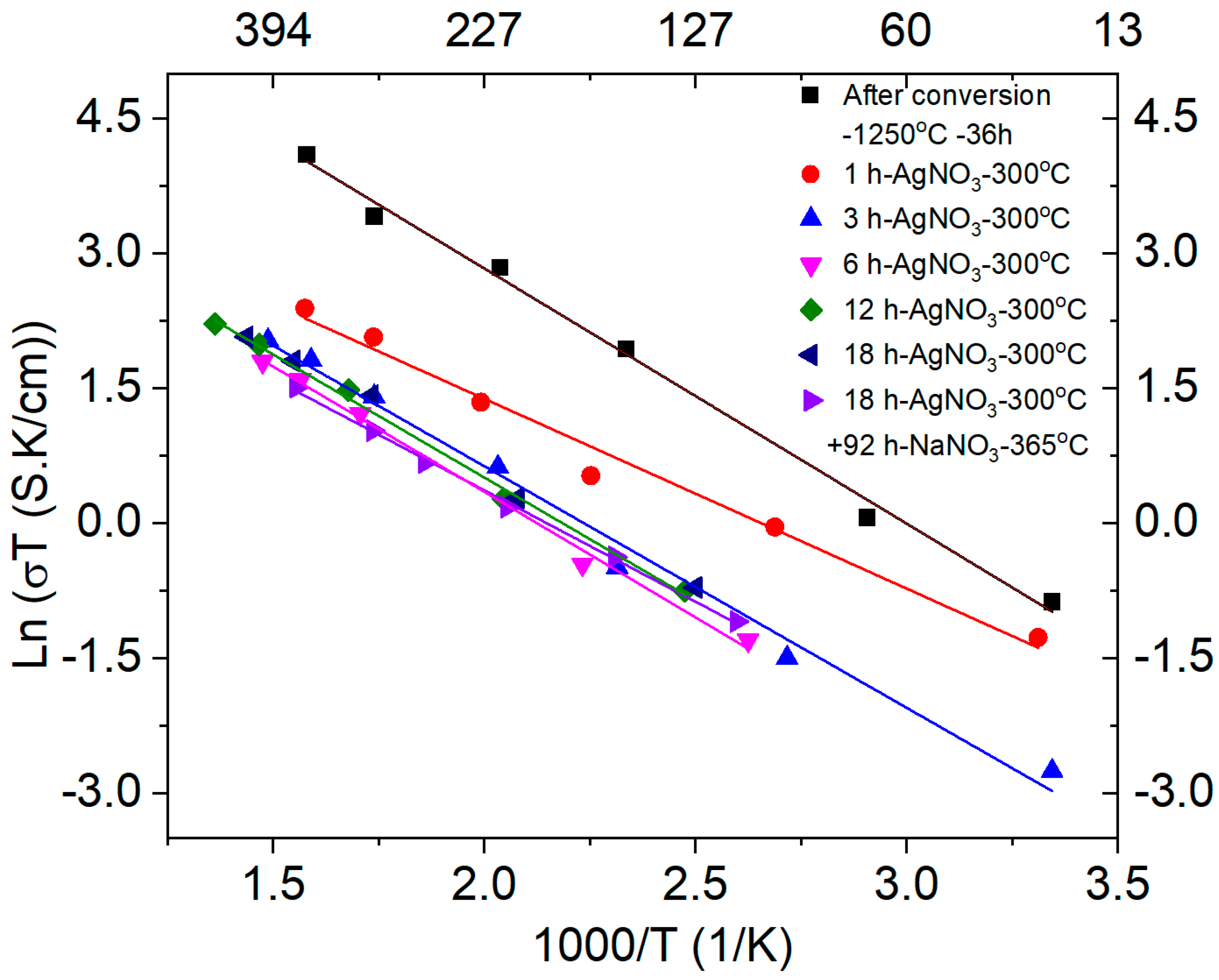
3.3. Conductivity of as Converted and Ion-Exchanged Samples
3.4. Stability of Ag-β″-Alumina + YSZ Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudworth, J.L.; Tilley, A.R. The Sodium Sulfur Battery; Chapman and Hall: London, UK, 1985. [Google Scholar]
- Sudworth, J.L. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Hunt, T.K.; Weber, N.; Cole, T. High efficiency thermoelectric conversion with beta″-alumina electrolytes, the sodium heat engine. Solid State Ion. 1981, 5, 263–265. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lu, X.; Bonnett, J.F.; Canfield, N.L.; Han, K.; Engelhard, M.H.; Jung, K.; Sprenkle, V.L.; Li, G. Decorating β′′-alumina solid-state electrolytes with micron Pb spherical particles for improving Na wettability at lower temperatures. J. Mater. Chem. A 2018, 6, 19703–19711. [Google Scholar] [CrossRef]
- Sparks, T.D.; Ghadbeigi, L. Anisotropic properties of Na-β″-alumina + YSZ composite synthesized by vapor phase method. J. Mater. Res. 2018, 33, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Yang, Y.; Zhang, X.; Liang, S. Highly oriented β″-alumina ceramics with excellent ionic conductivity and mechanical performance obtained by spark plasma sintering technique. J. Mater. Sci. 2020, 55, 8435–8443. [Google Scholar] [CrossRef]
- Ligon, S.C.; Bay, M.-C.; Heinz, M.V.F.; Battaglia, C.; Graule, T.; Blugan, G. Large planar Na-β″-Al(2)O(3) solid electrolytes for next generation na-batteries. Materials 2020, 13, 433. [Google Scholar] [CrossRef] [Green Version]
- Bay, M.-C.; Wang, M.; Grissa, R.; Heinz, M.V.F.; Sakamoto, J.; Battaglia, C. Sodium plating from Na-β″-alumina ceramics at room temperature, paving the way for fast-charging all-solid-state batteries. Adv. Energy Mater. 2020, 10, 1902899. [Google Scholar] [CrossRef] [Green Version]
- Weber, N.; Kummer, J.T. Sodium sulfur batteries. Annu. Power Sources Conf. 1967, 21, 37–39. [Google Scholar]
- Long, W.; Yuhao, L.; Jue, L.; Maowen, X.; Jinguang, C.; Dawei, Z.; Goodenough, J.B. A superior low-cost cathode for a Na-Ion battery. Angew. Chem. Int. Ed. 2013, 52, 1964–1967. [Google Scholar] [CrossRef]
- Jian, Z.; Han, W.; Lu, X.; Yang, H.; Hu, Y.-S.; Zhou, J.; Zhou, Z.; Li, J.; Chen, W.; Chen, D.; et al. Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries. Adv. Energy Mater. 2013, 3, 156–160. [Google Scholar] [CrossRef]
- Kubota, K.; Komaba, S. Review—Practical issues and future perspective for Na-Ion batteries. J. Electrochem. Soc. 2015, 162, A2538–A2550. [Google Scholar] [CrossRef]
