Production and Surfactant Properties of Tert-Butyl α-d-Glucopyranosides Catalyzed by Cyclodextrin Glucanotransferase
Abstract
:1. Introduction
2. Results and Discussion
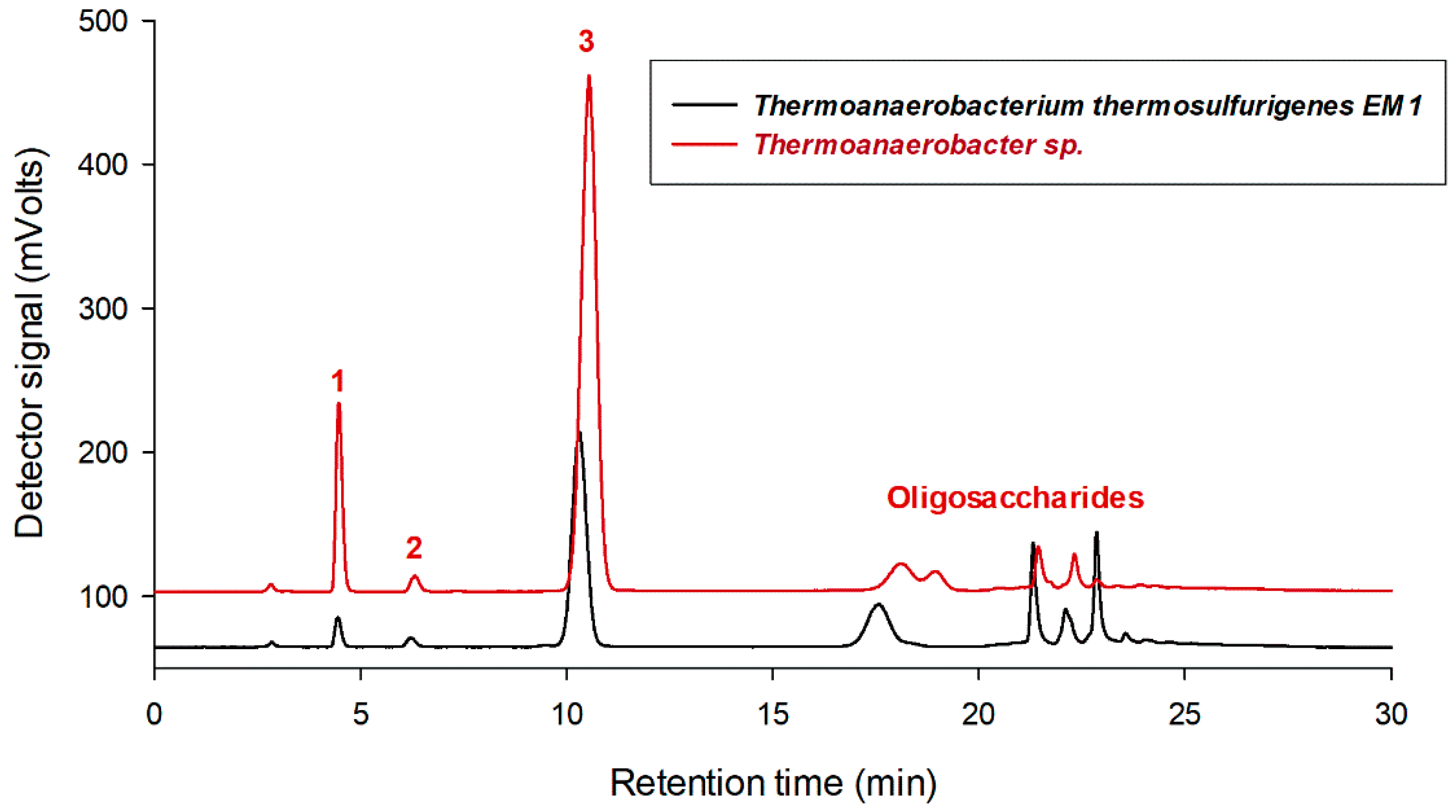
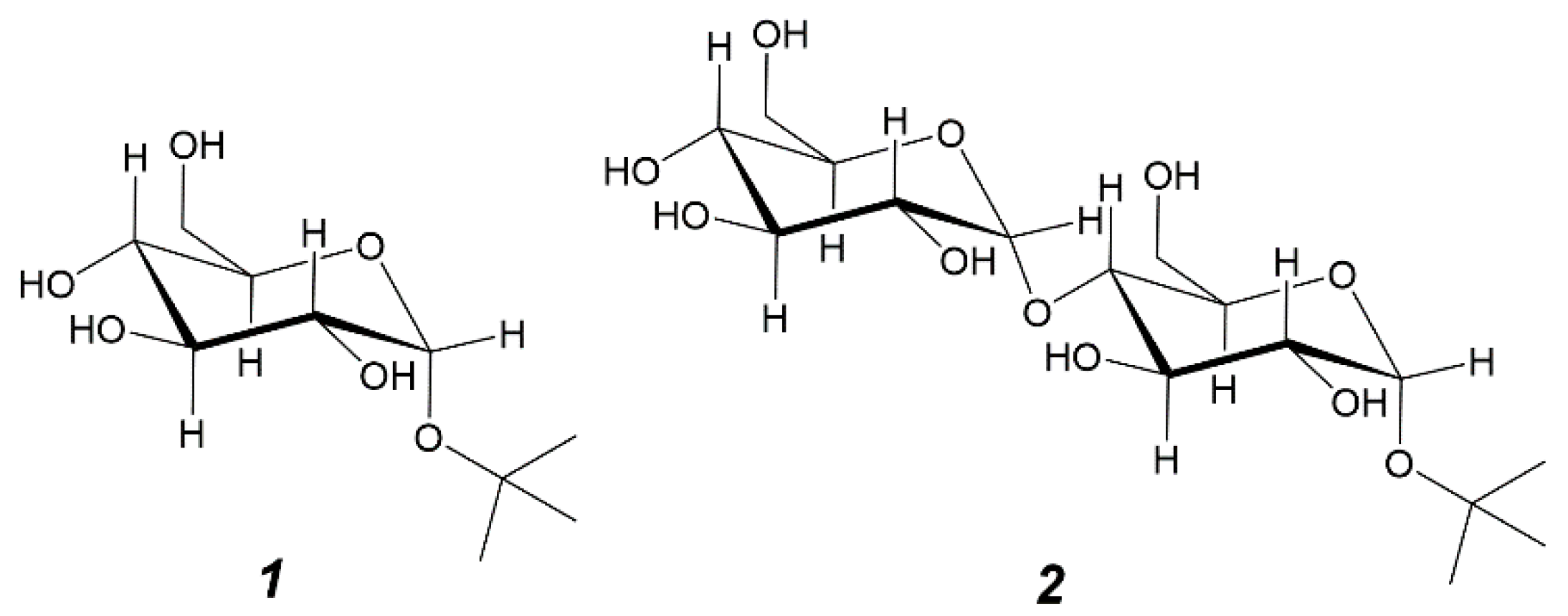
2.1. Glucosylation of Tert-Butyl Alcohol by CGTases and Characterization of Products
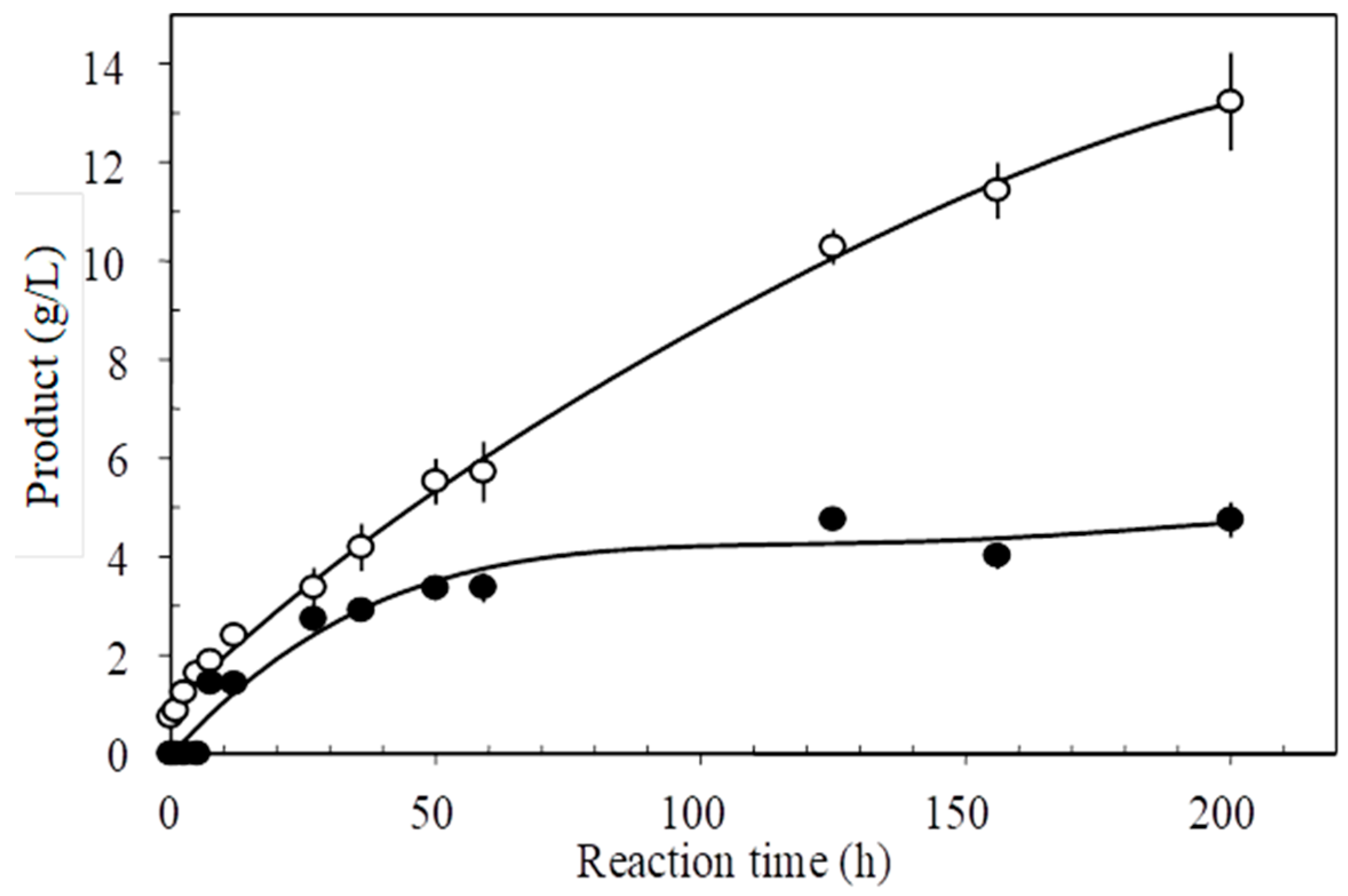
2.2. Progress of Tert-Butyl Alcohol Glucosylation by Thermoanaerobacter sp. CGTase
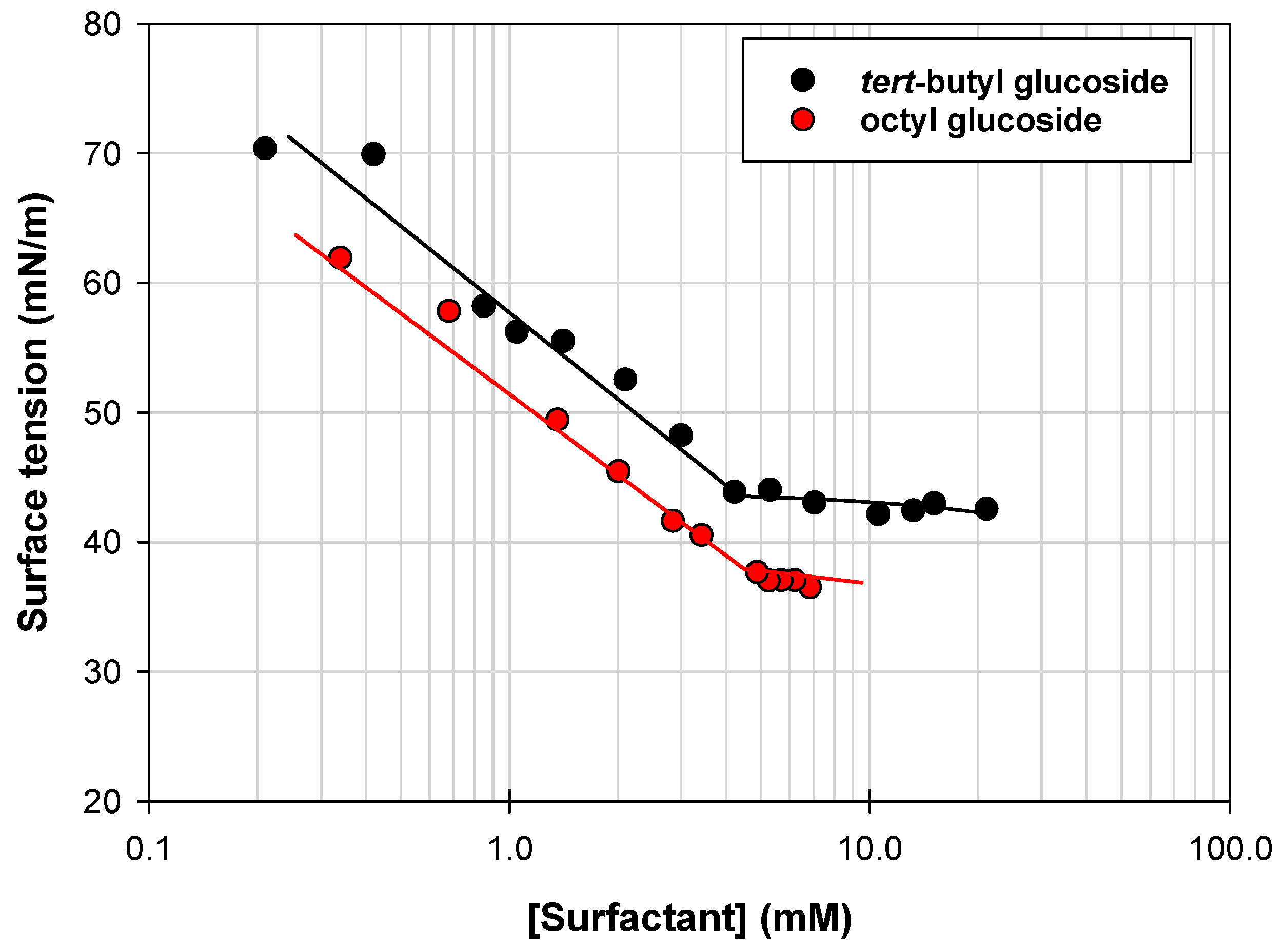
2.3. Surfactant Properties of Tert-Butyl Glucoside
3. Materials and Methods
3.1. Enzymes and Reagents
3.2. Activity Assay
3.3. Enzymatic Synthesis of Tert-Butyl Glucosides
3.4. TLC and HPLC Analyses
3.5. Purification of Tert-Butyl Glucosides
3.6. Mass Spectrometry (MS)
3.7. Nuclear Magnetic Resonance (NMR)
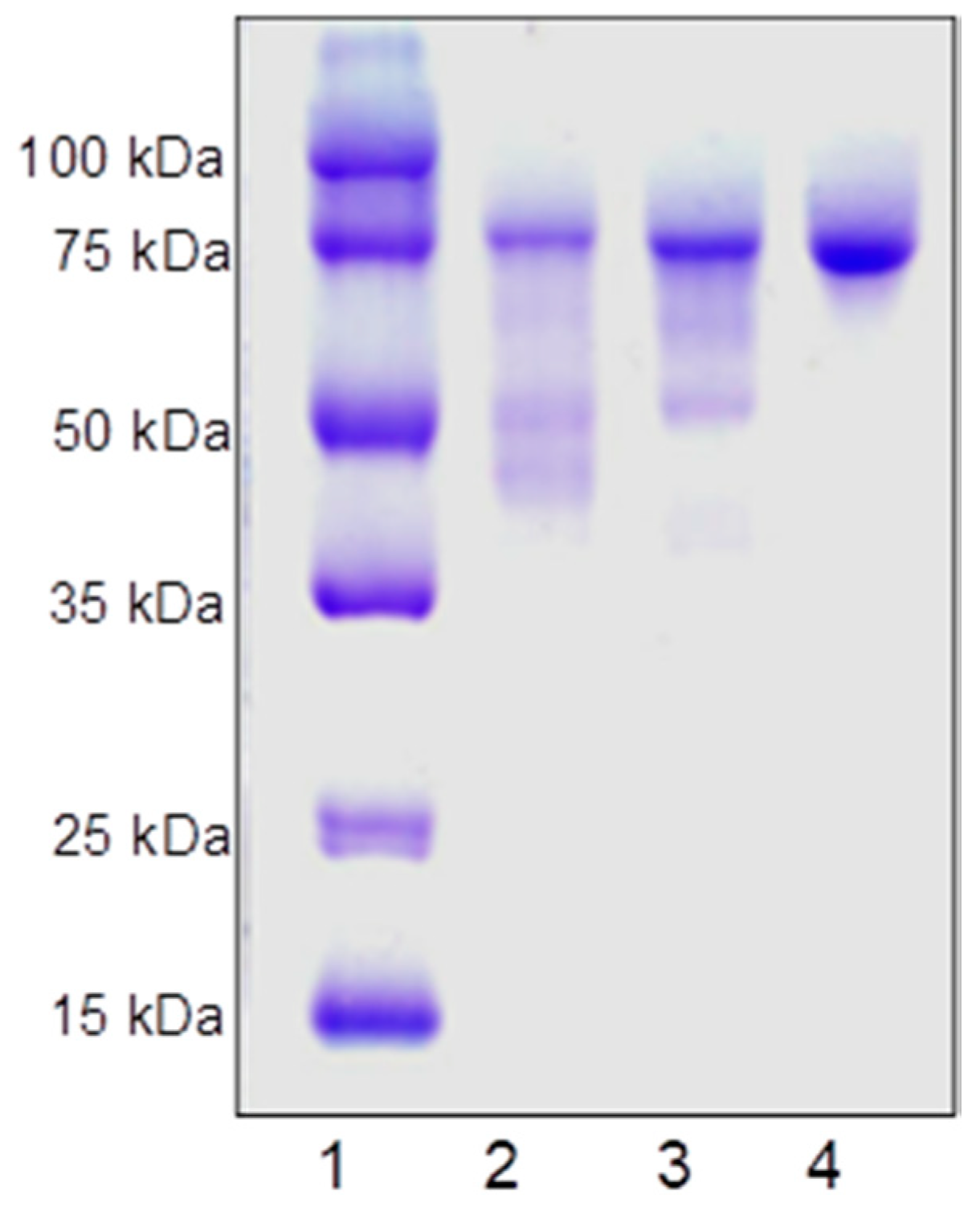
3.8. Enzyme Purification
3.9. Critical Micellar Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uitdehaag, J.C.M.; Van Der Veen, B.A.; Dijkhuizen, L.; Dijkstra, B.W. Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family. Enzyme Microb. Technol. 2002, 30, 295–304. [Google Scholar] [CrossRef]
- Leemhuis, H.; Kelly, R.M.; Dijkhuizen, L. Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl. Microbiol. Biotechnol. 2010, 85, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, É.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, M.; Plou, F.J.; Andersen, C.; Martin, M.T.; Pedersen, S.; Ballesteros, A. Chemical modification of lysine side chains of cyclodextrin glycosyltransferase from Thermoanaerobacter causes a shift from cyclodextrin glycosyltransferase to alpha-amylase specificity. FEBS Lett. 1999, 445, 333–337. [Google Scholar] [CrossRef]
- Wind, R.D.; Buitelaar, R.M.; Dijkhuizen, L. Engineering of factors determining alpha-amylase and cyclodextrin glycosyltransferase specificity in the cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1. Eur. J. Biochem. 1998, 253, 598–605. [Google Scholar] [CrossRef]
