AgBr/(Sr0.6Bi0.305)2Bi2O7 Heterostructured Composites: Fabrication, Characterization, and Significantly Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Composition of Catalysts
2.2. Morphology Analysis
2.3. Photophysical Properties of AgBr/SBO Composites
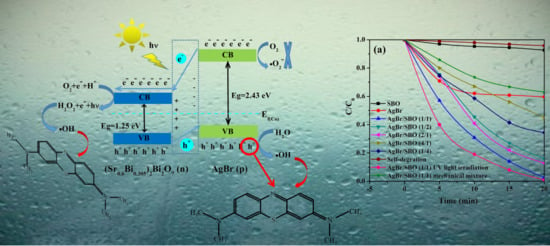
2.4. Photocatalytic Performance of AgBr/SBO Composites
2.5. Photocatalytic Mechanism of AgBr/SBO Catalysts
3. Materials and Methods
3.1. Preparation of Photocatalysts
3.2. Characterizations of Photocatalysts
3.3. Photocatalytic Performance Measurement
3.4. Photoelectrochemical Measurement
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kyung, H.; Lee, J.; Choi, W. Simultaneous and synergistic conversion of dyes and heavy metal ions in aqueous TiO2 suspensions under visible-light illumination. Environ. Sci. Technol. 2005, 39, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor photocatalysis mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performance. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yang, G.; Yang, B.; Zhao, Y. Construction of novel three dimensionally ordered macroporous carbon nitride for highly efficient photocatalytic activity. Appl. Catal. B Environ. 2016, 198, 276–285. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar]
- Kumar, S.G.; Devi, L.G. Review of modified TiO2 photocatalysis under UV/Visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Qu, J. Photoelectrocatalytic degradation of organic contaminants at Bi2O3/TiO2 nanotube array electrode. Appl. Surf. Sci. 2011, 257, 4621–4624. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Huang, F.; Zheng, C.; Wang, W. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Zhang, L. Preparation of BiOBr lamellar structure with high photocatalytic activity by CTAB as Br source and template. J. Hazard. Mater. 2009, 167, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, F.; Lü, X.; Chen, P.; Wan, D.; Xu, F. Improved visible-light photocatalysis of nano-Bi2Sn2O7 with dispersed s-bands. J. Mater. Chem. 2011, 21, 3872–3876. [Google Scholar] [CrossRef]
- Dong, F.; Ho, W.K.; Lee, S.C.; Wu, Z.B.; Fu, M.; Zou, S.C.; Huang, Y. Template-free fabrication and growth mechanism of uniform (BiO)2CO3 hierarchical hollow microspheres with outstanding photocatalytic activities under both UV and visible light irradiation. J. Mater. Chem. 2011, 21, 12428–12436. [Google Scholar] [CrossRef]
- Kako, T.; Zou, Z.; Katagiri, M.; Ye, J. Decomposition of organic compounds over NaBiO3 under visible Light irradiation. Chem. Mater. 2007, 19, 198–202. [Google Scholar] [CrossRef]
- Takei, T.; Haramoto, R.; Dong, Q.; Kumada, N.; Yonesaki, Y.; Kinomura, N.; Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Photocatalytic activities of various pentavalent bismuthates under visible light irradiation. J. Solid State Chem. 2011, 184, 2017–2022. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Liu, G.; Shen, Z.; Xia, D.; Wong, P.K.; Yu, J.C. Monoclinic dibismuth tetraoxide: A new visible-light-driven photocatalyst for environmental remediation. Appl. Catal. B Environ. 2015, 176, 444–453. [Google Scholar] [CrossRef]
- Kumada, N.; Hosoda, M.; Kinomura, N. Preparation of alkaline earth bismuth pyrochlores containing Bi5+ by low temperature hydrothermal reaction. J. Solid State Chem. 1993, 106, 476–484. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; An, H.; Zhong, Y.; Wang, D.; Tang, C.; Hu, C. (Sr0.6Bi0.305)2Bi2O7 as a new visible-light-responsive photocatalyst: An experimental and theoretical study. 2019; submitted. [Google Scholar]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Álvarez Docio, C.M.; Zapata-Ramírez, V.; Fernández, J.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez Docio, C.M.; del Campo, A.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO-TiO2 composites. Boletín Soc. Esp. Ceram. Vid. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Cao, J.; Luo, B.; Lin, H.; Xu, B.; Chen, S. Visible light photocatalytic activity enhancement and mechanism of AgBr/Ag3PO4 hybrids for degradation of methyl orange. J. Hazard. Mater. 2012, 217, 107–115. [Google Scholar] [CrossRef]
- Liang, K.; Zheng, J.; Lai, H.H.; Nicholls, R.J.; Xiao, T.; Jones, M.O.; Edwards, P.P. Unusual reactivity of visible-light-responsive AgBr–BiOBr heterojunction photocatalysts. J. Catal. 2012, 293, 116–125. [Google Scholar]
