Isomerization and Dehydroaromatization of R(+)-Limonene Over the Ti-MCM-41 Catalyst: Effect of Temperature, Reaction Time and Catalyst Content on Product Yield
Abstract
:1. Introduction
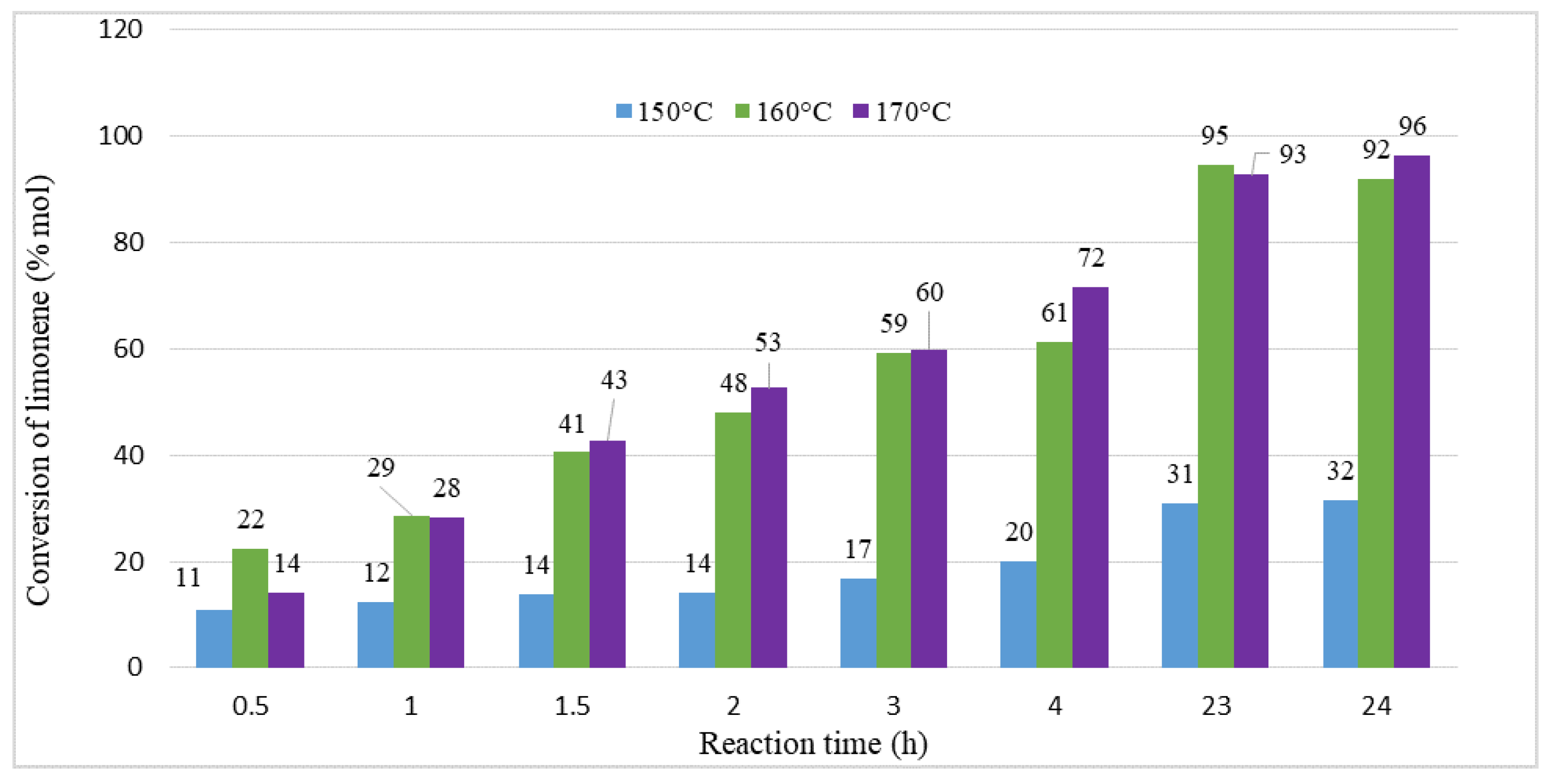