- Tonkova, A. Bacterial cyclodextrin glucanotransferase. Enzyme Microb. Technol. 1998, 22, 678–686. [Google Scholar] [CrossRef]
- Alcalde, M.; Plou, F.J.; Martin, M.T.; Valdes, I.; Mendez, E.; Ballesteros, A. Succinylation of cyclodextrin glycosyltransferase from Thermoanaerobacter sp. 501 enhances its transferase activity using starch as donor. J. Biotechnol. 2001, 86, 71–80. [Google Scholar] [CrossRef]
- Martin, M.T.; Cruces, M.A.; Alcalde, M.; Plou, F.J.; Bernabe, M.; Ballesteros, A. Synthesis of maltooligosyl fructofuranosides catalyzed by immobilized cyclodextrin glucosyltransferase using starch as donor. Tetrahedron 2004, 60, 529–534. [Google Scholar] [CrossRef]
- Torres, P.; Poveda, A.; Jimenez-Barbero, J.; Parra, J.L.; Comelles, F.; Ballesteros, A.O.; Plou, F.J. Enzymatic synthesis of α-glucosides of resveratrol with surfactant activity. Adv. Synth. Catal. 2011, 353, 1077–1086. [Google Scholar] [CrossRef]
- Mathew, S.; Adlercreutz, P. Regioselective glycosylation of hydroquinone to α-arbutin by cyclodextrin glucanotransferase from Thermoanaerobacter sp. Biochem. Eng. J. 2013, 79, 187–193. [Google Scholar] [CrossRef]
- Monthieu, C.; Guibert, A.; Taravel, F.R.; Nardin, R.; Combes, D. Purification and characterisation of polyglucosyl-fructosides produced by means of cyclodextrin glucosyl transferase. Biocatal. Biotransf. 2003, 21, 7–15. [Google Scholar] [CrossRef]
- Martin, M.T.; Alcalde, M.; Plou, F.J.; Dijkhuizen, L.; Ballesteros, A. Synthesis of malto-oligosaccharides via the acceptor reaction catalyzed by cyclodextrin glycosyltransferases. Biocatal. Biotransf. 2001, 19, 21–35. [Google Scholar] [CrossRef]
- Han, R.; Ge, B.; Jiang, M.; Xu, G.; Dong, J.; Ni, Y. High production of genistein diglucoside derivative using cyclodextrin glycosyltransferase from Paenibacillus macerans. J. Ind. Microbiol. Biotechnol. 2017, 44, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alfonso, J.L.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Plou, F.J. Efficient α-glucosylation of epigallocatechin gallate catalyzed by cyclodextrin glucanotransferase from Thermoanaerobacter sp. J. Agric. Food Chem. 2018, 66, 7402–7408. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.K.; Bae, K.M.; Kim, S.K. Production of 2-O-alpha-D-glucopyranosyl L-ascorbic acid using cyclodextrin glucanotransferase from Paenibacillus sp. Biotechnol. Lett. 2001, 23, 1793–1797. [Google Scholar] [CrossRef]
- Miranda-Molina, A.; Marquina-Bahena, S.; López-Munguía, A.; Álvarez, L.; Castillo, E. Regioselective glucosylation of inositols catalyzed by Thermoanaerobacter sp. CGTase. Carbohydr. Res. 2012, 360, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Kitahata, S. Application of cyclodextrin glucanotransferase to the synthesis of useful oligosaccharides and glycosides. In Handbook of Industrial Biocatalysts; Hou, C.T., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 419–435. [Google Scholar]
- Do, H.; Sato, T.; Kirimura, K.; Kino, K.; Usami, S. Enzymatic synthesis of L-menthyl alpha-maltoside and L-menthyl alpha-maltooligosides from L-menthyl alpha-glucoside by cyclodextrin glucanotransferase. J. Biosci. Bioeng. 2002, 94, 119–123. [Google Scholar] [CrossRef]
- Kelly, R.M.; Dijkhuizen, L.; Leemhuis, H. The evolution of cyclodextrin glucanotransferase product specificity. Appl. Microbiol. Biotechnol. 2009, 84, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Kurimoto, M.; Tabuchi, A.; Mandai, T.; Shibuya, T.; Chaen, H.; Fukuda, S.; Sugimoto, T.; Tsujisaka, Y. Synthesis of glycosyl-trehaloses by cyclomaltodextrin glucanotransferase through the transglycosylation reaction. Biosci. Biotechnol. Biochem. 1997, 61, 1146–1149. [Google Scholar] [CrossRef]
- Shimoda, K.; Hara, T.; Hamada, H.; Hamada, H. Synthesis of curcumin β-maltooligosaccharides through biocatalytic glycosylation with Strophanthus gratus cell culture and cyclodextrin glucanotransferase. Tetrahedron Lett. 2007, 48, 4029–4032. [Google Scholar] [CrossRef]