- Wang, D.; Xue, G.; Zhen, Y.; Fu, F.; Li, D. Monodispersed Ag nanoparticles loaded on the surface of spherical Bi2WO6 nanoarchitectures with enhanced photocatalytic activities. J. Mater. Chem. 2012, 22, 4751–4758. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, J.; Zhong, L.; Zhong, Y.; Wang, D.; Zhou, H. Significantly enhanced photocatalytic activity of visible light responsive AgBr/Bi2Sn2O7 heterostructured composites. Appl. Surf. Sci. 2017, 426, 1173–1181. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, C.; Zhuang, J.; Zhong, Y.; Wang, D.; Zhou, H. AgBr/MgBi2O6 heterostructured composites with highly efficient visible-light-driven photocatalytic activity. J. Phys. Chem. Solids 2018, 117, 94–100. [Google Scholar] [CrossRef]
- Tian, J.; Hao, P.; Wei, N.; Cui, H.; Liu, H. 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure: Enhanced photocatalytic activities and photoelectochemistry performance. ACS Catal. 2015, 5, 4530–4536. [Google Scholar] [CrossRef]
- Cai, H.; Cheng, L.; Xu, F.; Wang, H.; Xu, W.; Li, F. Fabrication of the heterojunction catalyst BiVO4/P25 and its visible-light photocatalytic activities. R. Soc. Open Sci. 2018, 5, 180752. [Google Scholar] [CrossRef]
- Ke, D.; Peng, T.; Ma, L. Effects of hydrothermal temperature on the microstructures of BiVO4 and its photocatalytic O2 evolution activity under visible light. Inorg. Chem. 2009, 48, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Zhao, J.; Yang, P. Assembled fabrication of α-Fe2O3/BiOCl heterojunctions with enhanced photocatalytic performance. Appl. Surf. Sci. 2018, 430, 585–594. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Fang, J.; Zhou, G.; Hong, X.; Wu, S.; Zhu, X.; Chen, Y.; Cen, C. CTAB-assisted hydrothermal synthesis of Bi2Sn2O7 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. Int. J. Photoenergy 2013, 7, 14502–14510. [Google Scholar]
- Xia, D.; Wang, W.; Yin, R.; Jiang, Z.; An, T.; Li, G.; Zhao, H.; Wong, P.K. Enhanced photocatalytic inactivation of Escherichia coli by a novel Z-scheme g-C3N4/m-Bi2O4 hybrid photocatalyst under visible light: The role of reactive oxygen species. Appl. Catal. B Environ. 2017, 214, 23–33. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Yan, T.; Ji, P.; Zhao, X.; Yuan, K.; Wei, D.; Du, B. Fabrication of heterostructured Bi2O2CO3/Bi2O4 photocatalyst and efficient photodegradation of organic contaminants under visible-light. J. Harzard. Mater. 2017, 333, 169–178. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Akple, M.S.; Low, J.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Zhang, J. Enhanced visible light photocatalytic H2-production of g-C3N4/WS2 composite heterostructures. Appl. Surf. Sci. 2015, 358, 193–203. [Google Scholar] [CrossRef]
- Lin, X.; Xing, J.; Wang, W. Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3: A strategy for the design of efficient combined photocatalysts. J. Phys. Chem. C 2007, 111, 18288–18293. [Google Scholar] [CrossRef]
- Zhang, S.W.; Li, J.X.; Zeng, M.Y.; Zhao, G.X.; Xu, J.Z.; Hu, W.P. In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. ACS Appl. Mater. Int. 2013, 5, 12735–12743. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Yang, Z.; Du, H.; Jiang, Y.; Shen, C. Enhanced visible-light photocatalytic activity of Z-scheme graphitic carbon nitride/oxygen vacancy-rich zinc oxide hybrid photocatalysts. Chin. J. Catal. 2015, 36, 2135–2144. [Google Scholar] [CrossRef]
- Lin, H.; Cao, J.; Luo, B.; Xu, B.; Chen, S. Synthesis of novel Z-scheme AgI/Ag/AgBr composite with enhanced visible light photocatalytic activity. Catal. Commun. 2012, 21, 91–95. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, K.; Chen, B. Graphene/TiO2/ZSM-5 composites synthesized by mixture design were used for photocatalytic degradation of oxytetracycline under visible light: Mechanism and biotoxicity. Appl. Surf. Sci. 2016, 362, 329–334. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zhu, Y. Visible photocatalytic activity enhancement of ZnWO4 by graphene hybridization. ACS Catal. 2012, 2, 2769–2778. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhu, D.; Zhong, Y.; Wang, D.; Hu, C. AgBr/(Sr0.6Bi0.305)2Bi2O7 Heterostructured Composites: Fabrication, Characterization, and Significantly Enhanced Photocatalytic Activity. Catalysts 2019, 9, 394. https://doi.org/10.3390/catal9050394
Wang X, Zhu D, Zhong Y, Wang D, Hu C. AgBr/(Sr0.6Bi0.305)2Bi2O7 Heterostructured Composites: Fabrication, Characterization, and Significantly Enhanced Photocatalytic Activity. Catalysts. 2019; 9(5):394. https://doi.org/10.3390/catal9050394
Chicago/Turabian StyleWang, Xinling, Di Zhu, Yan Zhong, Dianhui Wang, and Chaohao Hu. 2019. "AgBr/(Sr0.6Bi0.305)2Bi2O7 Heterostructured Composites: Fabrication, Characterization, and Significantly Enhanced Photocatalytic Activity" Catalysts 9, no. 5: 394. https://doi.org/10.3390/catal9050394