Metal Foams as Novel Catalyst Support in Environmental Processes
Abstract
:1. Introduction
2. Results
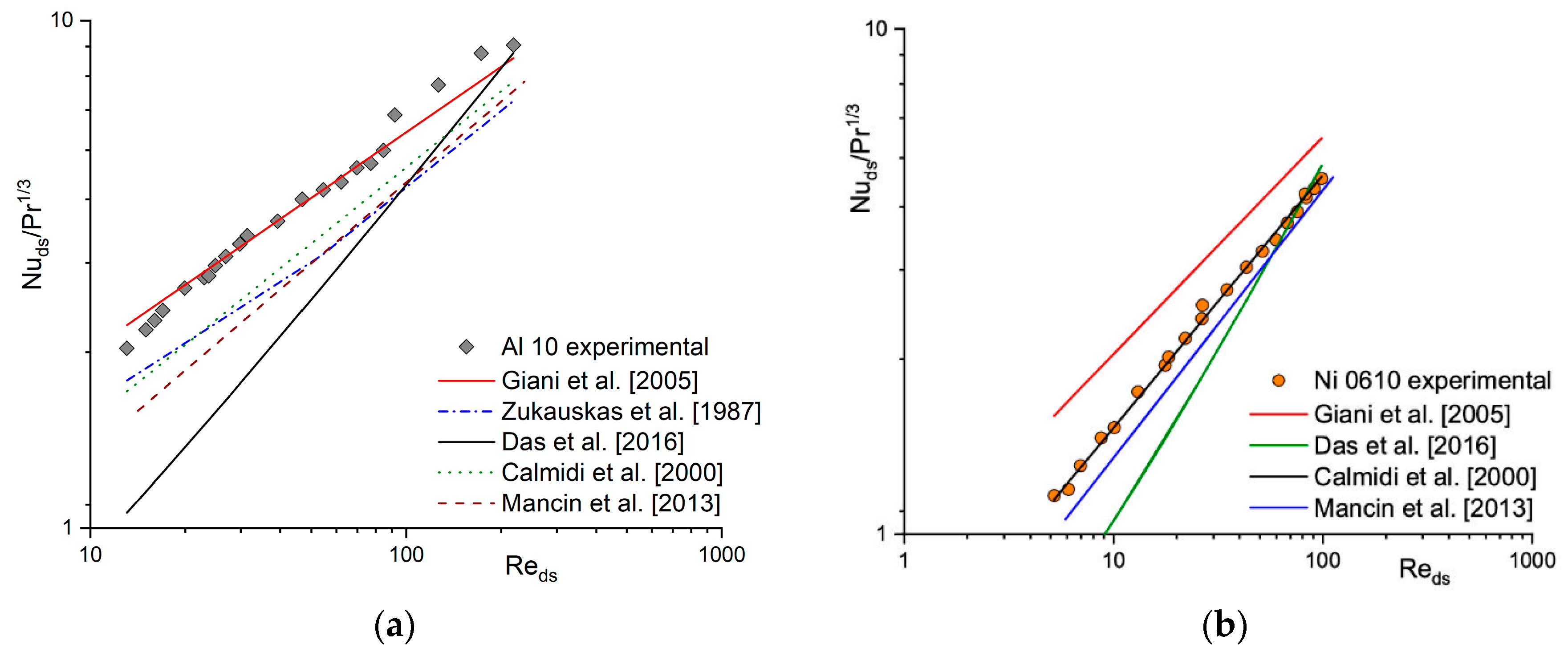