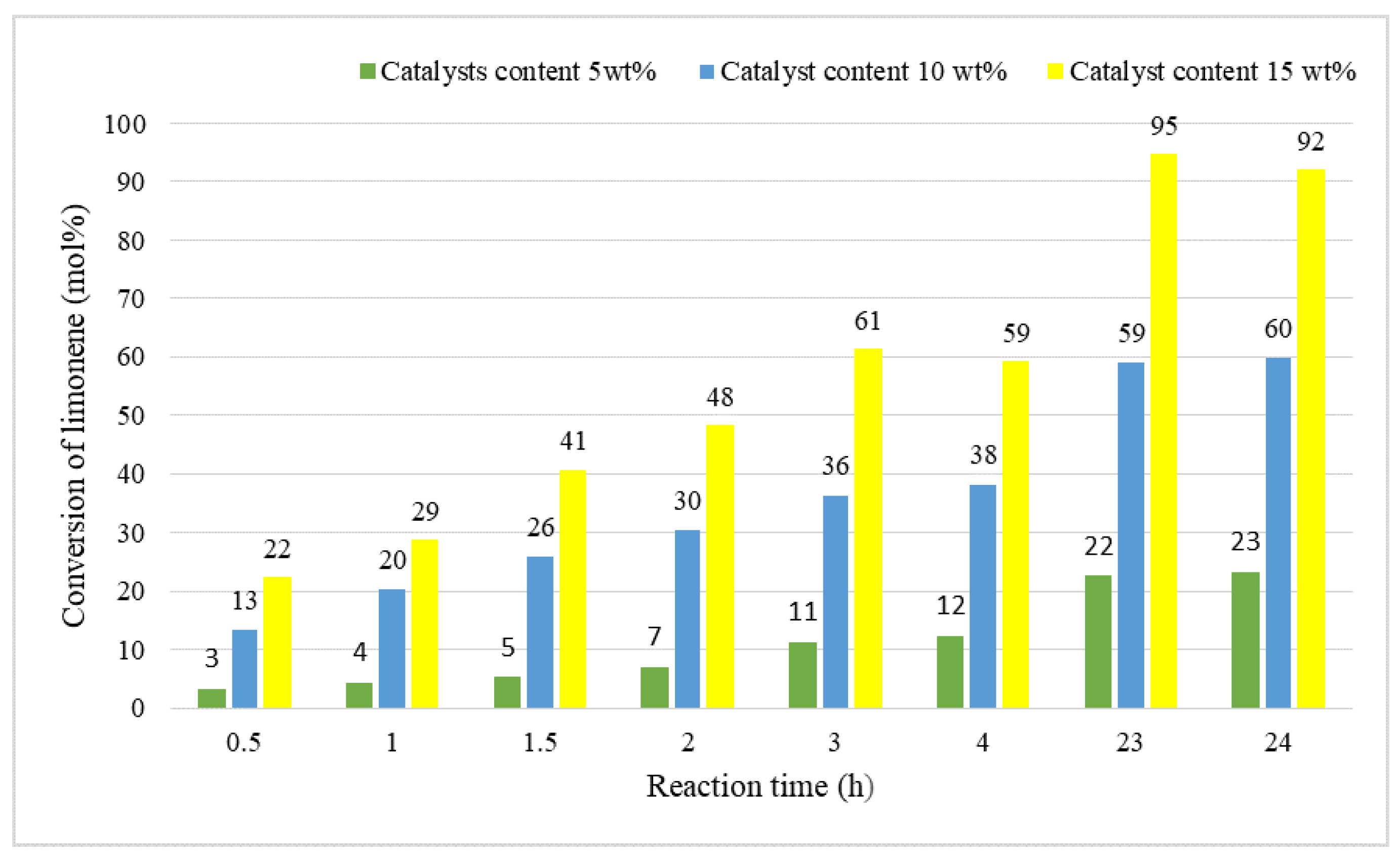
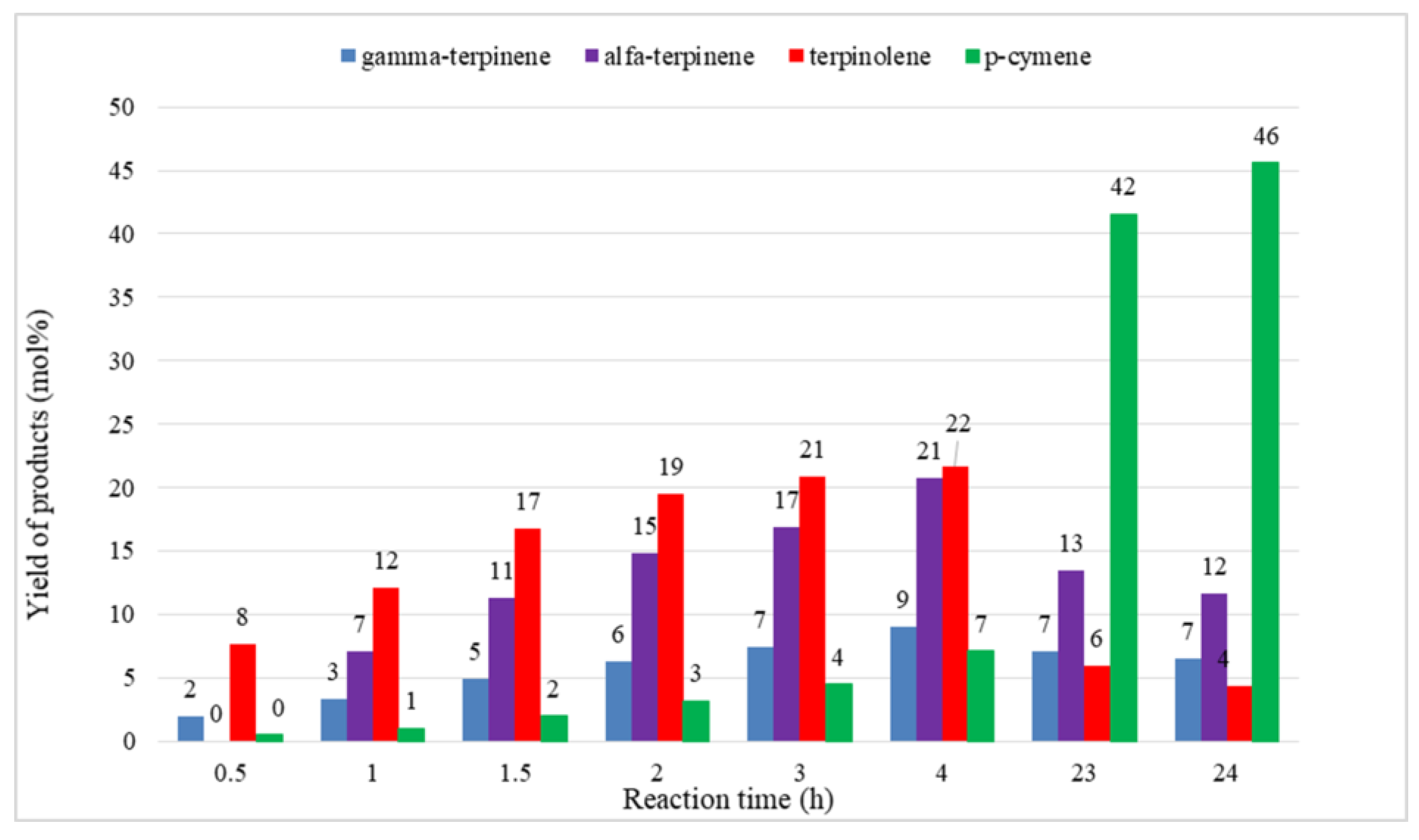
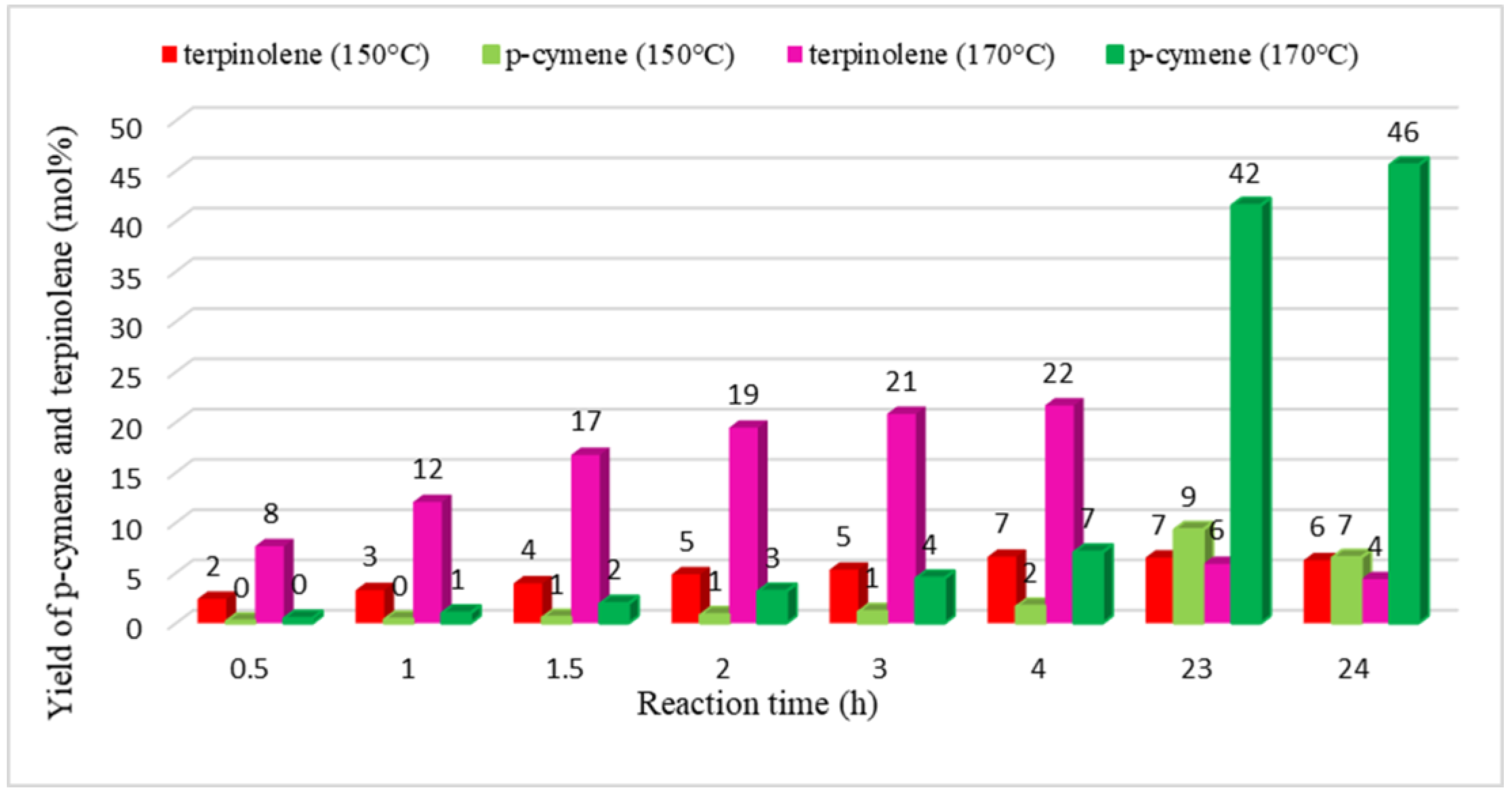
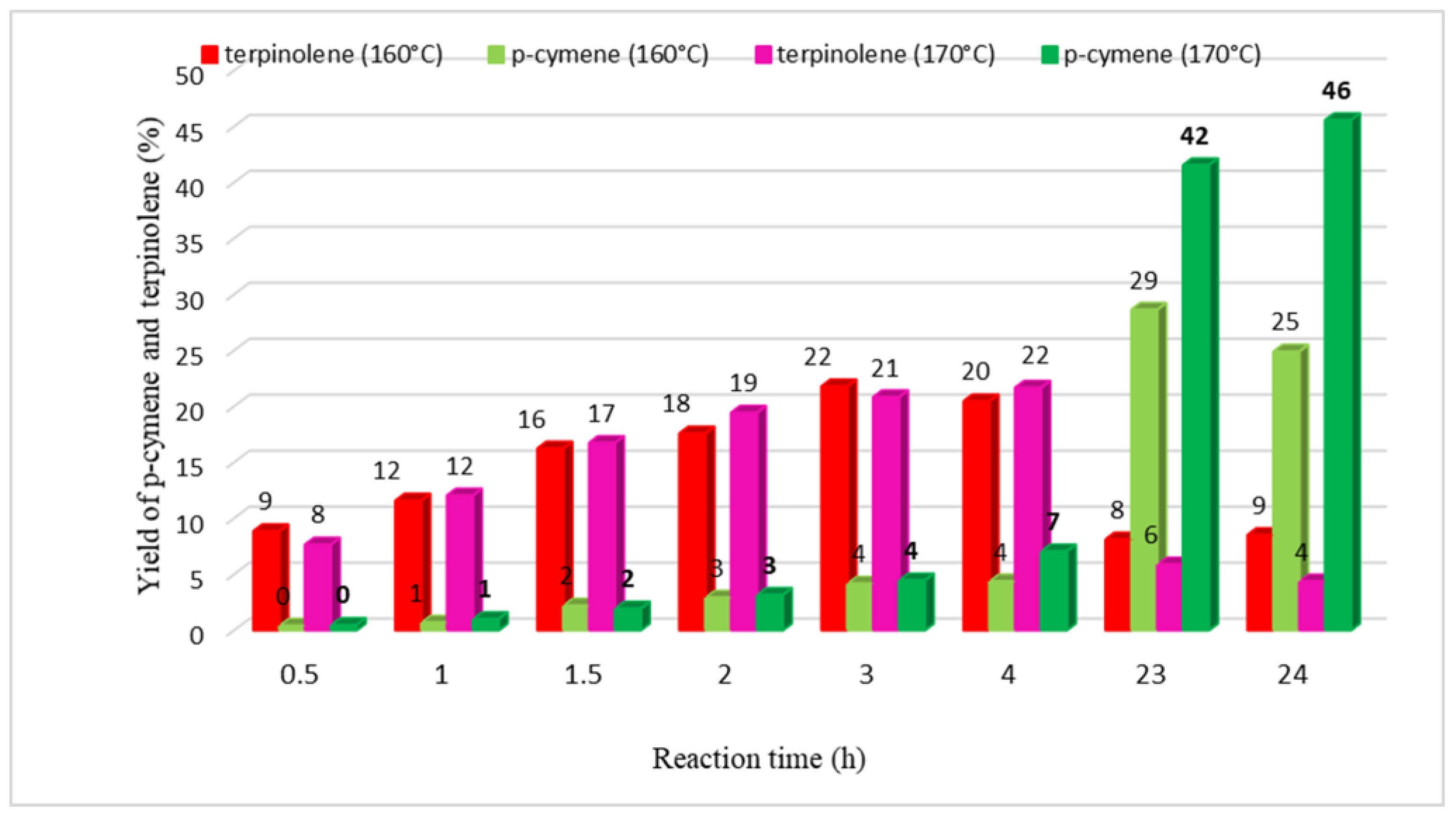
2. Results
3. Materials and Methods
3.1. Raw Materials
3.2. The Synthesis of the Ti-MCM-41 Catalyst
3.3. The Method of the Isomerization of Limonene Over the Ti-MCM-41 Catalyst
3.4. Identification of the Products of Isomerization by the Gas Chromatography Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Makuch, E.; Wróblewska, A. Budowa i charakterystyka uporządkowanego materiału typu SBA-15 o dwuwymiarowej strukturze (2D) heksagonalnej, I Krajowa Konferencja, Grafen i inne materiały 2D”, Szczecin, Poland 2015, 96-97. Available online: http://grafen2d.zut.edu.pl/fileadmin/downloads/Grafen2D-Szczecin2015-abstrakty.pdf (accessed on 3 June 2017).