- González-Alfonso, J.; Rodrigo-Frutos, D.; Belmonte-Reche, E.; Peñalver, P.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Hirose, Y.; Polaina, J.; Morales, J.; et al. Enzymatic synthesis of a novel pterostilbene α-glucoside by the combination of cyclodextrin glucanotransferase and amyloglucosidase. Molecules 2018, 23, 1271. [Google Scholar] [CrossRef] [PubMed]
- González-Alfonso, J.L.; Míguez, N.; Padilla, J.D.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A.O.; Sandoval, G.; Plou, F.J. Optimization of regioselective α-glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. Molecules 2018, 23, 2885. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liu, L.; Shin, H.; Chen, R.R.; Du, G.; Chen, J. Site-saturation engineering of lysine 47 in cyclodextrin glycosyltransferase from Paenibacillus macerans to enhance substrate specificity towards maltodextrin for enzymatic synthesis of 2-O-d-glucopyranosyl-L-ascorbic acid (AA-2G). Appl. Microbiol. Biotechnol. 2013, 97, 5851–5860. [Google Scholar] [CrossRef] [PubMed]
- Wind, R.D.; Uitdehaag, J.C.M.; Buitelaar, R.M.; Dijkstra, B.W.; Dijkhuizen, L. Engineering of cyclodextrin product specificity and pH optima of the thermostable cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1. J. Biol. Chem. 1998, 273, 5771–5779. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.S.; Lee, H.W.; Jeon, H.Y.; Choi, H.J.; Choung, W.J.; Shim, J.H. Development and characterization of cyclodextrin glucanotransferase as a maltoheptaose-producing enzyme using site-directed mutagenesis. Protein Eng. Des. Sel. 2015, 28, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.T.; Plou, F.J.; Alcalde, M.; Ballesteros, A. Immobilization on Eupergit C of cyclodextrin glucosyltransferase (CGTase) and properties of the immobilized biocatalyst. J. Mol. Catal. B Enzym. 2003, 21, 299–308. [Google Scholar] [CrossRef]
- Iyer, J.L.; Shetty, P.; Pai, J.S. Immobilisation of cyclodextrin glucanotransferase from Bacillus circulans ATCC 21783 on purified seasand. J. Ind. Microbiol. Biotechnol. 2003, 30, 47–51. [Google Scholar] [CrossRef]