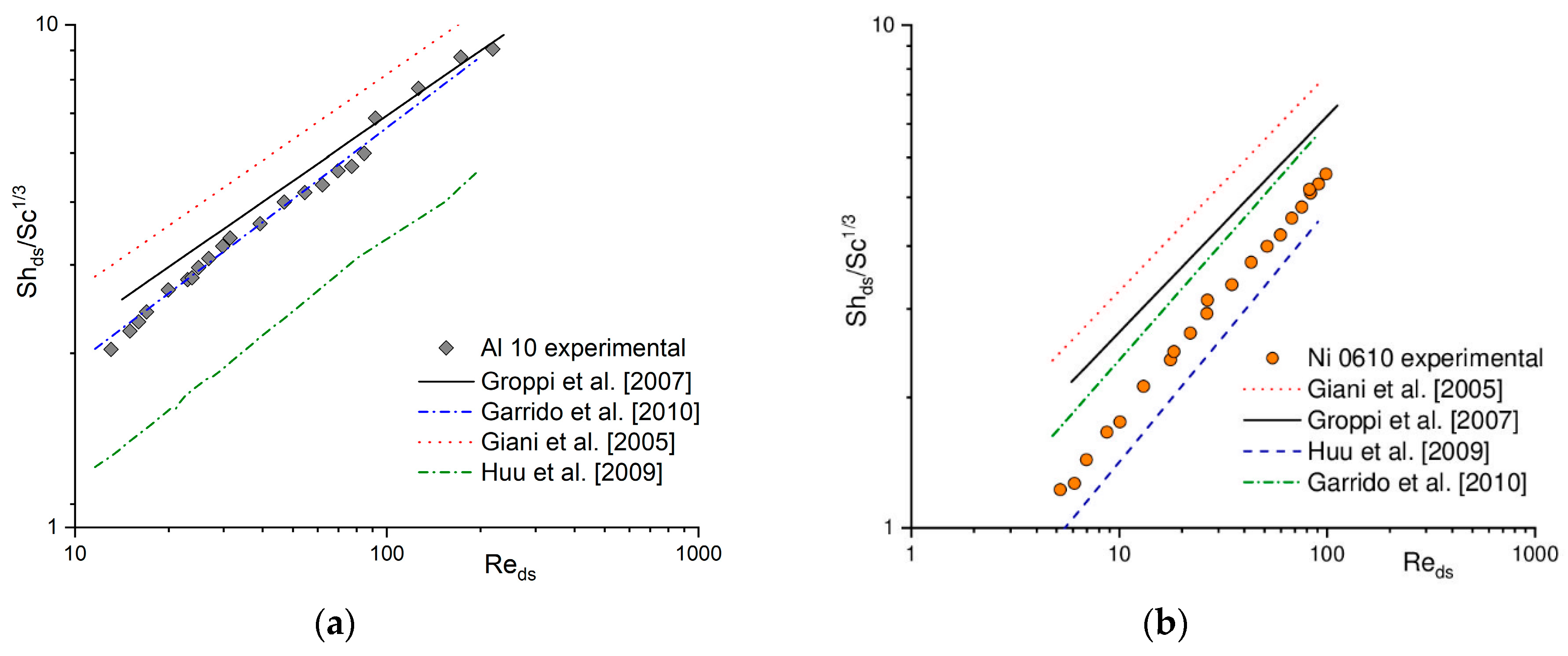
2.1. Heat and Mass Transfer
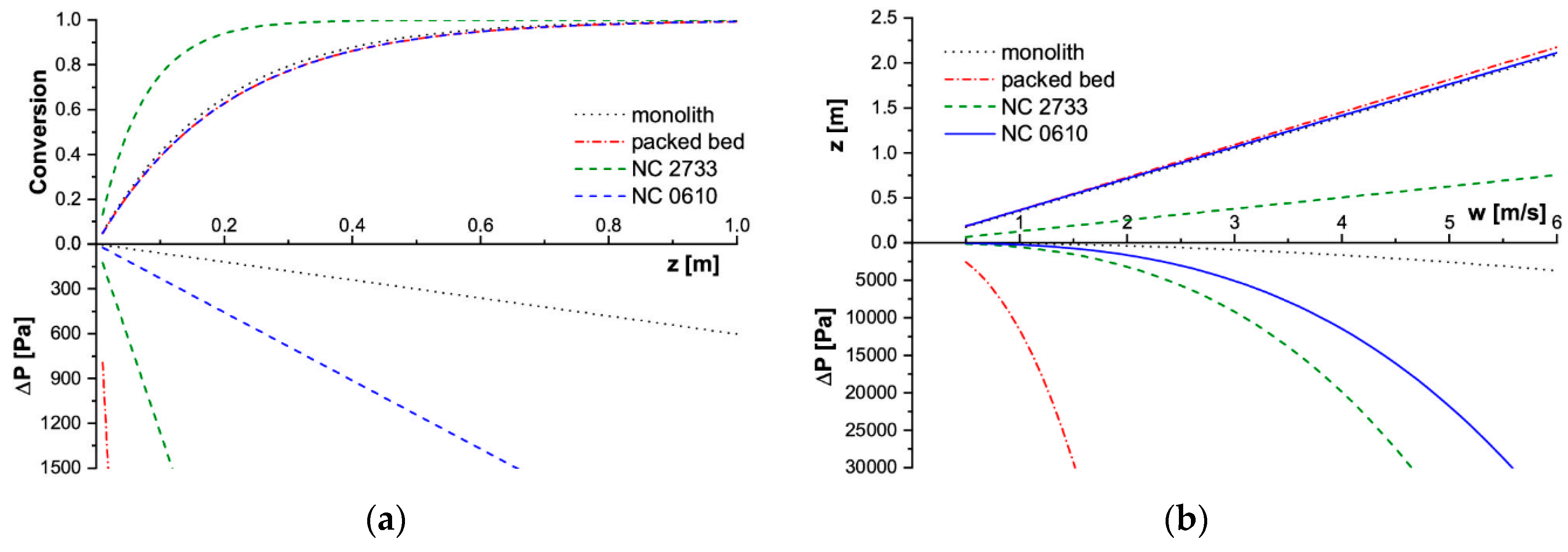
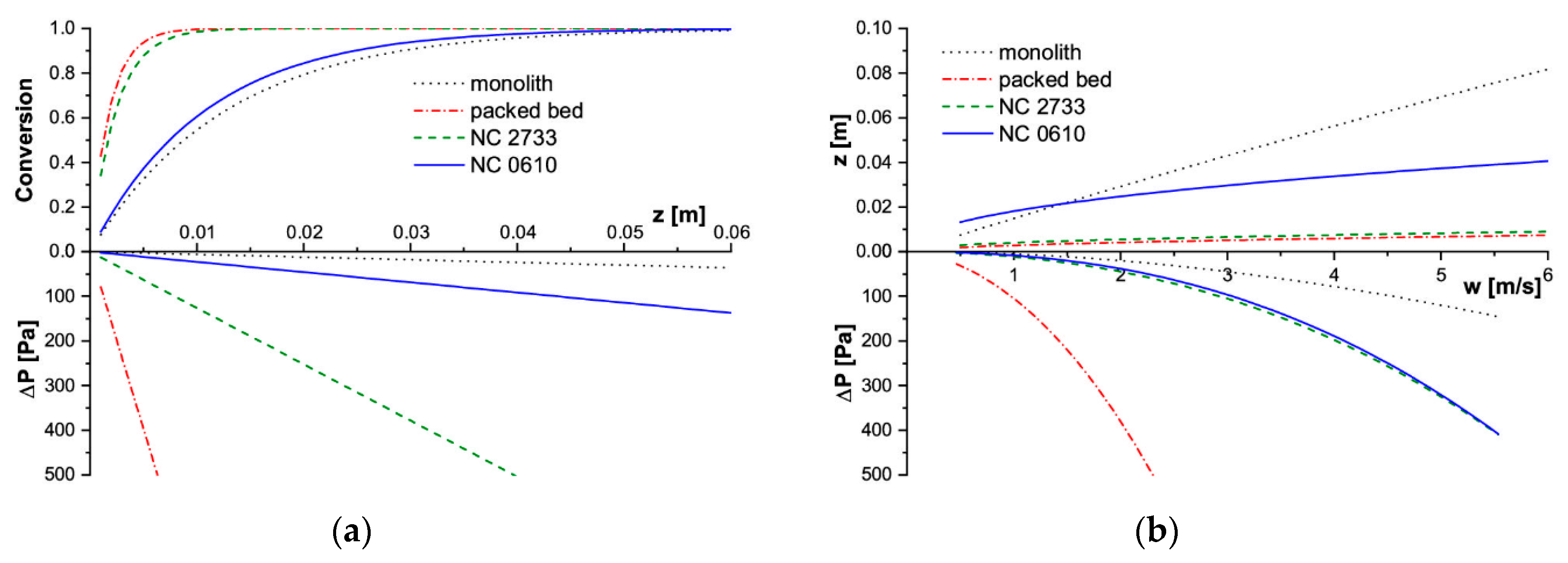
2.2. Assessment of Solid Foams as Catalyst Carriers
- the material balance in the gas phase:with boundary conditions: z = 0; CA = CA0.
