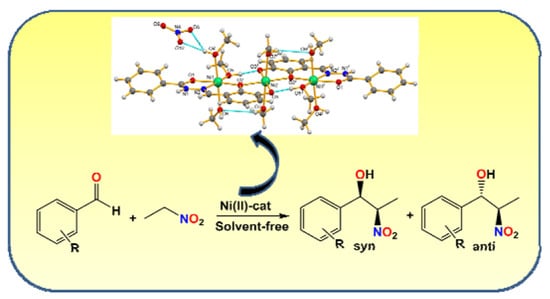
Ni(II)-Aroylhydrazone Complexes as Catalyst Precursors Towards Efficient Solvent-Free Nitroaldol Condensation Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterizations
2.2. General Description of the Crystal Structures
2.3. Catalytic Studies
3. Materials and Methods
3.1. Syntheses of the Pro-Ligand H2L
3.2. Synthesis of the Nickel(II) Complexes
- Synthesis of [Ni2(L1)2(MeOH)4] (1)
- Synthesis of [Ni2(L1)2(DMF)4] [Ni2(L1)2(DMF)2(H2O)2]·2DMF (1A)
- Synthesis of [Ni3(HL2)2(CH3OH)8]·(NO3)2 (2)
3.3. X-ray Measurements
3.4. Catalytic studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christensen, C.; Juhl, K.; Jorgensen, K.A. Catalytic Asymmetric Henry Reactions—A Simple Approach to Optically Active β-Nitro α-Hydroxy Esters. Chem. Commun. 2001, 2222–2223. [Google Scholar] [CrossRef]
- Ballini, R.; Palmieria, A.; Righi, P. Highly Efficient One- or Two-Step Sequences for the Synthesis of Fine Chemicals from Versatile Nitroalkanes. Tetrahedron. 2007, 63, 12099–12121. [Google Scholar] [CrossRef]
- Ballini, R.; Barboni, L.; Giarlo, G. Nitroalkanes in Aqueous Medium as an Efficient and Eco-Friendly Source for the One-Pot Synthesis of 1,4-Diketones, 1,4-Diols, δ-Nitroalkanols, and Hydroxytetrahydrofurans. J. Org. Chem. 2003, 68, 9173–9176. [Google Scholar] [CrossRef] [PubMed]
- Naïli, H.; Hajlaoui, F.; Mhiri, T.; Mac Leod, T.C.O.; Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J.L. 2-Dihydromethylpiperazinediium-MII (MII = CuII, FeII, CoII, ZnII) Double Sulfates and their Catalytic Activity in Diastereoselective Nitroaldol (Henry) Reaction. Dalton Trans. 2013, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Opanasenko, M.; Cejka, J.; Garcia, H. Metal Organic Frameworks as Solid Catalysts in Condensation Reactions of Carbonyl Groups. Adv. Synth. Catal. 2013, 355, 247–268. [Google Scholar] [CrossRef]
- Doyle, A.G.; Jacobsen, E.N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007, 107, 5713–5743. [Google Scholar] [CrossRef] [PubMed]
- Kopylovich, M.N.; Mac Leod, T.C.O.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Zinc(II) ortho-Hydroxyphenylhydrazo-β-diketonate Complexes and their Catalytic Ability Towards Diastereoselective Nitroaldol (Henry) Reaction. Dalton Trans. 2011, 40, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Kanai, M.; Matsunaga, S.; Kumagai, N. Multimetallic Multifunctional Catalysts for Asymmetric Reactions: In Bifunctional Molecular Catalysis; Ikariya, T., Shibasaki, M., Eds.; Topics in Organometallic Chemistry; Springer: Berlin, Germany, 2011; Volume 37. [Google Scholar]
- Kopylovich, M.N.; Mizar, A.; Guedes da Silva, M.F.C.; Mac Leod, T.C.O.; Mahmudov, K.T.; Pombeiro, A.J.L. Template Syntheses of Copper(II) Complexes from Arylhydrazones of Malononitrile and their Catalytic Activity towards Alcohol Oxidations and the Nitroaldol Reaction: Hydrogen Bond-Assisted Ligand Liberation and E/Z Isomerisation. Chem. Eur. J. 2013, 19, 588–600. [Google Scholar] [CrossRef]