- Delbourg, M.F.; Drouet, P.; Demoraes, F.; Thomas, D.; Barbotin, J.N. Effect of PEG and other additives on cyclodextrin production by Bacillus macerans cyclomaltodextrin-glycosyl-transferase. Biotechnol. Lett. 1993, 15, 157–162. [Google Scholar] [CrossRef]
- Morita, T.; Yoshida, N.; Karube, I. A novel synthesis method for cyclodextrins from maltose in water-organic solvent systems. Appl. Biochem. Biotechnol. 1996, 56, 311–324. [Google Scholar] [CrossRef]
- Blackwood, A.D.; Bucke, C. Addition of polar organic solvents can improve the product selectivity of cyclodextrin glycosyltransferase-Solvent effects on CGTase. Enzyme Microb. Technol. 2000, 27, 704–708. [Google Scholar] [CrossRef]
- Qi, Q.S.; Zimmermann, W. Cyclodextrin glucanotransferase: From gene to applications. Appl. Microbiol. Biotechnol. 2005, 66, 475–485. [Google Scholar] [CrossRef] [PubMed]
- van Rantwijk, F.; Oosterom, M.W.V.; Sheldon, R.A. Glycosidase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B Enzym. 1999, 6, 511–532. [Google Scholar] [CrossRef]
- Svasti, J.; Phongsak, T.; Sarnthima, R. Transglucosylation of tertiary alcohols using cassava beta-glucosidase. Biochem. Biophys. Res. Commun. 2003, 305, 470–475. [Google Scholar] [CrossRef]
- Huneke, F.U.; Bailey, D.; Nucci, R.; Cowan, D. Sulfolobus solfataricus β-glycosidase-catalysed synthesis of sugar-alcohol conjugates in the presence of organic solvents. Biocatal. Biotransf. 2000, 18, 291–299. [Google Scholar] [CrossRef]
- Mazzaferro, L.S.; Weiz, G.; Braun, L.; Kotik, M.; Pelantová, H.; Křen, V.; Breccia, J.D. Enzyme-mediated transglycosylation of rutinose (6-O-α-L-rhamnosyl-D-glucose) to phenolic compounds by a diglycosidase from Acremonium sp. DSM 24697. Biotechnol. Appl. Biochem. 2019, 66, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pérez, B.; Anankanbil, S.; Li, J.; Gao, R.; Guo, Z. Enhanced synthesis of alkyl galactopyranoside by Thermotoga naphthophila β-galactosidase catalyzed transglycosylation: Kinetic insight of a functionalized ionic liquid-mediated system. ACS Sustain. Chem. Eng. 2017, 5, 2006–2014. [Google Scholar] [CrossRef]
