Non-Isothermal Crystallization Behavior of Poly(vinylidene fluoride) in Dialkyl Phthalate Diluents during Thermally Induced Phase Separation Process
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Cloud Point and DSC Determination
2.4. Avrami Analysis Modified by Jeziorny
2.5. Mo’s Approach Analysis
2.6. Membrane Preparation and Sem Observation
3. Results and Discussion
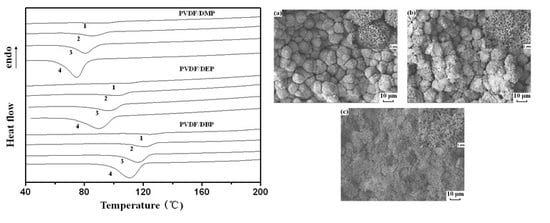
3.1. Non-Isothermal Crystallization Behavior
3.2. Avrami Analysis Modified by Jeziorny
3.3. Mo’s Approach
3.4. Membrane Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castro, J. Method for Making Microporous Products. U.S. Patent 4,247,498, 27 January 1981. [Google Scholar]
- Kim, S.S.; Lloyd, D.R. Thermodynamics of Polymer/Diluent Systems for Thermally Induced Phase Separation: 2.Solid-Liquid Phase Separation Systems. Polymer 1992, 33, 1036–1046. [Google Scholar] [CrossRef]
- Dohany, J.E. Fluorine-Containing Polymers, Poly(Vinylidene Fluoride). Kirk Othmer Encycl. Chem. Technol. 2000, S118–S119. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes–A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Maruyama, T.; Sotani, T.; Matsuyama, H. Preparation of PVDF hollow fiber membrane from a ternary polymer/solvent/nonsolvent system via thermally induced phase separation (TIPS) method. Sep. Purif. Technol. 2008, 63, 415–423. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, W.M.; Wang, X.L. Diluent selection of PVDF membrane prepared via thermally induced phase separation. Chem. J. Chin. Univ. Chin. 2008, 29, 1895–1900. [Google Scholar]
- Yang, J.; Li, D.W.; Lin, Y.K.; Wang, X.L.; Tian, F.; Wang, Z. Formation of a bicontinuous structure membrane of polyvinylidene fluoride in diphenyl ketone diluent via thermally induced phase separation. J. Appl. Polym. Sci. 2008, 110, 341–347. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Y.; Ma, H.; Yang, J.; Tian, Y.; Ma, W.; Wang, X. Formation of a bicontinuous structure membrane of polyvinylidene fluoride in diphenyl carbonate diluent via thermally induced phase separation. J. Appl. Polym. Sci. 2009, 114, 1523–1528. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Ma, W.; Tian, Y.; Yang, J.; Wang, X. Preparation of microporous PVDF membrane via tips method using binary diluent of DPK and PG. J. Appl. Polym. Sci. 2010, 118, 3518–3523. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Du, C.H.; Xu, Y.Y.; Ji, G.L.; Zhu, B.K. Preparation of porous PVdF membrane via thermally induced phase separation using sulfolane. J. Appl. Polym. Sci. 2008, 108, 272–280. [Google Scholar] [CrossRef]
- Ishigami, T.; Nii, Y.; Ohmukai, Y.; Rajabzadeh, S.; Matsuyama, H. Solidification Behavior of Polymer Solution during Membrane Preparation by Thermally Induced Phase Separation. Membranes 2014, 4, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Yang, W.; Zhang, J.; Li, Y.; Yuan, S. Fabrication of hollow fiber microfiltration membrane from PVDF/DBP/DBS system via thermally induced phase separation process. J. Polym. Eng. 2015, 35, 709–717. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J. An improved process for polyvinylidene fluoride membrane preparation by using a water soluble diluent via thermally induced phase separation technique. Mater. Des. 2015, 86, 204–214. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D.; Wang, X.; Meng, X.; Lv, Y.; Wang, X.; Miao, R. Preparation of PVDF membranes via the low-temperature TIPS method with diluent mixtures: The role of coagulation conditions and cooling rate. Desalination 2015, 361, 25–37. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Z.; Shen, H.; Zhu, Z.; Liu, L.; Yang, L.; Cheng, L. Morphology and performance of PVDF TIPS microfiltration hollow fiber membranes prepared from PVDF/DBP/DOP systems for industrial application. J. Chem. Technol. Biotechnol. 2016, 91, 1697–1708. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Shen, H.H. Effect of Diluent Mixing Ratio on PVDF Hollow Fiber Membrane Fabricated by Thermally Induced Phase Separation. China Plast. 2017, 31, 87–94. [Google Scholar]
- Cui, Z.; Xu, S.; Ding, J.; Zhang, J.; He, B.; Wang, H.; Li, J. The Effect of Diluent Mixture with Upper Critical Solution Temperature on Membrane Formation Process, Microstructure, and Performance of PVDF Hollow Fiber Membrane by TIPS Process. Polymers 2018, 10, 719. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Pan, Q.; Gao, J.; Xiao, C. Effect of Mixed Solvents on the Structure o f Polyvinylidene Fluoride Flat Membrane in Thermally Induced Phase Separation Method. J. Nanosci. Nanotechnol. 2019, 19, 5994–5998. [Google Scholar] [CrossRef]
- Ji, G.L.; Zhu, B.K.; Zhang, C.F.; Xu, Y.Y. Nonisothermal Crystallization Kinetics of Poly(vinylidene fluoride) in a Poly(vinylidene fluoride)/Dibutyl Phthalate/Di(2-ethylhexyl)phthalate System via Thermally Induced Phase Separation. J. Appl. Polym. Sci. 2008, 107, 2109–2117. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, J.; Wang, X.; Ma, W. Crystallization Behavior of PVDF-DMP System via Thermally Induced Phase Separation. J. Appl. Polym. Sci. 2006, 102, 3714–3719. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters Characterizing the Kinetics of the Non-isothermal Crystallization of Poly(ethylene terephthalate) Determined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic of Non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Qiu, Z.; Mo, Z.; Yu, Y.; Zhang, H.; Sheng, S.; Song, C. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ketone ether ketone ketone). J. Appl. Polym. Sci. 2000, 77, 2865–2871. [Google Scholar] [CrossRef]
- Liu, B.; Du, Q.G.; Yang, Y.L. The phase diagrams of mixtures of EVAL and PEG in relation to membrane formation. J. Membr. Sci. 2000, 180, 81–92. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters; CRC Press: Boca Raton, FL, USA, 2000; pp. 168–200. [Google Scholar]
- Nishi, T.; Wang, T. TMelting Point Depression and Kinetic Effects of Cooling on Crystallization in Poly(vinylidene fluoride)-Poly(methyl methacrylate) Mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Wang, T.T.; Nishi, T. Spherulitic Crystallization in Compatible Blends of Poly(vinylidene fluoride) and Poly(methyl methacrylate). Macromolecules 1977, 10, 421–425. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, Q.B.; Sun, C.Y. Nonisothermal crystallization kinetics of polypropylene/polyethersulfone blend. Polym. Bull. 2008, 60, 291–300. [Google Scholar] [CrossRef]