- the heat balance for the gas phase:with boundary conditions: z = 0; T = T0, and assuming no heat losses.
- the material balance at the catalyst surface:
- the heat balance at the catalyst surface:
- reaction conversion was calculated along the reactor axis (z) for T = const. and w = const.;
- necessary reactor length to reach 90% conversion was calculated for given flow velocity at T = const.
3. Discussion
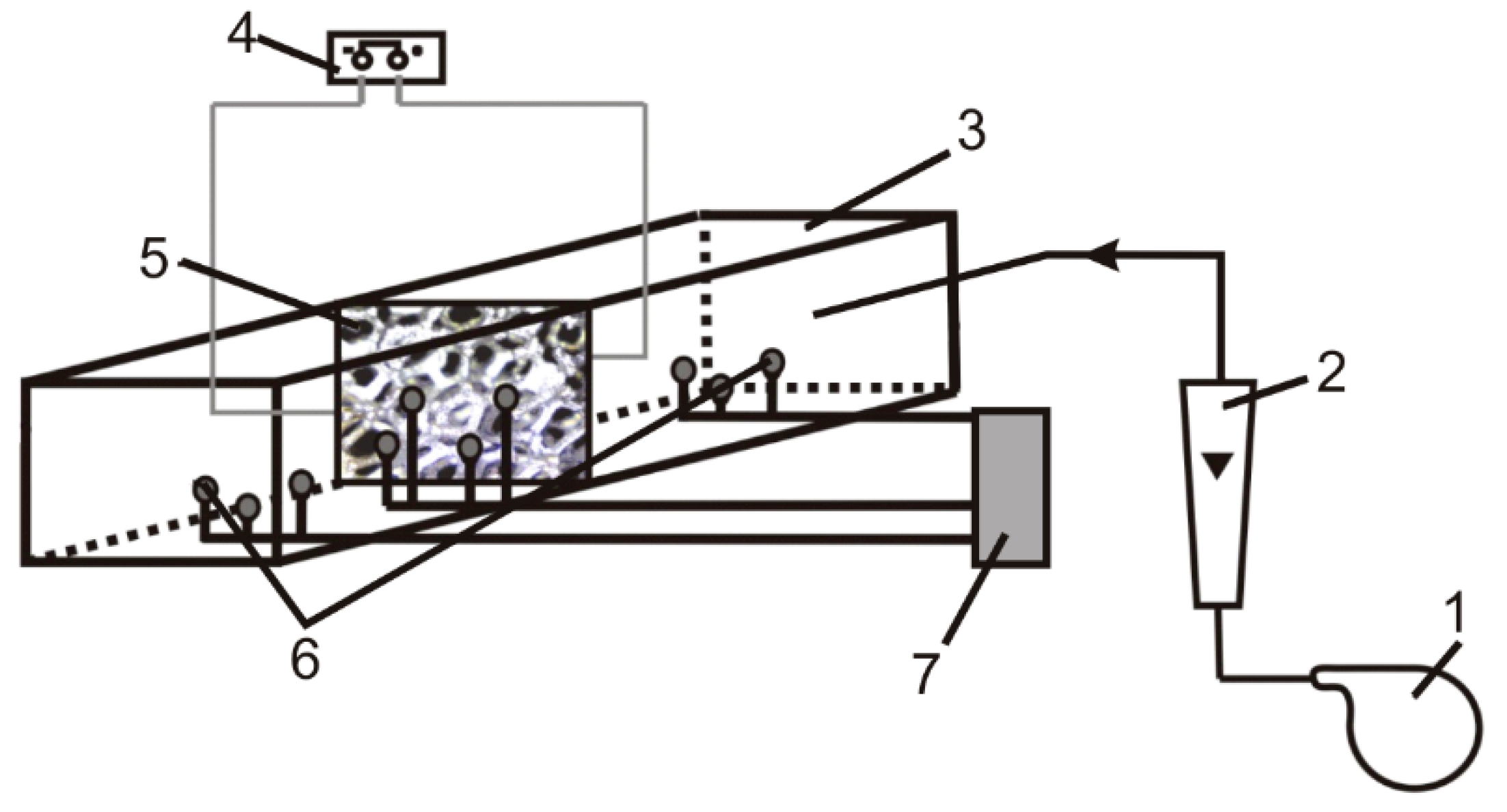
4. Materials and Methods
4.1. Foam Morphology
4.2. Heat and Mass Transfer
4.3. Kinetic Tests
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Symbols
| CA | reagent concentration in gas stream, mol/m3 |
| cp | specific heat, J/(kg∙K) |
| D | diameter, m |
| DA | diffusivity, m2/s |
| dc | cell diameter, m |
| dh | hydraulic diameter, m |
| ds | strut diameter, m |
| ey | average relative error, =, % |
| F | heat transfer area, m2 |
| f | Fanning friction factor, |
| h | heat transfer coefficient, W/(m2∙K) |
| k | thermal conductivity, W/(m∙K) |
| kC | mass transfer coefficient, m/s |
| kr | reaction rate constant |
| k∞ | pre-exponential coefficient in Arrhenius equation, m/s |
| L | bed or channel length, m |
| L+ | dimensionless length for the hydrodynamic entrance region, = L/(D∙Re) |
| L* | dimensionless length for the thermal entrance region, = L/(D∙Re∙Pr) |
| L*M | dimensionless length for the mass transfer entrance region, = L/(D∙Re∙Sc) |
| Nu | Nusselt number, = h∙D/k |
| Pr | Prandtl number, = Cp∙ μ/k |
| Q | heat flow rate, W |
| (−RA) | reaction rate, mol/(m2∙s) |
| Re | Reynolds number, = w∙D∙ρ/(μ∙ε) |
| Sc | Schmidt number, = μ/(ρ∙DA) |
| Sh | Sherwood number, = kC∙D/DA |
| Sv | specific surface area (external surface area per unit volume of bed) m2/m3 |
| T | temperature, K |
| w | superficial velocity, m/s |
| ΔHR | standard heat of reaction, J/mol |
| ΔP | pressure drop, Pa |
| ΔT | logarithmic mean temperature difference between gas and structure surface at the inlet and outlet sides |
| z | reactor axis, m |
| ε | porosity |
| η | effectiveness factor for catalyst |
| μ | dynamic viscosity (Pa∙s) |
| ρ | density (kg/m3) |
Subscripts
| ds | based on strut diameter |
| s | refers to the catalyst surface |
References