- García, C.; Hoyos, P.; Hernáiz, M.J. Enzymatic synthesis of carbohydrates and glycoconjugates using lipases and glycosidases in green solvents. Biocatal. Biotransf. 2018, 36, 131–140. [Google Scholar] [CrossRef]
- von Rybinski, W.; Hill, K. Alkyl polyglycosides-Properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Sarney, D.B.; Vulfson, E.N. Application of enzymes to the synthesis of surfactants. Trends Biotechnol. 1995, 13, 164–172. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Peñalver, P.; Ballesteros, A.O.; Morales, J.C.; Plou, F.J. Effect of α-glucosylation on the stability, antioxidant properties, toxicity, and neuroprotective activity of (–)-epigallocatechin gallate. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef]
- Plou, F.J.; Martin, M.T.; Gomez de Segura, A.; Alcalde, M.; Ballesteros, A. Glucosyltransferases acting on starch or sucrose for the synthesis of oligosaccharides. Can. J. Chem. 2002, 80, 743–752. [Google Scholar] [CrossRef]
- Svensson, D.; Ulvenlund, S.; Adlercreutz, P. Efficient synthesis of a long carbohydrate chain alkyl glycoside catalyzed by cyclodextrin glycosyltransferase (CGTase). Biotech. Bioeng. 2009, 104, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Kitahata, S.; Tominaga, Y.; Kiku, Y.; Ando, K.; Kawashima, Y.; Takenishi, S. Syntheses of glucosides with trimethylolpropane and 2 related polyol moieties by cyclodextrin glucanotransferase and their esterification by lipase. J. Ferment. Bioeng. 1992, 73, 237–238. [Google Scholar] [CrossRef]
- Larsson, J.; Svensson, D.; Adlercreutz, P. α-Amylase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B Enzym. 2005, 37, 84–87. [Google Scholar] [CrossRef]
- Otto, R.T.; Bornscheuer, U.T.; Syldatk, C.; Schmid, R.D. Synthesis of aromatic n-alkyl-glucoside esters in a coupled β-glucosidase and lipase reaction. Biotechnol. Lett. 1998, 20, 437–440. [Google Scholar] [CrossRef]
