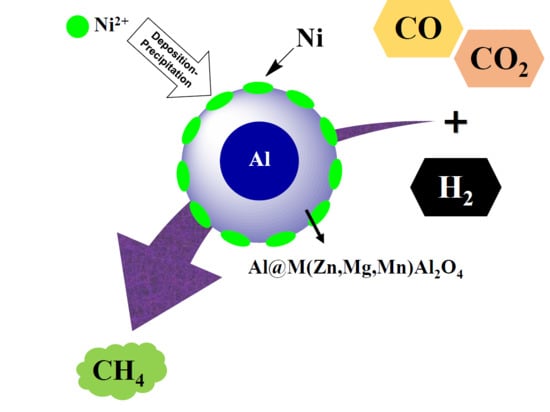
CO2 Methanation over Ni/Al@MAl2O4 (M = Zn, Mg, or Mn) Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Performance
3. Experimental
3.1. Support Synthesis
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Selective catalytic synthesis using the combination of carbon dioxide and hydrogen: Catalytic chess at the interface of energy and chemistry. Angew. Chem. Int. Ed. 2016, 55, 7296–7343. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. CO and CO2 methanation over Ni catalysts supported on alumina with different crystalline phases. Korean J. Chem. Eng. 2017, 34, 3085–3091. [Google Scholar] [CrossRef]
- Burger, T.; Koschany, F.; Thomys, O.; Köhler, K.; Hinrichsen, O. CO2 methanation over Fe-and Mn-promoted co-precipitated Ni-Al catalysts: Synthesis, characterization and catalysis study. Appl. Catal. A Gen. 2018, 558, 44–54. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Z.; Hu, M.; Zuo, C.; Traitangwong, A.; Meeyoo, V.; Li, C.; Zhang, S. Highly active Ni-based catalyst derived from double hydroxides precursor for low temperature CO2 methanation. Ind. Eng. Chem. Res. 2018, 57, 9102–9111. [Google Scholar] [CrossRef]
- Guo, X.; Traitangwong, A.; Hu, M.; Zuo, C.; Meeyoo, V.; Peng, Z.; Li, C. Carbon Dioxide methanation over Nickel-based catalysts supported on various mesoporous material. Energy Fuel. 2018, 32, 3681–3689. [Google Scholar] [CrossRef]
- Le, T.A.; Kang, J.K.; Park, E.D. CO and CO2 methanation over Ni/SiC and Ni/SiO2 catalysts. Top. Catal. 2018, 61, 1537–1544. [Google Scholar] [CrossRef]
- Li, M.; Amari, H.; van Veen, A.C. Metal-oxide interaction enhanced CO2 activation in methanation over ceria supported nickel nanocrystallites. Appl. Catal. B Environ. 2018, 239, 27–35. [Google Scholar] [CrossRef]
- Le, T.A.; Kang, J.K.; Lee, S.H.; Park, E.D. CO and CO2 Methanation over Ni/γ-Al2O3 prepared by deposition-precipitation method. J. Nanosci. Nanotechnol. 2019, 19, 3252–3262. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhuang, J.; Du, J.; Gu, F.; Xu, G.; Zhong, Z.; Liu, Q.; Su, F. Highly dispersed Ni nanocatalysts derived from NiMnAl-hydrotalcites as high-performing catalyst for low-temperature syngas methanation. Catalysts 2019, 9, 282. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Rui, N.; Zhao, B.; Liu, C.J. Improved activity of Ni/MgAl2O4 for CO2 methanation by the plasma decomposition. J. Energy Chem. 2015, 24, 655–659. [Google Scholar] [CrossRef]
- Atzori, L.; Rombi, E.; Meloni, D.; Sini, M.F.; Monaci, R.; Cutrufello, M.G. CO and CO2 co-methanation on Ni/CeO2-ZrO2 soft-templated catalysts. Catalysts 2019, 9, 415. [Google Scholar] [CrossRef]
- Jarenwattananon, N.N.; Glöggler, S.; Otto, T.; Melkonian, A.; Morris, W.; Burt, S.R.; Yaghi, O.M.; Bouchard, L.S. Thermal maps of gases in heterogeneous reactions. Nature 2013, 502, 537. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.; Remue, D.; Hung, J.K. Properties of ceramic foam catalyst supports: Mass and heat transfer. Appl. Catal. A Gen. 2003, 250, 319–329. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández, E.; Alfaro, S.; López Medina, R.; Valverde Aguilar, G.; Albiter, E.; Valenzuela, M.A. High selectivity and stability of Nickel catalysts for CO2 methanation: Support effects. Catalysts 2018, 9, 24. [Google Scholar] [CrossRef]
- Alihosseinzadeh, A.; Nematollahi, B.; Rezaei, M.; Lay, E.N. CO methanation over Ni catalysts supported on high surface area mesoporous nanocrystalline γ-Al2O3 for CO removal in H2-rich stream. Int. J. Hydrogen. Energy. 2015, 40, 1809–1819. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Gama, L.; Ribeiro, M.A.; Barros, B.S.; Kiminami, R.H.A.; Weber, I.T.; Costa, A.C.F.M. Synthesis and characterization of the NiAl2O4, CoAl2O4 and ZnAl2O4 spinels by the polymeric precursors method. J. Alloys. Compd. 2009, 483, 453–455. [Google Scholar] [CrossRef]
- Alvar, E.N.; Rezaei, M.; Alvar, H.N. Synthesis of mesoporous nanocrystalline MgAl2O4 spinel via surfactant assisted precipitation route. Powder. Technol. 2010, 198, 275–278. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Amini, E.; Nemati Lay, E. Preparation of high surface area Ni/MgAl2O4 nanocatalysts for CO selective methanation. Int. J. Hydrogen. Energy. 2018, 43, 772–780. [Google Scholar] [CrossRef]