- Xu, K.; Lai, G.; Zha, Z.; Pan, S.; Chen, H.; Wang, Z. A Highly anti-Selective Asymmetric Henry Reaction Catalyzed by a Chiral Copper Complex: Applications to the Syntheses of (+)-Spisulosine and a Pyrroloisoquinoline Derivative. Chem. Eur. J. 2012, 18, 12357–12362. [Google Scholar] [CrossRef]
- Yao, L.; Wei, Y.; Wang, P.; He, W.; Zhang, S. Promotion of Henry Reactions Using Cu(OTf)2 and a Sterically Hindered Schiff base: Access to Enantioenriched β-Hydroxynitroalkanes. Tetrahedron 2012, 68, 9119–9124. [Google Scholar] [CrossRef]
- Ono, N. The Nitro Group in Organic Synthesis Willey-VCH; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Yamaguchi, M.; Shiraishi, T.; Hirama, M. Asymmetric Michael Addition of Malonate Anions to Prochiral Acceptors Catalyzed by l-Proline Rubidium Salt. J. Org. Chem. 1996, 61, 3520–3530. [Google Scholar] [CrossRef]
- Varma, R.S.; Dahiya, R.; Kumar, S. Microwave-assisted Henry reaction: Solventless synthesis of conjugated nitroalkenes. Tetrahedron Lett. 1997, 38, 5131–5134. [Google Scholar] [CrossRef]
- Noland, W.E. The NEF Reaction. Chem. Rev. 1955, 55, 137–155. [Google Scholar] [CrossRef]
- Sasai, H.; Suzuki, T.; Arai, S.; Arai, T.; Shibasaki, M. Basic character of rare earth metal alkoxides. Utilization in catalytic carbon-carbon bond-forming reactions and catalytic asymmetric nitroaldol reactions. J. Am. Chem. Soc. 1992, 114, 4418–4420. [Google Scholar] [CrossRef]
- Das, A.; Kureshy, R.I.; Prathap, K.J.; Choudhary, M.K.; Rao, G.V.; Khan, N.-U.H.; Abdi, S.H.; Bajaj, H.C. Chiral recyclable Cu(II)-catalysts in nitroaldol reaction of aldehydes with various nitroalkanes and its application in the synthesis of a valuable drug (R)-isoproterenol. Appl. Catal. A: Gen. 2013, 459, 97–105. [Google Scholar] [CrossRef]
- Das, A.; Kureshy, R.I.; Subramanian, P.S.; Khan, N.-U.H.; Abdi, S.H.R.; Bajaj, H.C. Synthesis and characterization of chiral recyclable dimeric copper(ii)–salen complexes and their catalytic application in asymmetric nitroaldol (Henry) reaction. Catal. Sci. Technol. 2014, 4, 411–418. [Google Scholar] [CrossRef]
- Selvakumar, P.M.; Suresh, E.; Subramanian, P. Single stranded helical supramolecular architecture with a left handed helical water chain in ternary copper(II) tryptophan/diamine complexes. Polyhedron 2009, 28, 245–252. [Google Scholar] [CrossRef]
- Kitagaki, S.; Ueda, T.; Mukai, C. Planar chiral [2.2]paracyclophane-based bis(thiourea) catalyst: Application to asymmetric Henry reaction. Chem. Commun. 2013, 49, 4030. [Google Scholar] [CrossRef]
- Nitabaru, T.; Nojiri, A.; Kobayashi, M.; Kumagai, N.; Shibasaki, M. anti-Selective Catalytic Asymmetric Nitroaldol Reaction via a Heterobimetallic Heterogeneous Catalyst. J. Am. Chem. Soc. 2009, 131, 13860–13869. [Google Scholar] [CrossRef]
- Karmakar, A.; Da Silva, M.F.C.G.; Pombeiro, A.J.L. Zinc metal–organic frameworks: Efficient catalysts for the diastereoselective Henry reaction and transesterification. Dalton Trans. 2014, 43, 7795–7810. [Google Scholar] [CrossRef]