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Iuliean, V.; Asaftei, N.B.; Birsa, L.M. Sorption properties of MCM-41 mesoporous materials. Acta. Chem. Iasi. 2008, 16, 47–60. [Google Scholar]
- Galacho, C.; Carrott, M.M.L.R.; Carro, P.J.M. Structural and catalytic properties of Ti–MCM-41 synthesised at room temperature up to high Ti content. Micropor. Mesopor. Mater. 2007, 100, 312–321. [Google Scholar] [CrossRef]
- Kleestorfer, K.; Vinek, H.; Jentys, A. Structure simulation of MCM-41 type materials. J. Mol. Catal. A: Chem. 2007, 166, 53–57. [Google Scholar] [CrossRef]
- Makuch, E.; Wróblewska, A.; Miądlicki, P.; Retajczyk, M. Synteza i charakterystyka wysokouporządkowanych materiałów mezoporowatych o strukturze typu SBA-16 wykazujących symetrię sześcienną Im3m, Innowacje w Polskiej nauce w obszarze inżynierii i technologii; Wydawnictwo Nauka i Biznes: Brzeziny, Poland, 2016; pp. 66–74. [Google Scholar]
- Sinha, A.K.; Seelan, S.; Akita, T.; Tsubota, S.; Haruta, M. Vapor phase propylene epoxidation over Au/Ti-MCM-41 catalysts prepared by different Ti incorporation modes. App. Catal. A-Gen. 2003, 240, 243–252. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Ma, X. Microwave synthesis, characterization and transesterification activities of Ti-MCM-41. Micropor. Mesopor. Mater. 2012, 156, 22–28. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Modified Ti/MCM-41 catalysts for enantioselective epoxidation of styrene. J. Mol. Catalysis A-Chem. 2016, 420, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska, A.; Makuch, E. Studies on the deactivation of Ti-MCM-41 catalyst in the process of allyl alcohol epoxidation. Pol. J. Chem. Technol. 2013, 15, 111–115. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; He, M.; Tatsumi, T. A novel titanosilicate with MWW structure Catalytic properties in selective epoxidation of diallyl ether with hydrogen peroxide. J. Catal. 2004, 228, 183–191. [Google Scholar] [CrossRef]
- Walasek, W.; Wróblewska, A. Epoxidation of allyl-glycidyl ether with hydrogen peroxide over Ti-SBA-15 catalyst and in methanol medium. Pol. J. Chem. Technol. 2016, 18, 9–14. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Mi, Z. Epoxidation of allyl chloride and hydrogen peroxide over titanium silicalite-1 film on SiO2 pellet support. J. Chem. Technol. Biotechnol. 2007, 82, 414–420. [Google Scholar] [CrossRef]
- Gawarecka, A.; Wróblewska, A. Limonene oxidation over Ti-MCM-41 and Ti-MWW catalysts with t-butyl hydroperoxide as the oxidant. Reac. Kinet. Mech. Cat. 2018, 124, 523–543. [Google Scholar] [CrossRef] [Green Version]
- Retajczyk, M.; Wróblewska, A. The isomerization of limonene over the Ti-SBA-15 catalyst—the influence of reaction time, temperature, and catalyst content. Catalysts 2017, 7, 273. [Google Scholar] [CrossRef]
- Casuscelli, S.; Eimer, G.A.; Canepa, A.; Heredia, A.C.; Poncio, C.E.; Crivello, M.E.; Perez, C.F.; Aguilar, A.; Herrero, E.R. Ti-MCM-41 as catalyst for a-pinene oxidation Study of the effect of Ti content and H2O2 addition on activity and selectivity. Catal. Today 2008, 133, 678–683. [Google Scholar] [CrossRef]
