Single Crystals of the Isotypic Series BaLu2Ch4 (Ch = S, Se and Te) with CaFe2O4-Type Structure
Abstract
: Single crystals of ternary chalcogenides with the composition BaLu2Ch4 (Ch = S, Se and Te; orthorhombic, Pnma; a = 1211.4−1353.6, b = 395.6−438.5, c = 1427.8−1593.6 pm) could be obtained after attempts to synthesize ternary lutetium(III) nitride chalcogenides using the elements (Lu and Ch) along with BaN3Cl as a nitrogen source. Their crystal structures are isotypic with CaFe2O4 containing two sorts of chains built up of edge-linked [(Lu1)(Ch2)(Ch3)3(Ch4)2]9− and [(Lu2)(Ch1)3(Ch2)2(Ch4)]9− octahedra, respectively. A further interconnection via the chalcogenide anions (Ch3)2− and (Ch1)2− leads to double chains, where either (Lu1)3+ or (Lu2)3+ coordinates these chalcogenide anions as well. The three-dimensional framework emerges from the corner-linkage of the two kinds of double chains forming large channels apt to take up the Ba2+ cations. These divalent cations exhibit eight contacts to chalcogenide anions resulting in the formation of bicapped trigonal prisms [BaCh8]14−.1. Introduction
Up until now many nitride chalcogenides of the lanthanides with the formulae Ln3NCh3 (Ch = S, Se) [1-5] and Ln4N2Ch3 (Ch = S, Se, Te) [4,6-10] are known in the literature, mainly as compounds with the lighter representatives (Ln = La−Ho). Thus, the quest for a possible formation of nitride chalcogenides of the heaviest lanthanoid leads to formulation of the question, which nitrogen source would be most suitable for their synthesis? In order to test new educts for such experiments, the recently described barium azide chloride BaN3Cl [11] offers a promising option. Unfortunately, these experiments failed in the case of lutetium and ternary chalcogenides with the general composition BaLu2Ch4 were obtained instead of the primary target products Lu3NCh3 or Lu4N2Ch3 (Ch = S, Se, Te). This type of compound (M2+)(M3+)2(Ch2−)4 has been known for quite a long time with a huge range of combinations for chalcogenide anions (Ch2−), divalent (M2+) and trivalent cations (M3+), even for the lanthanides as rather large trivalent species (Ln3+). First studies within the systems alkaline-earth metals, rare-earth metals and chalcogens were carried out by Flahaut et al. [12] in the early 1960s. Since this time further groups have published many reports dealing with this class of compounds. Although several examples for lutetium-bearing chalcogenides were characterized, the combination of barium as divalent (Ba2+) and lutetium as trivalent cation (Lu3+) was barely investigated. The so far identified compounds MIILu2Ch4 crystallize in three different structure types: MgAl2O4 (for MII = Mg, Mn, Fe and Ch = S) [13-17], CaFe2O4 (for MII = Ca, Eu, Pb and Ch = O, S, Se) [18-23] and Th3P4 (for MII = Eu and Ch = S) [24]. Furthermore, besides BaY2S4 [25] and the defective crystal structure of Ba0.9Sm2S3.9 [26] no other examples for sulfide-containing compounds with barium and rare-earth metal cations are known with the CaFe2O4-type arrangement hitherto, while many representatives for the compositions BaLn2Se4 [27] and BaLn2Te4 [28] could be synthesized. In this short paper the crystal structures of the three isotypic barium lutetium chalcogenides BaLu2Ch4 (Ch = S, Se and Te) will be presented and discussed.
2. Results and Discussion
Initially, nitride chalcogenides of the heavy lanthanoids should be synthesized by using barium azide chloride BaN3Cl as a nitrogen source and fluxing agent. Instead of the target compounds several ternary chalcogenides with the formula type BaLn2Ch4 (Ln = Gd, Tb, Er, Tm, Lu; Ch = S, Se and Te) could be isolated and identified by X-ray single crystal and powder diffraction after washing the products with water. Although the first attempts to synthesize almost all of the compounds BaLn2Ch4 were successful and described by Bugaris and Ibers [27] and Narducci et al. [28], the lutetium representatives were not described in their papers.