- Kehat, T.; Portnoy, M. Polymer-Supported Proline-Decorated Dendrons: Dendritic Effect in Asymmetric Aldol Reaction. Chem. Commun. 2007, 2823–2825. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Steere, J.A.; Wolf, S. The ultrasound promoted Knoevenagel condensation of aromatic aldehydes. Tetrahedron Lett. 1998, 39, 8013–8016. [Google Scholar] [CrossRef]
- Neelakandeswari, N.; Sangami, G.; Emayavaramban, P.; Karvembu, R.; Dharmaraj, N.; Kim, H.Y. Mesoporous nickel hydroxyapatite nanocomposite for microwave-assisted Henry reaction. Tetrahedron Lett. 2012, 53, 2980–2984. [Google Scholar] [CrossRef]
- Hazra, S.; Karmakar, A.; Silva, M.D.F.C.G.D.; Dlháň, L.; Boča, R.; Pombeiro, A.J.L. Sulfonated Schiff base dinuclear and polymeric copper(ii) complexes: Crystal structures, magnetic properties and catalytic application in Henry reaction. New J. Chem. 2015, 39, 3424–3434. [Google Scholar] [CrossRef]
- Gupta, M.; De, D.; Pal, S.; Pal, T.K.; Tomar, K. A porous two-dimensional Zn(ii)-coordination polymer exhibiting SC–SC transmetalation with Cu(ii): Efficient heterogeneous catalysis for the Henry reaction and detection of nitro explosives. Dalton Trans. 2017, 46, 7619–7627. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.M.; Mahmudov, K.T.; Da Silva, M.F.C.G.; Martins, L.M.; Guseinov, F.I.; Pombeiro, A.J. 1D Zn(II) coordination polymer of arylhydrazone of 5,5-dimethylcyclohexane-1,3-dione as a pre-catalyst for the Henry reaction. Catal. Commun. 2016, 87, 49–52. [Google Scholar] [CrossRef]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef]
- Tanaka, K.; Hachiken, S. Enantioselective Henry reaction catalyzed by trianglamine–Cu(OAc)2 complex under solvent-free conditions. Tetrahedron Lett. 2008, 49, 2533–2536. [Google Scholar] [CrossRef]
- Angelini, T.; Ballerini, E.; Bonollo, S.; Curini, M.; Lanari, D. A new sustainable protocol for the synthesis of nitroaldol derivatives via Henry reaction under solvent-free conditions. Green Chem. Lett. Rev. 2014, 7, 11–17. [Google Scholar] [CrossRef]
- Huseynov, F.E.; Shamilov, N.T.; Voronina, A.A.; Buslaeva, T.M.; Mahmudov, K.T.; Da Silva, M.F.C.G.; Sutradhar, M.; Kopylovich, M.N.; Pombeiro, A.J.L. Lanthanide derivatives comprising arylhydrazones of β-diketones: Cooperative E/Z isomerization and catalytic activity in nitroaldol reaction. Dalton Trans. 2015, 44, 5602–5610. [Google Scholar]
- Rocha, B.G.M.; Mac Leod, T.C.O.; Guedes da Silva, M.F.C.; Luzyanin, K.V.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. NiII, CuII and ZnII Complexes with a Sterically Hindered Scorpionate Ligand (TpmsPh) and Catalytic Application in the Diasteroselective Nitroaldol (Henry) Reaction. Dalton Trans. 2014, 43, 15192–15200. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Marchetti, F.; Cerquetella, A.; Pettinari, R.; Monari, M.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Coordination chemistry of the (h6-cymene)ruthenium(II) fragment with bis-, tris-, and tetrakis(pyrazol-1-yl)borate ligands: Synthesis, structural, electrochemical and catalytic diastereoselective nitroaldol reaction studies. Organometallics 2011, 30, 1616–1626. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Mahmoud, A.G.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Nickel(II)-2-amino-4-alkoxy-1,3,5-triazapentadienato complexes as catalysts for Heck and Henry reactions. RSC Adv. 2016, 6, 29159–29163. [Google Scholar] [CrossRef]