- Youngblood, G.E.; Virkar, A.V.; Cannon, W.R.; Gordon, R.S. Sintering processes and heat treatment schedules for conductive, lithia-stabilized β′′-Al2O3. Am. Ceram. Soc. Bull. 1977, 56, 206–210. [Google Scholar]
- Virkar, A.V.; Jue, J.-F.; Fung, K.-Z. Alkali Metal β and β″-Alumina and gallate polycrystalline ceramics by a vapor phase method. US Patent 6,117,807, 12 September 2000. [Google Scholar]
- Parthasarthy, P.; Weber, N.; Virkar, A.V. High temperature sodium-zinc chloride batteries with sodium beta-alumina solid electrolyte. ECS Trans. 2007, 6, 67. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Virkar, A.V. Vapor phase conversion of α-alumina + zirconia composites into sodium ion conducting Na-β″-alumina + zirconia solid electrolytes. J. Electrochem. Soc. 2013, 160, A2268–A2280. [Google Scholar] [CrossRef]
- Yao, Y.; Kummer, J.T. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 1967, 29, 2453–2466. [Google Scholar]
- Koh, J.-H.; Weber, N.; Virkar, A.V. Synthesis of lithium-beta-alumina by various ion-exchange and conversion processes. Solid State Ion. 2012, 220, 32–38. [Google Scholar] [CrossRef]
- Roth, W.L.; Farrington, G.C. Lithium-Sodium Beta alumina: First of a Family of Co-ionic Conductors? Science 1977, 196, 1332. [Google Scholar] [CrossRef]
- Dunn, B.; Farrington, G.C. Trivalent ion exchange in beta″ alumina. Solid State Ion. 1983, 9–10, 223–225. [Google Scholar] [CrossRef]
- Dunn, B.; Farrington, G.C. Fast divalent ion conduction in Ba++, Cd++ and Sr++ beta″ aluminas. Mater. Res. Bull. 1980, 15, 1773–1777. [Google Scholar] [CrossRef]
- Chevalier, J.r.m.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Allen, J.L.; Wolfenstine, J.; Ramaswamy, E.; Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Zhang, L.; Virkar, A.V. On space charge and spatial distribution of defects in yttria-stabilized zirconia. J. Electrochem. Soc. 2017, 164, F1506–F1523. [Google Scholar] [CrossRef]




 |
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Virkar, A.V. Sodium, Silver and Lithium-Ion Conducting β″-Alumina + YSZ Composites, Ionic Conductivity and Stability. Crystals 2021, 11, 293. https://doi.org/10.3390/cryst11030293
Zhu L, Virkar AV. Sodium, Silver and Lithium-Ion Conducting β″-Alumina + YSZ Composites, Ionic Conductivity and Stability. Crystals. 2021; 11(3):293. https://doi.org/10.3390/cryst11030293
Chicago/Turabian StyleZhu, Liangzhu, and Anil V. Virkar. 2021. "Sodium, Silver and Lithium-Ion Conducting β″-Alumina + YSZ Composites, Ionic Conductivity and Stability" Crystals 11, no. 3: 293. https://doi.org/10.3390/cryst11030293