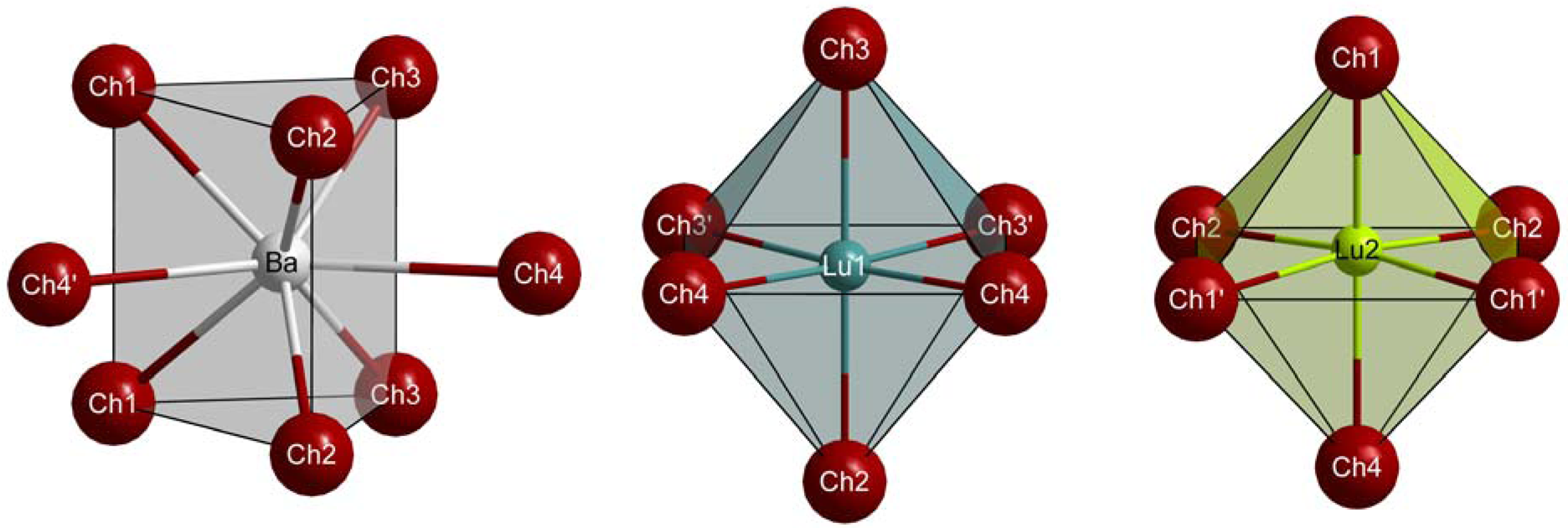
The ternary chalcogenides BaLu2Ch4 crystallize in the orthorhombic system with the space group Pnma (Ch = S: a = 1211.43(6), b = 395.56(2), c = 1427.81(4) pm; Ch = Se: a = 1261.32(4), b = 410.89(1), c = 1487.74(4) pm; Ch = Te: a = 1353.58(8), b = 438.47(3), c = 1593.62(8) pm) and four formula units per unit cell representing the CaFe2O4-structure type (see Table 1 for atomic coordinates). All seven crystallographically independent atoms reside at Wyckoff positions 4c with the site symmetry m. The two trivalent lutetium cations are surrounded by six chalcogenide anions forming slightly distorted [(Lu1)(Ch2)(Ch3)3(Ch4)2]9− and [(Lu2)(Ch1)3(Ch2)2(Ch4)]9− octahedra (Figure 1, mid and right), while the divalent barium cations are coordinated by eight chalcogenide anions in the shape of bicapped trigonal prisms (Figure 1, left). Each of the four crystallographically different chalcogenide anions shows three contacts to lutetium and just two bonds to barium. The polyhedra can be described as more or less distorted square pyramids for (Ch1)2−, (Ch2)2− and (Ch3)2− as well as distorted trigonal bipyramids for (Ch4)2−.
All bond lengths between Ba2+ and its eight Ch2− ligands (315−333 pm for Ch = S, 327−349 pm for Ch = Se, and 350−367 pm for Ch = Te, for details see Table 2) are similar to those obtained for the other representatives of the ternary barium sulfides BaLn2S4 (318−343 pm for Ln = Sm, Y) [25,26], selenides BaLn2Se4 (327–346 pm for Ln = Er−Yb) [28] and tellurides BaLn2Te4 (350−375 pm for Ln = Sm−Tm, Y) [27]. Comparable short distances between Lu3+ and Ch2− as in the BaLu2Ch4 series (264−272 pm for Ch = S, 277−285 pm for Ch = Se, 298−306 pm for Ch = Te) can also be observed in Lu2S3 (E-type: 264−274 pm) [30], CaLu2S4 (265−277 pm) [19], Lu2Se3 (Z-type: 279−286 pm) [31], EuLu2Se4 (275−282 pm) [21], CsCdLuTe3 (307−311 pm) [32] and CsCuLu2Te4 (297–314 pm) [33]. A periodic effect regarding ionic radii and volume increments can be stated by a comparison of their influence on the molar volumes Vm of the title compounds in the order of increasing radii of the chalcogenide anions: sulfide (r = 184 pm), selenide (r = 198 pm) and telluride (r = 221 pm) [34]. The difference of the volume increments of these chalcogenide anions (16 cm3/mol between 4 × S2− and 4 × Se2− as well as 30 cm3/mol between 4 × Se2− and 4 × Te2−) [35] are very similar to the obtained Vm differences of the ternary compounds (13 cm3/mol between BaLu2S4 and BaLu2Se4, 26 cm3/mol between BaLu2Se4 and BaLu2Te4).