- Makuch, E.; Wróblewska, A. Przebieg procesu izomeryzacji eugenolu na heterogenicznych katalizatorach tytanowo-silikatowych TS-1 i Ti-MCM-41 o stosunku molowym Si/Ti w żelu krystalizacyjnym równym 15, Innowacje w Polskiej nauce w obszarze inżynierii i technologii; Wydawnictwo Nauka i Biznes: Brzeziny, Poland, 2016; pp. 85–92. [Google Scholar]
- Marin-Astorga, N.; Martinez, J.J.; Borda, G.; Cubillos, J.; Suarez, D.N.; Rojas, H. Control of the chemoselectivity in the oxidation of geraniol over lanthanum, titanium and niobium catalysts supported on mesoporous silica MCM-41. Top. Catal. 2012, 55, 620–624. [Google Scholar] [CrossRef]
- Arizaga, B.; Leon, A.; Burgueño, N.; López, A.; Paz, D.; Martínez, N.; Lorenzo, D.; Dellacassa, E.; Bussi, J. A clean process for the production of oxygenated limonene derivatives starting from orange oil. J. Chem. Technol. Biotechnol. 2007, 82, 532–538. [Google Scholar] [CrossRef]
- Zhang, Z.; Vriesekoop, F.; Yuan, Q.; Liang, H. Effects of nisin on the antimicrobial activity of D-limonene and its nanoemulsion. Food Chem. 2014, 150, 307–312. [Google Scholar] [CrossRef]
- Byrne, C.M.; Allen, S.D.; Lobkovsky, E.B.; Coates, G.W. Alternating copolymerization of limonene oxide and carbon dioxide. J. Am. Chem. Soc. 2004, 126, 11404–11405. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. d-Limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco. Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A. Lecznicze zastosowanie limonenu. Pom. J. Life Sci. 2018, 64, 51–57. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Parikh, P.A.; Subrahmanyam, N.; Bhat, Y.S.; Halgeri, A.B. Toluene isopropylation over zeolite beta and metallosiliates of MFI structure. Appl. Catal. A-Gen. 1992, 90, 1–10. [Google Scholar] [CrossRef]
- Selvaraj, M.; Pandurangan, A.; Seshadri, K.S.; Sinha, P.K.; Krishnasamy, V.; Lal, K.B. Comparison of mesoporous Al-MCM-41 molecular sieves in the production of p-cymene for isopropylation of toluene. J. Mol. Catal. A: Chem. 2002, 186, 173–186. [Google Scholar] [CrossRef]
- Phillips, M.; Gibbs, H.D. A Synthesis of thymol from p-cymene. J. Ind. Eng. Chem. 1920, 12, 733–734. [Google Scholar] [CrossRef]
- Granato, A.V.; Santos, A.G.; Dos Santos, E.N. p-Cymene as solvent for olefin metathesis: matching efficiency and sustainability. Chem. Sus. Chem. 2017, 10, 1832–1837. [Google Scholar] [CrossRef]
- Johnson, W.E. Process for the isomerization of limonene to terpinolene. Patent US 4551570A, 5 November 1985. [Google Scholar]
- Vieira Sobral, M.; Xavier, A.L.; Lima, T.C.; Pergentino de Sousa, D. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 953451, 1–35. [Google Scholar]
- Ito, K.; Ito, M. The sedative effect of inhaled terpinolene in mice and its structure–activity relationships. J. Nat. Med. 2013, 67, 833–837. [Google Scholar] [CrossRef]