- Fischer, L.; Bromann, R.; Kengen, S.W.M.; de Vos, W.M.; Wagner, F. Catalytical potency of β-glucosidase from the extremophile Pyrococcus furiosus in glucoconjugate synthesis. Bio/Technology 1996, 14, 88–91. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, Y.; Li, L.; Yu, X.; Kusakabe, I.; Kitaoka, M.; Hayashi, K. Transglycosylation reaction of xylanase B from the hyperthermophilic Thermotoga maritima with the ability of synthesis of tertiary alkyl β-d-xylobiosides and xylosides. J. Biotechnol. 2004, 114, 125–134. [Google Scholar] [CrossRef]
- Kongsaeree, P.T.; Ratananikom, K.; Choengpanya, K.; Tongtubtim, N.; Sujiwattanarat, P.; Porncharoennop, C.; Onpium, A.; Svasti, J. Substrate specificity in hydrolysis and transglucosylation by family 1 β-glucosidases from cassava and Thai rosewood. J. Mol. Catal. B Enzym. 2010, 67, 257–265. [Google Scholar] [CrossRef]
- Simerská, P.; Kuzma, M.; Monti, D.; Riva, S.; Macková, M.; Křen, V. Unique transglycosylation potential of extracellular α-D-galactosidase from Talaromyces flavus. J. Mol. Catal. B Enzym. 2006, 39, 128–134. [Google Scholar] [CrossRef]
- Tanaka, T.; Kikuta, N.; Kimura, Y.; Shoda, S.I. Metal-catalyzed stereoselective and protecting-group-free synthesis of 1,2-cis-glycosides using 4,6-dimethoxy-1,3,5-triazin-2-yl glycosides as glycosyl donors. Chem. Lett. 2015, 44, 846–848. [Google Scholar] [CrossRef]
- Zamost, B.L.; Nielsen, H.K.; Starnes, R.L. Thermostable enzymes for industrial applications. J. Ind. Microbiol. 1991, 8, 71–81. [Google Scholar] [CrossRef]
- De Roode, B.M.; Franssen, M.C.R.; Van Der Padt, A.; Boom, R.M. Perspectives for the industrial enzymatic production of glycosides. Biotechnol. Prog. 2003, 19, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Wasylewski, Z.; Kozik, A. Protein-nonionic detergent interaction-interaction of bovine serum-albumin with alkyl glucosides studied by equilibrium dialysis and infrared spectroscopy. Eur. J. Biochem. 1979, 95, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Haga, K.; Yamane, K. The transglycosylation reaction of cyclodextrin glucanotransferase is operated by a ping-pong mechanism. FEBS Lett. 1994, 337, 66–70. [Google Scholar] [CrossRef]