- Sutradhar, M.; Da Silva, M.F.C.G.; Pombeiro, A.J. A new cyclic binuclear Ni(II) complex as a catalyst towards nitroaldol (Henry) reaction. Catal. Commun. 2014, 57, 103–106. [Google Scholar] [CrossRef]
- Arunachalam, R.; Aswathi, C.S.; Das, A.; Kureshy, R.I.; Subramanian, P.S. ChemInform Abstract: Diastereoselective Nitroaldol Reaction Catalyzed by Binuclear Copper(II) Complexes in Aqueous Medium. ChemPlusChem 2015, 80, 209–216. [Google Scholar] [CrossRef]
- Karmakar, A.; Da Silva, M.F.C.G.; Hazra, S.; Pombeiro, A.J.L. Zinc amidoisophthalate complexes and their catalytic application in the diastereoselective Henry reaction. New J. Chem. 2015, 39, 3004–3014. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Da Silva, M.F.C.G.; Mahmudov, K.T.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear Cu II Structural Isomers: Coordination, Magnetism, Electrochemistry and Catalytic Activity towards the Oxidation of Alkanes. Eur. J. Inorg. Chem. 2015, 2015, 3959–3969. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium Complexes: Recent Progress in Oxidation Catalysis. Coord. Chem. Rev. 2015, 301–302, 200–239. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J.L. Coordination Chemistry of Non-oxido, Oxido and Dioxidovanadium(IV/V) Complexes with Azine Fragment Ligands. Coord. Chem. Rev. 2014, 265, 89–124. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Ghosh, S.; Drew, M.G. Synthesis of a mononuclear oxidovanadium(V) complex by bridge-splitting of the corresponding binuclear precursor. J. Mol. Struct. 2012, 1020, 148–152. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Mahmudov, K.T.; Da Silva, M.F.C.G.; Pombeiro, A.J.L. Iron(iii) and cobalt(iii) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: Catalysts for the microwave assisted oxidation of alcohols. RSC Adv. 2016, 6, 8079–8088. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Mukherjee, G.; Drew, M.G.; Ghosh, S. Synthesis, structural characterization and electrochemical activity of oxidovanadium(IV/V) complexes of a diprotic ONS chelating ligand. Inorganica Chim. Acta 2010, 363, 3376–3383. [Google Scholar] [CrossRef]
- Sutradhar, M.; Carrella, L.M.; Rentschler, E. A Discrete μ4-Oxido Tetranuclear Iron(III) Cluster. Eur. J. Inorg. Chem. 2012, 2012, 4273–4278. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Silva, M.F.C.G.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Silva, T.F.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Org. Chem. 2011, 24, 764–773. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Da Silva, M.F.C.G.; Pombeiro, A.J. Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.; Da Silva, M.F.C.G.; Alegria, E.C.B.A.; Liu, C.-M.; Pombeiro, A.J.L. Dinuclear Mn(ii,ii) complexes: Magnetic properties and microwave assisted oxidation of alcohols. Dalton Trans. 2014, 43, 3966. [Google Scholar] [CrossRef]
- Bruker, APEX2 & SAINT; AXS Inc.: Madison, WI, USA, 2004.