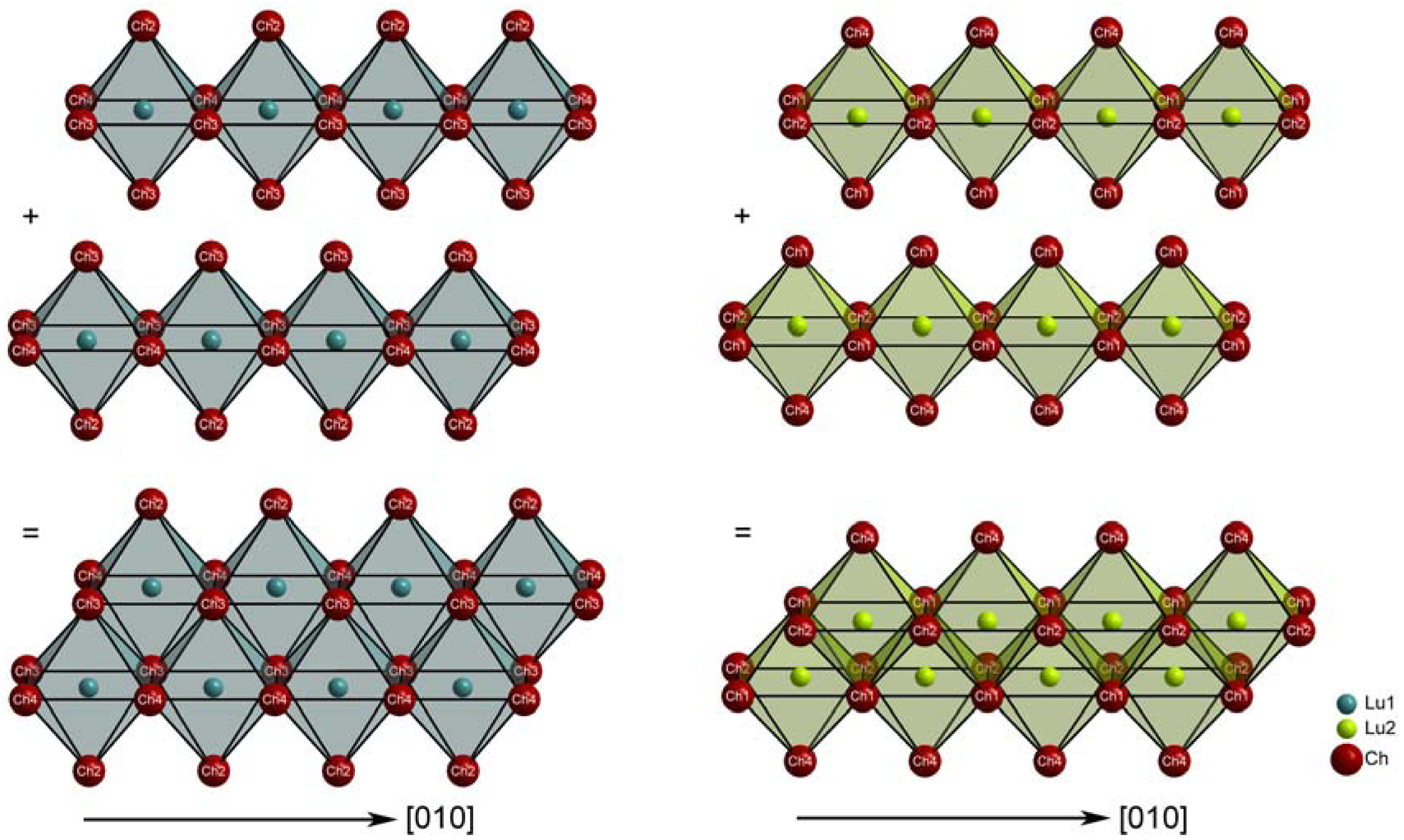
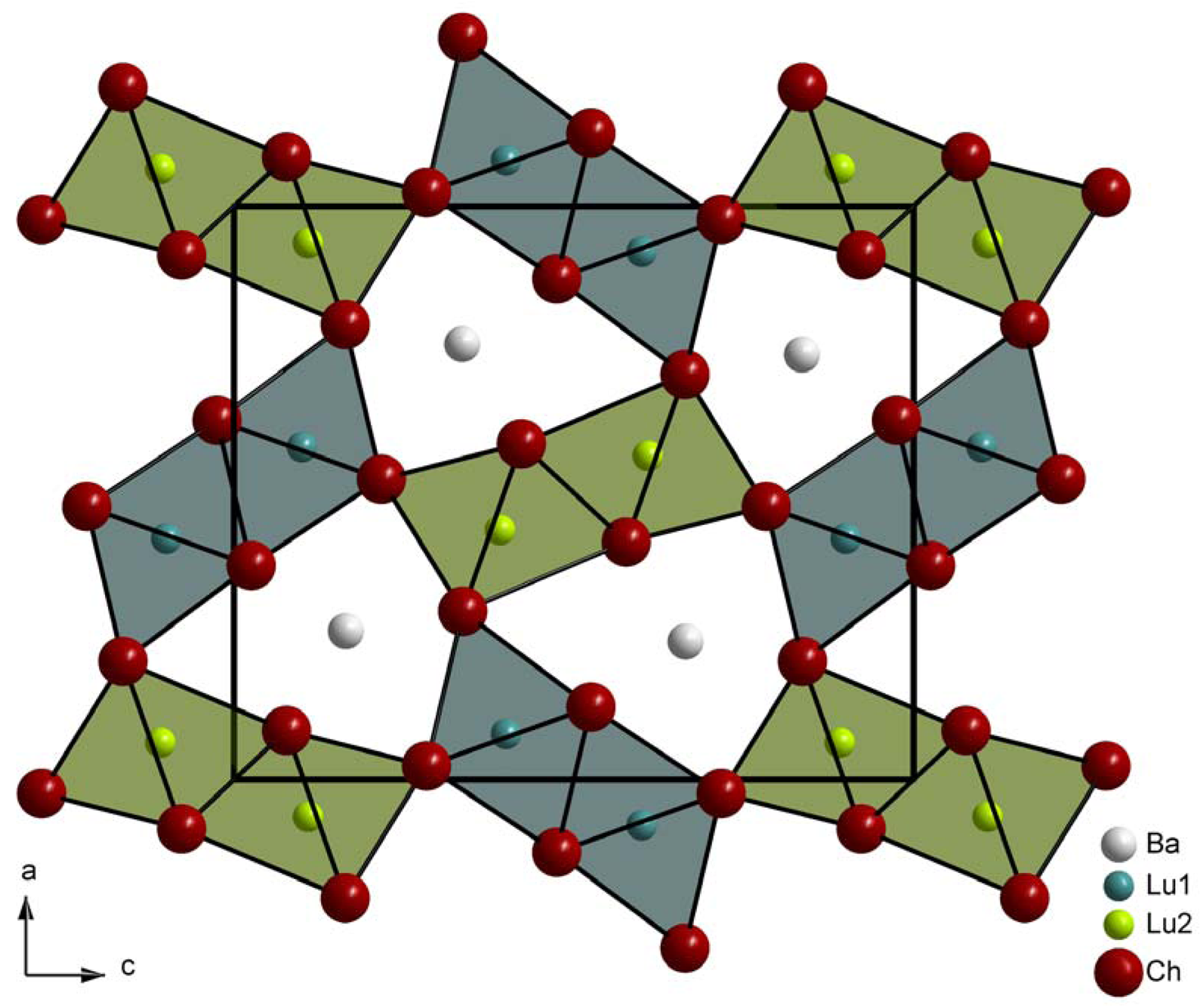
The different surrounding of the two crystallographically distinct lutetium cations is manifested in the crystal structure as the [(Lu1)(Ch2)(Ch3)3(Ch4)2]9− and [(Lu2)(Ch1)3(Ch2)2(Ch4)]9− octahedra form two different kinds of chains by edge linkage. Interestingly, the chalcogenide anions (Ch3)2− connect two strands to double chains running parallel to the [010] direction (Figure 2, left). The same holds for the (Ch1)2− anions in the case of the (Lu2)2+-bearing congeners (Figure 2, right) and so, besides two extra Ba2+ contacts for all chalcogenide anions, (Ch1)2− and (Ch3)2− exhibit three contacts to only one sort of trivalent lutetium cation, while the (Ch2)2− and (Ch4)2− anions are bonded to both types of Lu3+. Both kinds of double chains are finally corner-connected according to the pattern in Figure 3, forming a three-dimensional framework with channels that are occupied by the octacoordinated barium cations.