- Giani, L.; Groppi, G.; Tronconi, E. Mass-transfer characterization of metallic foams as supports for structured catalysts. Ind. Eng. Chem. Res. 2005, 44, 4993–5002. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Iwaniszyn, M.; Piatek, M.; Korpys, M.; Sindera, K.; Jodlowski, P.J.; Lojewska, J.; Kolodziej, A. Catalytic combustion of low-concentration methane on structured catalyst supports. Ind. Eng. Chem. Res. 2018, 57, 10281–10291. [Google Scholar] [CrossRef]
- Patcas, F.C.; Garrido, G.I.; Kraushaar-Czarnetzki, B. Co oxidation over structured carriers: A comparison of ceramic foams, honeycombs and beads. Chem. Eng. Sci. 2007, 62, 3984–3990. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Yurchenko, E.N.; Feofilov, A.E. Foam-metal catalysts for purification of waste gases and neutralization of automotive emissions. Catal. Today 1996, 29, 67–70. [Google Scholar] [CrossRef]
- Ochońska-Kryca, J.; Iwaniszyn, M.; Piątek, M.; Jodłowski, P.J.; Thomas, J.; Kołodziej, A.; Łojewska, J. Mass transport and kinetics in structured steel foam reactor with cu-zsm-5 catalyst for scr of nox with ammonia. Catal. Today 2013, 216, 135–141. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Li, H.R.; Cai, S.X.; Hu, H.; Fang, C.; Shi, L.Y.; Zhang, D.S. Rational design and in situ fabrication of MnO2@NiCO2O4 nanowire arrays on ni foam as high-performance monolith de-nox catalysts. J. Mater. Chem. A 2015, 3, 11543–11553. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for pd/γ-Al2O3 deposition on metallic foams. Appl. Catal. B 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Totarella, G.; Barison, S.; Musiani, M.; Verlato, E. Highly stable core–shell pt-CeO2 nanoparticles electrochemically deposited onto fecralloy foam reactors for the catalytic oxidation of co. J. Ind. Eng. Chem. 2018, 66, 404–410. [Google Scholar] [CrossRef]
- Cimino, S.; Gerbasi, R.; Lisi, L.; Mancino, G.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Oxidation of co and ch4 on pd–fecralloy foam catalysts prepared by spontaneous deposition. Chem. Eng. J. 2013, 230, 422–431. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Balzarotti, R.; Cristiani, C.; Groppi, G.; Tronconi, E. The influence of the washcoat deposition process on high pore density open cell foams activation for co catalytic combustion. Catalysts 2018, 8, 510. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Ketov, A.A.; Ismagilov, Z.R.; Ushakov, V.A.; Bos, A.; Veringa, H.J. Metal foam supported perovskite catalysts. React. Kinet. Catal. Lett. 1997, 60, 243–250. [Google Scholar] [CrossRef]