- Altomare, A.; Camalli, M.; Giacovazzo, C.; Moliterni, A.G.; Polidori, G.; Spagna, R.; Moliterni, A.G.G.; Burla, M.C.; Cascarano, G.L.; Guagliardi, A.; et al. SIR 97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Cwik, A.; Fuchs, A.; Hell, Z.; Clacens, J.-M.; Clacens, J. Nitroaldol-Reaction of Aldehydes in the Presence of Non-Activated Mg:Al 2:1 Hydrotalcite: A Possible New Mechanism for the Formation of 2-Aryl-1,3-dinitropropanes. Tetrahedron 2005, 36, 4015–4021. [Google Scholar] [CrossRef]
- Bulbule, V.J.; Deshpande, V.H.; Velu, S.; Sudalai, A.; Sivasankar, S.; Sathe, V. Heterogeneous Henry reaction of aldehydes: Diastereoselective synthesis of nitroalcohol derivatives over Mg-Al hydrotalcites. Tetrahedron 1999, 55, 9325–9332. [Google Scholar] [CrossRef]






| 1A | 2 | |
|---|---|---|
| Empirical formula | C20H24N4NiO5.50 | C18H26N3Ni1.50O10 |
| Formula Weight | 467.14 | 532.48 |
| Crystal system | Triclinic | Triclinic |
| Space group | P¯1 | P¯1 |
| Temperature/K | 296 (2) | 296 (2) |
| a/Å | 12.766 (3) | 9.114 (3) |
| b/Å | 13.714 (4) | 10.821 (3) |
| c/Å | 14.344 (4) | 13.221 (4) |
| α/° | 84.87 (1) | 71.721 (10) |
| β/° | 63.594 (10) | 83.309 (11) |
| γ/° | 79.445 (9) | 71.328 (10) |
| V (Å3) | 2211.2 (10) | 1172.8 (6) |
| Z | 4 | 2 |
| Dcalc (g cm−3) | 1.403 | 1.508 |
| μ(Mo Kα) (mm−1) | 0.92 | 1.27 |
| Rfls. collected/unique/observed | 35885/8156/6683 | 10924/4209/2646 |
| Rint | 0.029 | 0.091 |
| Final R1 a, wR2 b (I ≥ 2σ) | 0.038, 0.108 | 0.093, 0.224 |
| Goodness-of-fit on F2 | 1.03 | 1.05 |
| 1A | |||||
|---|---|---|---|---|---|
| Ni1—N1 | 1.995 (2) | N1—Ni1—O1 | 89.97 (8) | N5—Ni2—O6 | 89.33 (8) |
| Ni1—O1 | 2.0231 (17) | N1—Ni1—O1i | 170.34 (8) | N5—Ni2—O6ii | 169.38 (8) |
| Ni1—O1i | 2.0256 (16) | O1—Ni1—O1i | 80.43 (7) | O6—Ni2—O6ii | 80.07 (8) |
| Ni1—O2 | 2.039 (2) | N1—Ni1—O2 | 78.97 (9) | N5—Ni2—O7 | 79.06 (8) |
| Ni1—O4 | 2.128 (2) | O1—Ni1—O2 | 168.93 (7) | O6—Ni2—O7 | 168.18 (8) |
| Ni1—O5 | 2.1753 (18) | O1i—Ni1—O2 | 110.62 (7) | O6ii—Ni2—O7 | 111.52 (8) |
| Ni2—N5 | 2.009 (2) | N1—Ni1—O4 | 95.68 (9) | N5—Ni2—O9 | 92.27 (8) |
| Ni2—O6 | 2.0342 (18) | O1—Ni1—O4 | 89.38 (8) | O6—Ni2—O9 | 93.83 (8) |
| Ni2—O6ii | 2.0488 (18) | O1i—Ni1—O4 | 85.34 (8) | O6ii—Ni2—O9 | 88.04 (8) |