3. Experimental Section
Single crystals of BaLu2S4, BaLu2Se4 and BaLu2Te4 were accidentally (but reproducible) obtained after heating mixtures of lutetium metal, its trichloride, chalcogen and barium azide chloride in molar ratios of 26:2:27:6 designed to produce Lu3NCh3 [1-5] or Lu4N2Ch3 [4,6-10] along with an excess of barium chloride as flux at 920 °C for ten days.
All three water- and air-stable products exhibit the shape of needles with different colors (BaLu2S4: colorless, BaLu2Se4: dark red, BaLu2Te4: black) and were characterized by single crystal X-ray diffraction (κ-CCD, Bruker-Nonius, Mo-Kα radiation with graphite monochromator: λ = 71.01 pm) at room temperature. Essential information about the structure solutions and refinements for the BaLu2Ch4 series (Ch = S, Se, Te) by using the program package SHELXS-97 and SHELX-97 [36] as well as X-SHAPE for correction for absorption [37] and scattering factors from the International Tables, Vol. C [38], is available in Table 3. Further details may be obtained from the Fachinformationszentrum (FIZ) Karlsruhe, D-76344 Eggenstein-Leopoldshafen, Germany (Fax: (+49)7247-808-666, E-mail: [email protected]), on quoting the depository numbers CSD-422891 (BaLu2S4), CSD-422892 (BaLu2Se4) and CSD-422893 (BaLu2Te4).
4. Conclusions
The crystal structures of all three barium lutetium chalcogenides BaLu2Ch4 (Ch = S, Se and Te) exhibit a CaFe2O4-type arrangement. As a particularity, the two crystallographically distinct trivalent lutetium cations can be found in two different types of chains. These first build double chains by edge-linkage, which are corner-connected further to form a three-dimensional framework apt to embed divalent barium cations. It should be noted that these compounds were obtained in order to produce lutetium nitride chalcogenides (Lu3NCh3 or Lu4N2Ch3) by using barium azide chloride as flux and a nitrogen source, which leads to the assumption that this starting material is not suitable as a source of nitrogen for these kinds of synthetic experiments.
| Atom | Wyckoff site | x/a | y/b | z/c | Ueqa) | Ch |
|---|---|---|---|---|---|---|
| Ba | 4c | 0.24128(12) | ¼ | 0.66285(11) | 154(4) | S |
| 0.23994(5) | ¼ | 0.66557(4) | 64(2) | Se | ||
| 0.23844(13) | ¼ | 0.66986(11) | 143(4) | Te | ||
| Lu1 | 4c | 0.07937(8) | ¼ | 0.39920(7) | 140(3) | S |
| 0.07980(4) | ¼ | 0.40077(3) | 47(1) | Se | ||
| 0.08131(9) | ¼ | 0.40350(7) | 137(3) | Te | ||
| Lu2 | 4c | 0.56482(8) | ¼ | 0.60849(8) | 146(3) | S |
| 0.56246(4) | ¼ | 0.60870(3) | 49(1) | Se | ||
| 0.55812(9) | ¼ | 0.60972(7) | 130(3) | Te | ||
| Ch1 | 4c | 0.0822(5) | ¼ | 0.0759(4) | 142(12) | S |
| 0.08590(7) | ¼ | 0.07604(6) | 45(2) | Se | ||
| 0.09048(14) | ¼ | 0.07529(12) | 125(4) | Te | ||
| Ch2 | 4c | 0.2929(5) | ¼ | 0.3412(5) | 149(12) | S |
| 0.29430(7) | ¼ | 0.34133(6) | 61(2) | Se | ||
| 0.29558(14) | ¼ | 0.34259(12) | 140(4) | Te | ||
| Ch3 | 4c | 0.3766(5) | ¼ | 0.0236(4) | 132(12) | S |
| 0.37538(7) | ¼ | 0.02521(6) | 46(2) | Se | ||
| 0.37455(14) | ¼ | 0.02823(12) | 125(4) | Te | ||
| Ch4 | 4c | 0.4772(5) | ¼ | 0.7830(4) | 137(12) | S |
| 0.47488(7) | ¼ | 0.78338(6) | 58(2) | Se | ||
| 0.47228(14) | ¼ | 0.78407(12) | 138(4) | Te | ||
a)Ueq = ⅓(U11 + U22 + U33) [29].