- International Standard ISO 304-1985. Surface active agents. Determination of surface tension by drawing up liquid films. Available online: https://www.iso.org/standard/4238.html (accessed on 29 June 2019).
| Source | Protein (mg/mL) | Transglycosylation Activity (U/mL) a |
|---|---|---|
| Thermoanaerobacter sp. | 2.88 | 37.1 |
| Thermoanaerobacterium thermosulfurigenes EM1 | 0.77 | 43.1 |
| Geobacillus sp. | 2.63 | 66.4 |
| Bacillus circulans 251 | 2.79 | 152.7 |
| Property | Tert-butyl-α-glucoside | Octyl-α-glucoside |
|---|---|---|
| CMC (mM) | 4.0–4.5 | 4.8–5.0 |
| Surface tension at CMC (mN/m) | 43.9 | 37.0 |
| pC20a | 2.72 | 3.00 |
| A (Å2)b | 66.5 | 45.1 |
| Time | Acetonitrile | Water |
|---|---|---|
| 0–12 min | 78% | 22% |
| 12–15 min | 78% → 50% | 22% → 50% |
| 15–20 min | 50% | 50% |
| 20–21 min | 50% → 78% | 50% → 22% |
| 21–30 min | 78% | 22% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Arellano, H.; Gonzalez-Alfonso, J.L.; Ubilla, C.; Comelles, F.; Alcalde, M.; Bernabé, M.; Parra, J.-L.; Ballesteros, A.O.; Plou, F.J. Production and Surfactant Properties of Tert-Butyl α-d-Glucopyranosides Catalyzed by Cyclodextrin Glucanotransferase. Catalysts 2019, 9, 575. https://doi.org/10.3390/catal9070575
Garcia-Arellano H, Gonzalez-Alfonso JL, Ubilla C, Comelles F, Alcalde M, Bernabé M, Parra J-L, Ballesteros AO, Plou FJ. Production and Surfactant Properties of Tert-Butyl α-d-Glucopyranosides Catalyzed by Cyclodextrin Glucanotransferase. Catalysts. 2019; 9(7):575. https://doi.org/10.3390/catal9070575
Chicago/Turabian StyleGarcia-Arellano, Humberto, Jose L. Gonzalez-Alfonso, Claudia Ubilla, Francesc Comelles, Miguel Alcalde, Manuel Bernabé, José-Luis Parra, Antonio O. Ballesteros, and Francisco J. Plou. 2019. "Production and Surfactant Properties of Tert-Butyl α-d-Glucopyranosides Catalyzed by Cyclodextrin Glucanotransferase" Catalysts 9, no. 7: 575. https://doi.org/10.3390/catal9070575