- Balzarotti, R.; Beretta, A.; Groppi, G.; Tronconi, E. A comparison between washcoated and packed copper foams for the intensification of methane steam reforming. React. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Fyodorov, A.A.; Shurov, V.A.; Gaisinovich, M.S.; Fyodorova, I.V. Foam metal catalysts with intermediate support for deep oxidation of hydrocarbons. React. Kinet. Catal. Lett. 1994, 53, 347–352. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Fyodorov, A.A.; Gaisinovich, M.S.; Shurov, V.P.; Fyodorova, I.V.; Gubaydulina, T.A. Metal foam catalysts with supported active phase for deep oxidation of hydrocarbons. React. Kinet. Catal. Lett. 1995, 54, 167–172. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Heat transfer characterization of metallic foams. Ind. Eng. Chem. Res. 2005, 44, 9078–9085. [Google Scholar] [CrossRef]
- Das, S.; Deen, N.G.; Kuipers, J.A.M. Direct numerical simulation for flow and heat transfer through random open-cell solid foams: Development of an ibm based cfd model. Catal. Today 2016, 273, 140–150. [Google Scholar] [CrossRef]
- Calmidi, V.V.; Mahajan, R.L. Forced convection in high porositymetal foams. J. Heat Transf. 2000, 122, 557–565. [Google Scholar] [CrossRef]
- Mancin, S.; Zilio, C.; Diani, A.; Rossetto, L. Air forced convection through metal foams: Experimental results and modeling. Int. J. Heat Mass Transf. 2013, 62, 112–123. [Google Scholar] [CrossRef]
- Huu, T.T.; Lacroix, M.; Huu, C.P.; Schweich, D.; Edouard, D. Towards a more realistic modeling of solid foam: Use of the pentagonal dodecahedron geometry. Chem. Eng. Sci. 2009, 64, 5131–5142. [Google Scholar] [CrossRef]
- Garrido, G.I.; Kraushaar-Czarnetzki, B. A general correlation for mass transfer in isotropic and anisotropic solid foams. Chem. Eng. Sci. 2010, 65, 2255–2257. [Google Scholar] [CrossRef]
- Groppi, G.; Giani, L.; Tronconi, E. Generalized correlation for gas/solid mass-transfer coefficients in metallic and ceramic foams. Ind. Eng. Chem. Res. 2007, 46, 3955–3958. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Iwaniszyn, M.; Piątek, M.; Sindera, K.; Korpyś, M.; Jodłowski, P.J.; Łojewska, J.; Kołodziej, A. Interfacial heat and momentum transfer relation for porous media. Int. J. Therm. Sci. 2018, 132, 42–51. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Piatek, M.; Iwaniszyn, M.; Jodlowski, P.J.; Lojewska, J.; Kowalska, J.; Kolodziej, A. In search of governing gas flow mechanism through metal solid foams. Catalysts 2017, 7, 124. [Google Scholar] [CrossRef]
