Liquid-Phase Catalytic Oxidation of Limonene to Carvone over ZIF-67(Co)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ZIF-67(Co)
2.2. Catalytic Experiments
2.2.1. The Effect of Various Catalysts on the Reaction
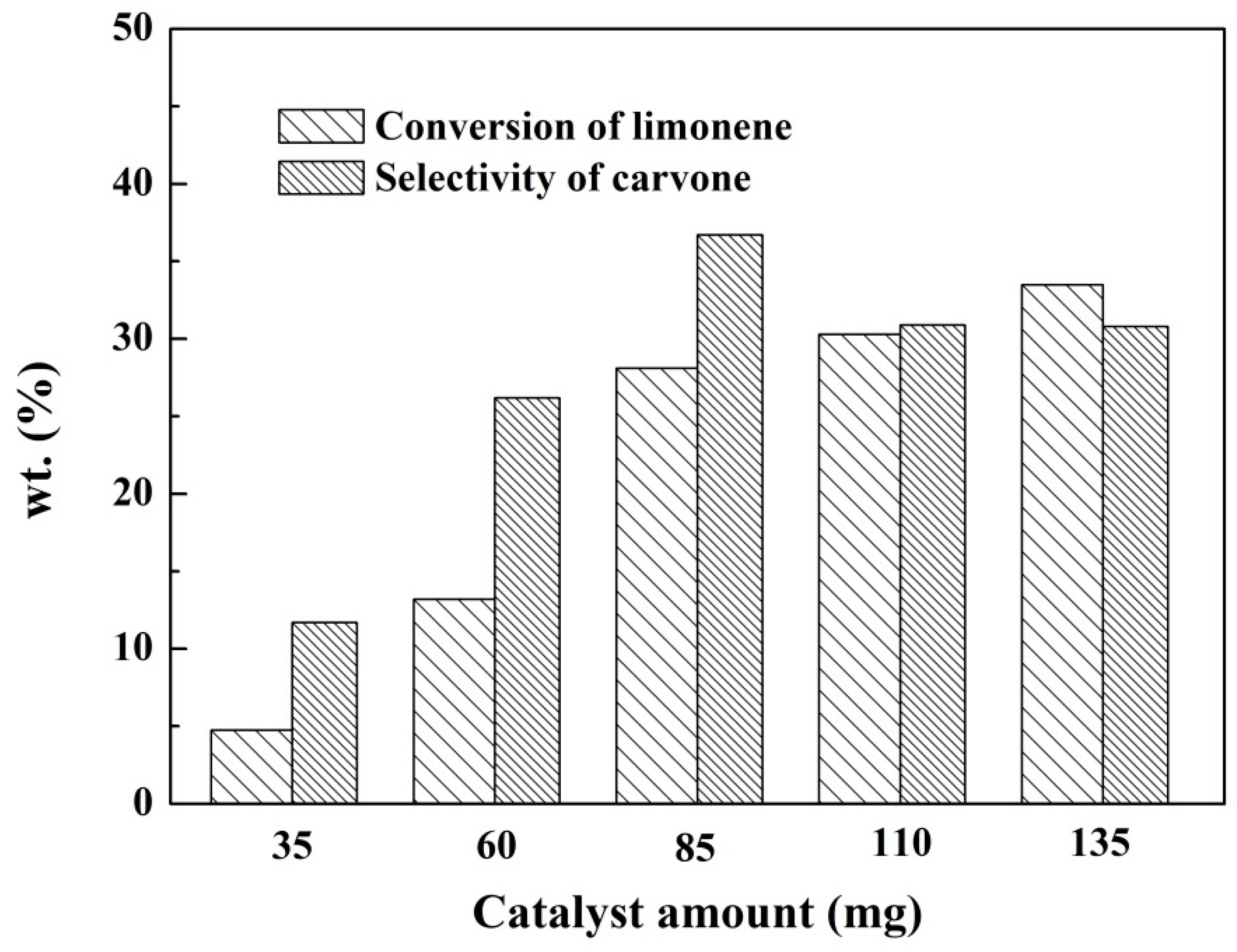