| Ch = S | Ch = Se | Ch = Te | |||
|---|---|---|---|---|---|
| Ba | − Ch3 | (2×) | 314.7(5) | 327.1(1) | 349.8(2) |
| − Ch1 | (2×) | 316.7(5) | 329.0(1) | 352.7(2) | |
| − Ch2 | (2×) | 325.1(5) | 335.3(1) | 354.9(2) | |
| − Ch4 | (1×) | 329.1(6) | 342.8(1) | 365.1(3) | |
| − Ch4′ | (1×) | 333.3(6) | 344.3(1) | 367.7(3) | |
| Lu1 | − Ch4 | (2×) | 267.2(4) | 278.3(1) | 299.3(1) |
| − Ch3 | (1×) | 269.2(6) | 280.4(1) | 300.3(2) | |
| − Ch3′ | (2×) | 271.1(4) | 282.3(1) | 301.9(1) | |
| − Ch2 | (1×) | 271.7(6) | 284.6(1) | 305.8(2) | |
| Lu2 | − Ch1 | (1×) | 264.0(6) | 276.4(1) | 298.1(2) |
| − Ch4 | (1×) | 270.8(6) | 282.1(1) | 301.2(2) | |
| − Ch 1′ | (2×) | 270.2(4) | 282.4(1) | 302.5(2) | |
| − Ch2 | (2×) | 272.0(4) | 283.5(1) | 305.1(2) | |
| BaLu2Ch4 | Ch = S | Ch = Se | Ch = Te |
|---|---|---|---|
| Crystal system | orthorhombic | orthorhombic | Orthorhombic |
| Space group | Pnma | Pnma | Pnma |
| a (pm) | 1211.43(8) | 1261.32(8) | 1353.58(8) |
| b (pm) | 395.56(3) | 410.89(3) | 438.47(3) |
| c (pm) | 1427.81(9) | 1487.74(9) | 1593.62(9) |
| Vm (cm3/mol)/Dx (g/cm3) | 103.007/5.975 | 116.081/6.918 | 142.394/7.006 |
| Formula units (Z) | 4 | 4 | 4 |
| F(000)/θmax | 1048/28.2 | 1336/28.2 | 1624/28.2 |
| ±h/±k/±l | 1 6/5/1 8 | 16/5 /19 | 17/5/21 |
| Reflections (independent) | 12319 (955) | 10976 (1087) | 17321 (1312) |
| (μ/mm−1) | 35.42 | 49.23 | 36.83 |
| Rint/Rσ | 0.078/0.047 | 0.062/0.036 | 0.094/0.061 |
| R1/wR2 | 0.074/0.136 | 0.030/0.058 | 0.090/0.103 |
| GooF | 1.186 | 1.053 | 1.181 |
Acknowledgments
The authors want to thank Raphael Marx, Alexander Schwenger and Pia Talmon-Gros for their practical work as well as Falk Lissner for the X-ray diffraction data collection. This research was supported by the federal state of Baden-Württemberg (Stuttgart) and the Deutsche Forschungsgemeinschaft (DFG, Bonn).