- Son, I.H.; Kwon, S.; Park, J.H.; Lee, S.J. High coke-resistance MgAl2O4 islands decorated catalyst with minimizing sintering in carbon dioxide reforming of methane. Nano Energy 2016, 19, 58–67. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Pomonis, P.J. Low-temperature synthesis of spinels MAl2O4 (M=Mg, Co, Ni, Cu, Zn) prepared by a sol-gel method. In Progress in Colloid and Polymer Science; Koutsoukos, P.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 17–21. [Google Scholar]
- Mierczynski, P.; Maniukiewicz, W.; Maniecki, T. Comparative studies of Pd, Ru, Ni, Cu/ZnAl2O4 catalysts for the water gas shift reaction. Open Chem. 2013, 11, 912–919. [Google Scholar]
- Maniecki, T.P.; Mierczyński, P.; Maniukiewicz, W.; Gebauer, D.; Jozwiak, W.K. The effect of spinel type support FeAlO3, ZnAl2O4, CrAl3O6 on physicochemical properties of Cu, Ag, Au, Ru supported catalysts for methanol synthesis. Kinet. Catal. 2009, 50, 228–234. [Google Scholar] [CrossRef]
- Mimani, T. Instant synthesis of nanoscale spinel aluminates. J. Alloys. Compd. 2001, 315, 123–128. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D. Core–shell metal–ceramic microstructures: Mechanism of hydrothermal formation and properties as catalyst materials. Chem. Mater. 2016, 28, 2786–2794. [Google Scholar] [CrossRef]
- Louis, C. Deposition-precipitation synthesis of supported metal catalysts. ChemInform 2007, 38. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Maurin, G.; Llewellyn, P. 1—Introduction. In Adsorption by Powders and Porous Solids, 2nd ed.; Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G., Eds.; Academic Press: Oxford, UK, 2014; pp. 1–24. [Google Scholar]
- Pan, Q.; Peng, J.; Sun, T.; Wang, S.; Wang, S. Insight into the reaction route of CO2 methanation: Promotion effect of medium basic sites. Catal. Commun. 2014, 45, 74–78. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Ewald, S.; Hinrichsen, O. On the interaction of CO2 with Ni-Al catalysts. Appl. Catal. A Gen. 2019, 580, 71–80. [Google Scholar] [CrossRef]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Eckle, S.; Anfang, H.G.; Behm, R.J. Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases. J. Phys. Chem. C 2011, 115, 1361–1367. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Louvier-Hernandez, J.F.; Almendarez-Camarillo, A.; Fierro-Gonzalez, J.C. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni. Appl. Catal. B Environ. 2017, 218, 611–620. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Micro- and mesoporous supports for CO2 methanation catalysts: A comparison between SBA-15, MCM-41 and USY zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Y.; Yang, Y.; Lapkin, A.A. Improved stability of Y2O3 supported Ni catalysts for CO2 methanation by precursor-determined metal-support interaction. Appl. Catal. B Environ. 2018, 237, 504–512. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Kwak, J.H.; Szanyi, J. Mechanism of CO2 hydrogenation on Pd/Al2O3 catalysts: Kinetics and transient DRIFTS-MS studies. ACS Catal. 2015, 5, 6337–6349. [Google Scholar] [CrossRef]









| Catalyst | Ni Content a (wt.%) | SBET b (m²/g) | VPore b (cm³/g) | DPore b (nm) | Ni Dispersion c (%) | CASA c (m²/gcat.) | CO2 Uptake d (μmol/gcat.) |
|---|---|---|---|---|---|---|---|
| Ni/γ-Al2O3 (WI) [12] | 10 | 94 | 0.22 | 9.2 | 1.7 | 1.2 | 28 |
| Ni/Al@ZnAl2O4 (WI) | 8 | 36 | 0.10 | 9.5 | 1.3 | 0.9 | 23 |
| Ni/Al@MgAl2O4 (WI) | 10 | 124 | 0.24 | 7.8 | 3.0 | 2.0 | 47 |
| Ni/Al@MnAl2O4 (WI) | 10 | 97 | 0.13 | 5.3 | 3.4 | 2.3 | 41 |
| Ni/ZnAl2O4 (DP) | 9 | 163 | 0.31 | 7.6 | 3.6 | 2.3 | 29 |
| Ni/MgAl2O4 (DP) | 8 | 179 | 0.42 | 9.5 | 7.4 | 4.9 | 46 |
| Ni/Al@ZnAl2O4 (DP) | 8 | 119 | 0.22 | 7.4 | 6.1 | 4.1 | 29 |
| Ni/Al@MgAl2O4 (DP) | 9 | 171 | 0.28 | 6.6 | 9.2 | 6.1 | 57 |
| Ni/Al@MnAl2O4 (DP) | 9 | 129 | 0.22 | 7.0 | 9.7 | 6.4 | 48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.A.; Kim, J.; Jeong, Y.R.; Park, E.D. CO2 Methanation over Ni/Al@MAl2O4 (M = Zn, Mg, or Mn) Catalysts. Catalysts 2019, 9, 599. https://doi.org/10.3390/catal9070599
Le TA, Kim J, Jeong YR, Park ED. CO2 Methanation over Ni/Al@MAl2O4 (M = Zn, Mg, or Mn) Catalysts. Catalysts. 2019; 9(7):599. https://doi.org/10.3390/catal9070599
Chicago/Turabian StyleLe, Thien An, Jieun Kim, Yu Ri Jeong, and Eun Duck Park. 2019. "CO2 Methanation over Ni/Al@MAl2O4 (M = Zn, Mg, or Mn) Catalysts" Catalysts 9, no. 7: 599. https://doi.org/10.3390/catal9070599