- Deck, P.; Birkert, O. Addition of H2S to terpenes for producing novel molar mass regulators for radical polymerisations. Patent US 20100010267A1, 14 January 2010. [Google Scholar]
- Okumura, N.; Yoshida, H.; Nishimura, Y.; Kitagishi, Y.; Matsuda, S. Terpinolene, a component of herbal sage, downregulates AKT1 expression in K562 cells. Oncol. Lett. 2012, 3, 321–324. [Google Scholar] [CrossRef]
- Ma, Y.; Marston, G. Formation of organic acids from the gas-phase ozonolysis of terpinolene. Phys. Chem. Chem. Phys. 2009, 11, 4198–4209. [Google Scholar]
- Ikeda, Y.; Takamatsu, C.; Matsuyama, K. Raw-material for pregellation, method for preparation of pregel, method for production of molded material, and molded material. Patent EP0376662 A2, 22 September 1992. [Google Scholar]
- Han, X.; Bourne, R.A.; Poliakoff, M.; George, M.W. Immobilised photosensitisers for continuous flow reactions of singlet oxygen in supercritical carbon dioxide. Chem. Sci. 2011, 2, 1059–1063. [Google Scholar] [CrossRef]
- Pape, M. Industrial Applications of photochemistry, BASF AG, Hauptiaboratorium, 67 Ludwigshafen, GFR, 535-558. Available online: https://old.iupac.org/publications/pac/1975/pdf/4104x0535.pdf (accessed on 28 June 2017).
- Dembitsky, V.; Shkrobb, I.; Hanusa, L.O. Ascaridole and related peroxides from the genus chenopodium. Biomed. Pap. Med Fac. Univ. Palacky. Olomouc. Czech Repub. 2008, 152, 209–215. [Google Scholar] [CrossRef]
- Kerns, T. Environmentally Induced illnesses. Ethics, Risk, Assessment and Human Rights; McFarland&Company, Publishers: Jefferson, NC, USA; London, UK, 2001. [Google Scholar]
- Grun, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel pathways for the preparation of mesoporous MCM-41 materials: control of porosity and morphology. Micropor. Mesopor. Mater. 1999, 27, 207–216. [Google Scholar] [CrossRef]
- Wróblewska, A.; Miądlicki, P.; Tołpa, J.; Sreńscek-Nazzal, J.; Koren, Z.C.; Michalkiewicz, B. Influence of the titanium content in the Ti-MCM-41 catalyst on the course of the α-pinene isomerization process. Catalysts 2019, 9, 396. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retajczyk, M.; Wróblewska, A. Isomerization and Dehydroaromatization of R(+)-Limonene Over the Ti-MCM-41 Catalyst: Effect of Temperature, Reaction Time and Catalyst Content on Product Yield. Catalysts 2019, 9, 508. https://doi.org/10.3390/catal9060508
Retajczyk M, Wróblewska A. Isomerization and Dehydroaromatization of R(+)-Limonene Over the Ti-MCM-41 Catalyst: Effect of Temperature, Reaction Time and Catalyst Content on Product Yield. Catalysts. 2019; 9(6):508. https://doi.org/10.3390/catal9060508
Chicago/Turabian StyleRetajczyk, Monika, and Agnieszka Wróblewska. 2019. "Isomerization and Dehydroaromatization of R(+)-Limonene Over the Ti-MCM-41 Catalyst: Effect of Temperature, Reaction Time and Catalyst Content on Product Yield" Catalysts 9, no. 6: 508. https://doi.org/10.3390/catal9060508