| Ni2—O7 | 2.059 (2) | O2—Ni1—O4 | 92.13 (8) | O7—Ni2—O9 | 84.51 (8) |
| Ni2—O9 | 2.0975 (19) | N1—Ni1—O5 | 91.79 (8) | N5—Ni2—O10 | 92.08 (8) |
| Ni2—O10 | 2.1161 (19) | O1—Ni1—O5 | 90.17 (7) | O6—Ni2—O10 | 94.69 (8) |
| N1—N2 | 1.385 (3) | O1i—Ni1—O5 | 87.23 (7) | O6ii—Ni2—O10 | 89.24 (8) |
| N5—N6 | 1.397 (3) | O2—Ni1—O5 | 89.74 (8) | O7—Ni2—O10 | 88.00 (8) |
| O2—C8 | 1.276 (3) | O4—Ni1—O5 | 172.52 (7) | O9—Ni2—O10 | 170.47 (9) |
| O7—C28 | 1.267 (3) | Ni1—O1—Ni1i | 99.57 (7) | Ni2—O6—Ni2ii | 99.93 (8) |
| 2 | |||||
| Ni1—O4 | 2.089 (7) | N2—Ni1—O5 | 170.5 (2) | O3—Ni2—O3iii | 180.0 |
| Ni1—N2 | 1.991 (6) | N2—Ni1—O2 | 90.7 (2) | O3—Ni2—O2iii | 99.4 (2) |
| Ni1—O5 | 2.025 (6) | O5—Ni1—O2 | 98.7 (2) | O3iii—Ni2—O2iii | 80.6 (2) |
| Ni1—O2 | 2.031 (5) | N2—Ni1—O1 | 78.7 (2) | O3—Ni2—O2 | 80.6 (2) |
| Ni1—O1 | 2.088 (6) | O5—Ni1—O1 | 91.9 (2) | O3iii—Ni2—O2 | 99.4 (2) |
| Ni1—O6 | 2.092 (6) | O2—Ni1—O1 | 169.4 (2) | O2iii—Ni2—O2 | 180.0 |
| Ni2—O3 | 1.948 (6) | N2—Ni1—O4 | 87.6 (3) | O3—Ni2—O7 | 88.8 (3) |
| Ni2—O3iii | 1.948 (5) | O5—Ni1—O4 | 93.5 (3) | O3iii—Ni2—O7 | 91.2 (3) |
| Ni2—O2iii | 2.108 (5) | O2—Ni1—O4 | 93.1 (2) | O2iii—Ni2—O7 | 93.3 (2) |
| Ni2—O2 | 2.108 (5) | O1—Ni1—O4 | 86.8 (2) | O2—Ni2—O7 | 86.7 (2) |
| Ni2—O7 | 2.143 (8) | N2—Ni1—O6 | 91.9 (3) | O3—Ni2—O7iii | 91.2 (3) |
| Ni2—O7iii | 2.143 (8) | O5—Ni1—O6 | 86.7 (3) | O3iii—Ni2—O7iii | 88.8 (3) |
| N1—N2 | 1.387 (8) | O2—Ni1—O6 | 88.8 (2) | O2iii—Ni2—O7iii | 86.7 (2) |
| C1—O1 | 1.250 (9) | O1—Ni1—O6 | 91.3 (3) | O2—Ni2—O7iii | 93.3 (2) |
| - | - | O4—Ni1—O6 | 178.0 (3) | O7—Ni2—O7iii | 180.0 |
| - | - | Ni1—O2—Ni2 | 125.2 (2) | - | - |
| Entry. | Catalyst | Time (h) | Amount of Catalyst (mol%) | Temp. (°C) | Solvent | Yield or Conversion (%) b | Selectivity c syn: anti | TOF/h−1 d |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 24 | 1.0 | ambient | - | 86.6 | 70:30 | 3.6 |
| 2 | 1 | 24 | 1.0 | ambient | H2O | 80.2 | 70:30 | 3.3 |
| 3 | 1 | 24 | 1.0 | ambient | MeOH | 72.2 | 64:36 | 3.0 |
| 4 | 1 | 24 | 1.0 | ambient | MeCN | 56.4 | 58:42 | 2.4 |
| 5 | 2 | 24 | 1.0 | ambient | - | 89.2 | 72:28 | 3.7 |
| 6 | 2 | 24 | 1.0 | ambient | H2O | 82.4 | 70:30 | 3.4 |
| 7 | 2 | 24 | 1.0 | ambient | MeOH | 72.6 | 66:34 | 3.0 |