References and Notes
- Lissner, F.; Schleid, Th. M3NS3, die ersten Nitridsulfide der Lanthanide (M = La–Nd, Sm). Z. Anorg. Allg. Chem. 1993, 619, 1771–1776. [Google Scholar]
- Lissner, F.; Schleid, Th. Ce3NSe3: Ein Cer(III)-Nitridselenid mit eckenverknüpften [NCe4]9+-Tetraedern. Z. Anorg. Allg. Chem. 2004, 630, 1741. [Google Scholar]
- Lissner, F.; Meyer, M.; Kremer, R.K.; Schleid, Th. M3NS3 (M = La−Nd, Sm, Gd−Dy): Struktur und Magnetismus von 3:1:3-Typ-Nitridsulfiden dreiwertiger Lanthanide. Z. Anorg. Allg. Chem. 2006, 632, 1995–2002. [Google Scholar]
- Lissner, F.; Schleid, Th. Lanthanido ammonium cations [NM4]9+ as main structural features in lanthanide (III) nitride chalcogenides and their derivatives. J. Alloys Compounds 2008, 451, 610–616. [Google Scholar]
- Lissner, F.; Schleid, Th. Die nicht-isotypen Nitridselenide Dy3NSe3 und Ho3NSe3: Ketten und Dimere. Z. Anorg. Allg. Chem. 2009, 635, 815–821. [Google Scholar]
- Lissner, F.; Schleid, Th. Ein neues Samariumnitridsulfid: Sm4N2S3. Z. Anorg. Allg. Chem. 1994, 620, 2003–2007. [Google Scholar]
- Lissner, F.; Schleid, Th. Nd4N2Se3 und Tb4N2Se3: Zwei nicht-isotype Lanthanoid(III)- Nitridselenide. Z. Anorg. Allg. Chem. 2003, 629, 1027–1032. [Google Scholar]
- Lissner, F.; Schleid, Th. Pr4N2S3 und Pr4N2Se3: Zwei nicht-isotype Praseodymium(III)- Nitridchalkogenide. Z. Anorg. Allg. Chem. 2005, 631, 427–432. [Google Scholar]
- Lissner, F.; Schleid, Th. M4N2Te3 (M = La−Nd): Die ersten Nitridtelluride der dreiwertigen Lanthanide. Z. Anorg. Allg. Chem. 2005, 631, 1119–1124. [Google Scholar]
- Lissner, F.; Schleid, Th. La4N2S3: Ein neues Nitridsulfid des Lanthans mit beispielloser Kristallstruktur. Z. Anorg. Allg. Chem. 2006, 632, 1167–1172. [Google Scholar]
- Blaschkowski, B.; Balzer, G.; Keller, H.L.; Schleid, Th. BaN3Cl: Synthesis, Crystal Structure, Vibrational Spectra and Thermal Decomposition of Barium Azide Chloride. Z. Anorg. Allg. Chem. 2008, 634, 2276–2280. [Google Scholar]
- Flahaut, J.; Domange, L.; Patrie, M. Combinaisons formées par les sulfures des elements du groupe des terres rares. Etude cristallographique des phases ayant le type structural du phosphure de thorium Th3P4. Bull. Soc. Chim. Fr. 1962, 1962, 2048–2054. [Google Scholar]
- Patrie, M.; Flahaut, J.; Domange, L. Sur une nouvelle serie de spinelles soufres, contenant des terres rares ou du scandium. C. R. Hebd. Seances Acad. Sci. 1964, 258, 2585–2586. [Google Scholar]
- Guittard, M.; Flahaut, J.; Souleau, C.; Farsam, H. Sur une nouvelle serie de spinelles selenies du terres rares, de l'yttrium et du scandium. C. R. Hebd. Seances Acad. Sci. 1964, 258, 2847–2849. [Google Scholar]
- Fujii, H. Crystallographic, magnetic and electric properties of rare earth chalcogenide spinels. J. Sci. Hiroshima Univ. Ser. A-I 1972, 36, 67–75. [Google Scholar]
- Tomas, A.; Brossard, L.; Guittard, M. Structural Studies by X-Ray Diffraction and Moessbauer Spectroscopy of Cubic FeYb2S4 and FeLu2S4. J. Solid State Chem. 1980, 34, 11–16. [Google Scholar]
- Pawlak, L.; Duczmal, M. Magnetic and structural properties of iron and manganese lanthanide spinels. J. Alloys Compounds 1992, 184, 203–209. [Google Scholar]
- Müller-Buschbaum, H.K.; von Schenk, R. Untersuchungen an SrYb2O4, CaYb2O4 und CaLu2O4: Ein Beitrag zur Kristallstruktur des Calciumferrat(III)-Typs. Z. Anorg. Allg. Chem. 1970, 377, 70–78. [Google Scholar]
- Rodier, N.; Tien, V. Structure du sulfure mixte de calcium et de lutetium CaLu2S4. C. R. Hebd. Seances Acad. Sci. 1977, 284, 909–911. [Google Scholar]
- Gulay, L.D.; Wolcyrz, M.; Pietraszko, A.; Olekseyuk, I.D. Investigations of the Tm2Se3-Cu2Se- PbSe and Lu2Se3-Cu2Se-PbSe systems at 870 K. Pol. J. Chem. 2006, 80, 1703–1704. [Google Scholar]