- Piatek, M.; Gancarczyk, A.; Iwaniszyn, M.; Jodlowski, P.J.; Lojewska, J.; Kolodziej, A. Gas-phase flow resistance of metal foams: Experiments and modeling. ALChE J. 2017, 63, 1799–1803. [Google Scholar] [CrossRef]
- Žkauskas, A. Heat transfer from tubes in crossflow. Adv. Heat Transf. 1987, 18, 87–159. [Google Scholar]
- Hawthorne, R.D. Afterburner catalysis—Effects of heat and mass transfer between gas and catalyst surface. ALChE Symp. Ser. 1974, 70, 428–438. [Google Scholar]
- Bird, R.B. Transport Phenomena; Wiley: New York, NY, USA, 1960; 780p. [Google Scholar]
- Wakao, N.; Kaguei, S. Heat and Mass Transfer in Packed Beds; Gordon and Breach Science Publisher: New York, NY, USA, 1982. [Google Scholar]
- Kołodziej, A.; Łojewska, J.; Tyczkowski, J.; Jodłowski, P.; Redzynia, W.; Iwaniszyn, M.; Zapotoczny, S.; Kuśtrowski, P. Coupled engineering and chemical approach to the design of a catalytic structured reactor for combustion of vocs: Cobalt oxide catalyst on knitted wire gauzes. Chem. Eng. J. 2012, 200, 329–337. [Google Scholar] [CrossRef]
- Kolodziej, A.; Lojewska, J. Prospect of compact afterburners based on metallic microstructures. Design and modelling. Top. Catal. 2007, 42, 475–480. [Google Scholar] [CrossRef]
- Jodlowski, P.J.; Kuterasinski, L.; Jedrzejczyk, R.J.; Chlebda, D.; Gancarczyk, A.; Basag, S.; Chmielarz, L. Deno(x) abatement modelling over sonically prepared copper usy and zsm5 structured catalysts. Catalysts 2017, 7, 205. [Google Scholar] [CrossRef]
- Leszczyński, B.; Gancarczyk, A.; Wróbel, A.; Piątek, M.; Łojewska, J.; Kołodziej, A.; Pędrys, R. Global and local thresholding methods applied to x-ray microtomographic analysis of metallic foams. J. Nondestruc. Eval. 2016, 35, 35. [Google Scholar] [CrossRef]
- Kołodziej, A.; Łojewska, J.; Ochońska, J.; Łojewski, T. Short-channel structured reactor: Experiments versus previous theoretical design. Chem. Eng. Process. 2011, 50, 869–876. [Google Scholar] [CrossRef]