| 8 | 2 | 24 | 1.0 | ambient | MeCN | 58.0 | 60:40 | 2.4 |
| 9 | 2 | 24 | 1.0 | 40 | - | 90.8 | 72:28 | 3.8 |
| 10 | 2 | 24 | 1.0 | 60 | - | 94.0 | 77:23 | 3.9 |
| 11 | 2 | 24 | 1.0 | 75 | - | 90.6 | 76:24 | 3.8 |
| 12 | Blank | 24 | − | 60 | - | - | - | - |
| 13 | Ni(OAc)2 | 24 | 1.0 | 60 | - | 9 | 62:38 | 0.3 |
| 14 | 2 | 24 | 2.0 | 60 | - | 94.2 | 76:24 | 1.9 |
| 15 | 2 | 24 | 3.0 | 60 | - | 92.8 | 74:26 | 1.3 |
| 16 | 2 | 24 | 5.0 | 60 | - | 91.6 | 71:29 | 0.8 |
| 17 | 2 | 6 | 1.0 | 60 | - | 44.4 | 68:32 | 7.4 |
| 18 | 2 | 12 | 1.0 | 60 | - | 60.8 | 72:28 | 5.1 |
| 19 | 2 | 48 | 1.0 | 60 | - | 92.6 | 74:26 | 1.9 |
| Entry | Substrate | Yield or Conversion (%) b | syn: anti ratio c | TOF/h−1 d |
|---|---|---|---|---|
| 1 |  | 94.0 | 77:23 | 3.9 |
| 2 |  | 60.8 | 72:28 | 2.5 |
| 3 |  | 56.4 | 70:30 | 2.3 |
| 4 |  | 74.0 | 76:24 | 3.1 |
| 5 |  | 88.4 | 74:26 | 3.7 |
| 6 |  | 70.2 | 70:30 | 2.9 |
| 7 |  | 97.0 | 80:20 | 4.0 |
| 8 | CH3CHO | 66.6 | 72:28 | 2.8 |
| 9 | CH3CH2CHO | 78.2 | 74:26 | 3.2 |
| Entry | Aldehyde | Nitroalkane | Yield or Conversion /% b | syn: anti ratio c | TOF/h−1 d |
|---|---|---|---|---|---|
| 1 |  | nitromethane | 94.2 | - | 3.9 |
| 2 | nitroethane | 94.0 | 77:23 | 3.9 | |
| 3 | nitropropane | 54.2 | 68:32 | 2.5 | |
| 4 |  | nitromethane | 56.6 | - | 2.3 |
| 5 | nitroethane | 44.4 | 66:34 | 1.8 | |
| 6 | nitropropane | 38.4 | 58:42 | 1.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, M.; Roy Barman, T.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Ni(II)-Aroylhydrazone Complexes as Catalyst Precursors Towards Efficient Solvent-Free Nitroaldol Condensation Reaction. Catalysts 2019, 9, 554. https://doi.org/10.3390/catal9060554
Sutradhar M, Roy Barman T, Pombeiro AJL, Martins LMDRS. Ni(II)-Aroylhydrazone Complexes as Catalyst Precursors Towards Efficient Solvent-Free Nitroaldol Condensation Reaction. Catalysts. 2019; 9(6):554. https://doi.org/10.3390/catal9060554
Chicago/Turabian StyleSutradhar, Manas, Tannistha Roy Barman, Armando J. L. Pombeiro, and Luísa M.D.R.S. Martins. 2019. "Ni(II)-Aroylhydrazone Complexes as Catalyst Precursors Towards Efficient Solvent-Free Nitroaldol Condensation Reaction" Catalysts 9, no. 6: 554. https://doi.org/10.3390/catal9060554