| Sample | (°/min) | (°C) | (°C) | (°C) | (°C) | (°C) | (°C) | (J/g) | (%) | (min) |
|---|---|---|---|---|---|---|---|---|---|---|
| PVDF/DMP | 2 | 108.5 | 94.7 | 75.4 | 100.2 | 122.5 | 135.2 | 62.7 | 60.0 | 8.0 |
| PVDF/DEP | 2 | 121.7 | 109.5 | 88.4 | 109.6 | 131.4 | 144.1 | 59.8 | 57.2 | 7.2 |
| PVDF/DBP | 2 | 138.8 | 125.4 | 106.8 | 125.3 | 148.0 | 159.0 | 58.4 | 55.9 | 7.1 |
| PVDF/DMP | 5 | 100.4 | 85.3 | 63.5 | 100.4 | 120.6 | 133.7 | 61.3 | 58.6 | 3.2 |
| PVDF/DEP | 5 | 114.3 | 102.8 | 76.1 | 107.6 | 129.5 | 141.6 | 60.4 | 57.8 | 3.0 |
| PVDF/DBP | 5 | 133.9 | 120.6 | 98.7 | 123.6 | 146.4 | 157.5 | 58.2 | 55.7 | 2.5 |
| PVDF/DMP | 10 | 98.5 | 80.7 | 57.9 | 95.3 | 116.7 | 133.2 | 65.6 | 62.7 | 1.9 |
| PVDF/DEP | 10 | 111.1 | 97.0 | 68.7 | 105.5 | 127.9 | 140.9 | 61.7 | 59.0 | 1.7 |
| PVDF/DBP | 10 | 129.0 | 116.6 | 91.6 | 123.0 | 145.2 | 155.6 | 57.0 | 54.5 | 1.4 |
| PVDF/DMP | 20 | 91.2 | 75.0 | 52.6 | 97.4 | 115.5 | 133.0 | 62.7 | 60.0 | 1.0 |
| PVDF/DEP | 20 | 107.9 | 90.0 | 62.89 | 105.7 | 126.4 | 140.9 | 60.7 | 58.1 | 0.9 |
| PVDF/DBP | 20 | 126.0 | 110.8 | 84.6 | 123.4 | 144.0 | 155.8 | 57.2 | 54.8 | 0.8 |
| Substance | δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) | (cm3/mol) | |
|---|---|---|---|---|---|
| PVDF | 17.0 | 12.1 | 10.2 | - | - |
| DMP | 18.6 | 10.8 | 4.9 | 163.0 | 0.66 |
| DEP | 17.6 | 9.6 | 4.5 | 180.4 | 0.73 |
| DBP | 17.8 | 8.6 | 4.1 | 266.4 | 1.40 |
| Sample (°C/min) | |||
|---|---|---|---|
| PVDF/DMP | |||
| 2 | 2.98 | 0.001 | 0.031 |
| 5 | 3.16 | 0.019 | 0.453 |
| 10 | 3.42 | 0.064 | 0.760 |
| 20 | 3.69 | 0.820 | 0.990 |
| PVDF/DEP | |||
| 2 | 2.96 | 0.001 | 0.035 |
| 5 | 3.26 | 0.029 | 0.492 |
| 10 | 3.20 | 0.122 | 0.810 |
| 20 | 2.83 | 0.989 | 0.999 |
| PVDF/DBP | |||
| 2 | 3.33 | 0.001 | 0.038 |
| 5 | 3.35 | 0.039 | 0.522 |
| 10 | 3.44 | 0.296 | 0.885 |
| 20 | 3.67 | 2.149 | 1.039 |
| 20% | 40% | 60% | 80% | |
|---|---|---|---|---|
| PVDF/DMP | ||||
| 1.17 | 1.10 | 1.06 | 1.03 | |
| 13.42 | 16.69 | 19.63 | 23.60 | |
| PVDF/DEP | ||||
| 1.11 | 1.07 | 1.04 | 1.03 | |
| 11.24 | 14.83 | 17.89 | 21.75 | |
| PVDF/DBP | ||||
| 1.03 | 1.02 | 1.01 | 1.02 | |
| 9.52 | 12.55 | 15.62 | 19.89 | |
| 20% | 40% | 60% | 80% | |
| PVDF/DMP | ||||
| 1.17 | 1.10 | 1.06 | 1.03 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Tang, Y.; Wang, L.; Wang, X. Non-Isothermal Crystallization Behavior of Poly(vinylidene fluoride) in Dialkyl Phthalate Diluents during Thermally Induced Phase Separation Process. Crystals 2020, 10, 782. https://doi.org/10.3390/cryst10090782
Lin Y, Tang Y, Wang L, Wang X. Non-Isothermal Crystallization Behavior of Poly(vinylidene fluoride) in Dialkyl Phthalate Diluents during Thermally Induced Phase Separation Process. Crystals. 2020; 10(9):782. https://doi.org/10.3390/cryst10090782
Chicago/Turabian StyleLin, Yakai, Yuanhui Tang, Lin Wang, and Xiaolin Wang. 2020. "Non-Isothermal Crystallization Behavior of Poly(vinylidene fluoride) in Dialkyl Phthalate Diluents during Thermally Induced Phase Separation Process" Crystals 10, no. 9: 782. https://doi.org/10.3390/cryst10090782