- Jodlowski, P.J.; Jedrzejczyk, R.J.; Chlebda, D.K.; Dziedzicka, A.; Kuterasinski, L.; Gancarczyk, A.; Sitarz, M. Non-noble metal oxide catalysts for methane catalytic combustion: Sonochemical synthesis and characterisation. Nanomaterials 2017, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Jodłowski, P.J.; Jędrzejczyk, R.J.; Gancarczyk, A.; Łojewska, J.; Kołodziej, A. New method of determination of intrinsic kinetic and mass transport parameters from typical catalyst activity tests: Problem of mass transfer resistance and diffusional limitation of reaction rate. Chem. Eng. Sci. 2017, 162, 322–331. [Google Scholar] [CrossRef]



| Catalyst Support | Sv (m2/m3) | dh (m) | Correlations 1 |
|---|---|---|---|
| NC 0610 | 1298 | 2.73 × 10−3 | |
| NC 2733 | 3616 | 9.61 × 10−3 | |
| monolith [26] | 1339 | 2.15 × 10−3 | |
| packed bed [27,28] | 1240 | 1.23 × 10−3 | |
| Catalyst | Metal Content (wt %) | Pre-Exponential Factor, k∞ (m/s) | Activation Energy, Ea, (kJ/mol) | Effectiveness Factor, η 1 |
|---|---|---|---|---|
| Cu/USY/s [31] | 3.70 ± 0.04 | 3.32 | 36.21 | 1 |
| Pd/Al2O3 [2] | 3.43 ± 0.9 | 1.07 × 1010 | 110.4 | 0.32 |
| Foam | PPI 1 | ds (m) | dh (m) | ε | Sv (m2/m3) |
|---|---|---|---|---|---|
| Al 10 | 10 | 4.49 × 10−4 | 4.12 × 10−3 | 0.89 | 860.98 |
| Al 20 | 20 | 3.73 × 10−4 | 3.94 × 10−3 | 0.91 | 926.49 |
| Al 40 | 40 | 3.04 × 10−4 | 3.19 × 10−3 | 0.91 | 1139.6 |
| Fecralloy 20 | 8 PPC 2 | 2.17 × 10−4 | 1.55 × 10−3 | 0.83 | 2084.63 |
| Ni 0610 | 6…10 | 4.71 × 10−4 | 4.05 × 10−3 | 0.92 | 908.73 |
| NC 0610 | 6…10 | 5.29 × 10−4 | 2.73 × 10−3 | 0.88 | 1297.72 |
| NC 2733 | 27…33 | 1.35 × 10−4 | 9.61 × 10−3 | 0.87 | 3615.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gancarczyk, A.; Sindera, K.; Iwaniszyn, M.; Piątek, M.; Macek, W.; Jodłowski, P.J.; Wroński, S.; Sitarz, M.; Łojewska, J.; Kołodziej, A. Metal Foams as Novel Catalyst Support in Environmental Processes. Catalysts 2019, 9, 587. https://doi.org/10.3390/catal9070587
Gancarczyk A, Sindera K, Iwaniszyn M, Piątek M, Macek W, Jodłowski PJ, Wroński S, Sitarz M, Łojewska J, Kołodziej A. Metal Foams as Novel Catalyst Support in Environmental Processes. Catalysts. 2019; 9(7):587. https://doi.org/10.3390/catal9070587
Chicago/Turabian StyleGancarczyk, Anna, Katarzyna Sindera, Marzena Iwaniszyn, Marcin Piątek, Wojciech Macek, Przemysław J. Jodłowski, Sebastian Wroński, Maciej Sitarz, Joanna Łojewska, and Andrzej Kołodziej. 2019. "Metal Foams as Novel Catalyst Support in Environmental Processes" Catalysts 9, no. 7: 587. https://doi.org/10.3390/catal9070587