- Gengbang, J.; Sang, C.E.; Guertin, R.P.; Albrecht-Schmitt, T.E. An investigation of structural parameters and magnetic and optical properties of EuLn2Q4 (Ln = Tb-Lu, Q = S, Se). J. Solid State Chem. 2008, 181, 14–19. [Google Scholar]
- Gulay, L.D.; Daszkiewicz, M.; Shemet, V.Ya.; Pietraszko, A. Crystal structure of the R2PbS4 (R = Yb and Lu) compounds. J. Alloys Compounds 2008, 453, 143–146. [Google Scholar]
- Hirose, K.; Doi, Y.; Hinatsu, Y. Magnetic properties of EuLn2O4 (Ln = rare earth). J. Solid State Chem. 2009, 182, 1624–1630. [Google Scholar]
- Ishida, Y.; Kinomura, N.; Miyamoto, Y.; Kume, S.; Koizumi, M. Syntheses of EuLn2S4 and SrLn2S4 (Ln = Lu, Yb, Er, Y) with Th3P4 type structure. High Pressure Sci. Technol. 1979, 1, 1026–1032. [Google Scholar]
- Lowe-Ma, C.K.; Vanderah, T.A.; Albrecht-Schmitt, T.E. The ternary yttrium sulfides, CaY2S4, SrY2S4, and BaY2S4: structures and properties. J. Solid State Chem. 1995, 117, 363–372. [Google Scholar]
- Carpenter, J.D.; Hwu, S.-J. Single-crystal structure of BaSm2S4. Acta Crystallogr. 1992, C48, 1164–1167. [Google Scholar]
- Bugaris, D.E.; Ibers, J.A. BaLn2Se4 (Ln = Er, Tm and Yb). Acta Crystallogr. 2009, C65, i60–i62. [Google Scholar]
- Narducci, A.A.; Yang, Y.; Digman, M.A.; Sipes, A.B.; Ibers, J.A. An investigation of rare-earth telluride system BaLn2Te4 (Ln = Sm-Tm, Y): syntheses, crystal structures, and magnetic properties. J. Alloys Compounds 2000, 303-304, 432–439. [Google Scholar]
- Fischer, R.X.; Tillmanns, E. The equivalent isotropic displacement factor. Acta Crystallogr. 1988, C44, 775–776. [Google Scholar]
- Schleid, Th.; Lissner, F. Einkristalle von A-Nd2S3, U-Ho2S3, D-Er2S3 und E-Lu2S3 durch Oxidation reduzierter Chloride der Lanthanide mit Schwefel. Z. Anorg. Allg. Chem. 1999, 625, 1700–1706. [Google Scholar]
- Folchnandt, M.; Schneck, C.; Schleid, Th. Über Sesquiselenide der Lanthanoide: Einkristalle von Ce2Se3 im C-, Gd2Se3 im U- und Lu2Se3 im Z-Typ. Z. Anorg. Allg. Chem. 2004, 630, 149–155. [Google Scholar]
- Liu, Y.; Chen, L.; Wu, L.; Chan, G.H.; van Duyne, R.P. Synthesis, crystal and band structures, and magnetic and optical properties of new CsLnCdTe3 (Ln = La, Pr, Nd, Sm, Gd−Tm, and Lu). Inorg. Chem. 2008, 47, 855–862. [Google Scholar]
- Babo, J.-M. Syntheses and Crystal Structures of Quaternary Chalcogenides Containing Rare-Earth and Coinage Metals. PhD Thesis, University of Stuttgart, Germany, 2010; pp. 48–80. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar]
- Biltz, W. Raumchemie der festen Stoffe; Verlag von Leopold Voss: Leipzig, Germany, 1934; pp. 181–195. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Herrendorf, W.; Bärnighausen, H. HABITUS: A Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE, version 1.06; Fa. Stoe, Darmstadt: Karlsruhe, Germany, 1996. [Google Scholar]
- Hahn, Th.; Wilson, A.J.C. International Tables for Crystallography, 2nd ed.; Kluwer Academic Publishers: Boston, MA, USA, 1992; volume C. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schurz, C.M.; Schleid, T. Single Crystals of the Isotypic Series BaLu2Ch4 (Ch = S, Se and Te) with CaFe2O4-Type Structure. Crystals 2011, 1, 78-86. https://doi.org/10.3390/cryst1030078
Schurz CM, Schleid T. Single Crystals of the Isotypic Series BaLu2Ch4 (Ch = S, Se and Te) with CaFe2O4-Type Structure. Crystals. 2011; 1(3):78-86. https://doi.org/10.3390/cryst1030078
Chicago/Turabian StyleSchurz, Christian M., and Thomas Schleid. 2011. "Single Crystals of the Isotypic Series BaLu2Ch4 (Ch = S, Se and Te) with CaFe2O4-Type Structure" Crystals 1, no. 3: 78-86. https://doi.org/10.3390/cryst1030078