2.2.2. The Effect of the Catalyst Dosage on the Reaction
2.2.3. The Effects of Various Oxidizing Agents on the Reaction
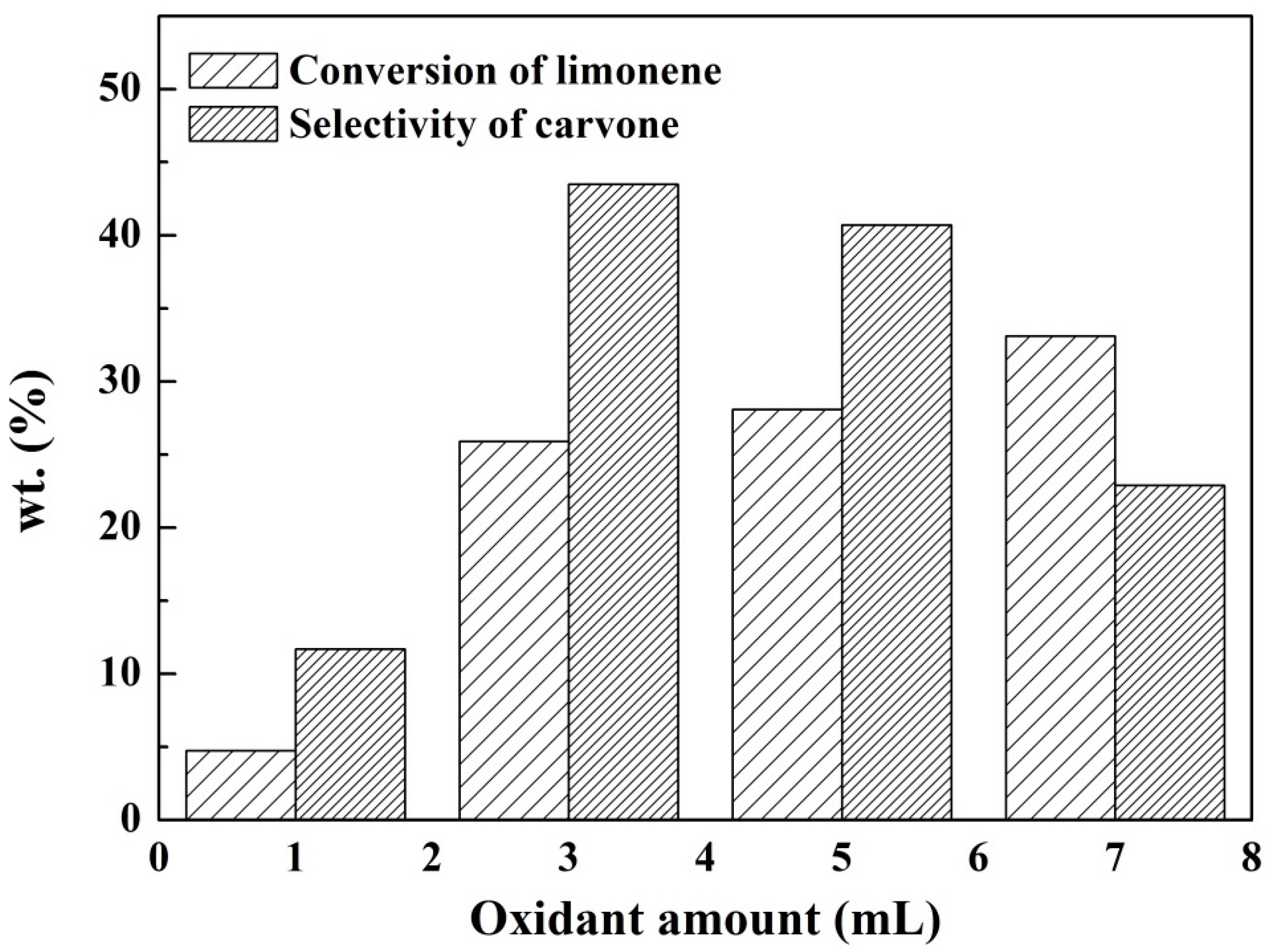
2.2.4. The Effect of the Dosage of the Oxidant on the Reaction
2.2.5. The Effects of Different Solvents on the Reaction
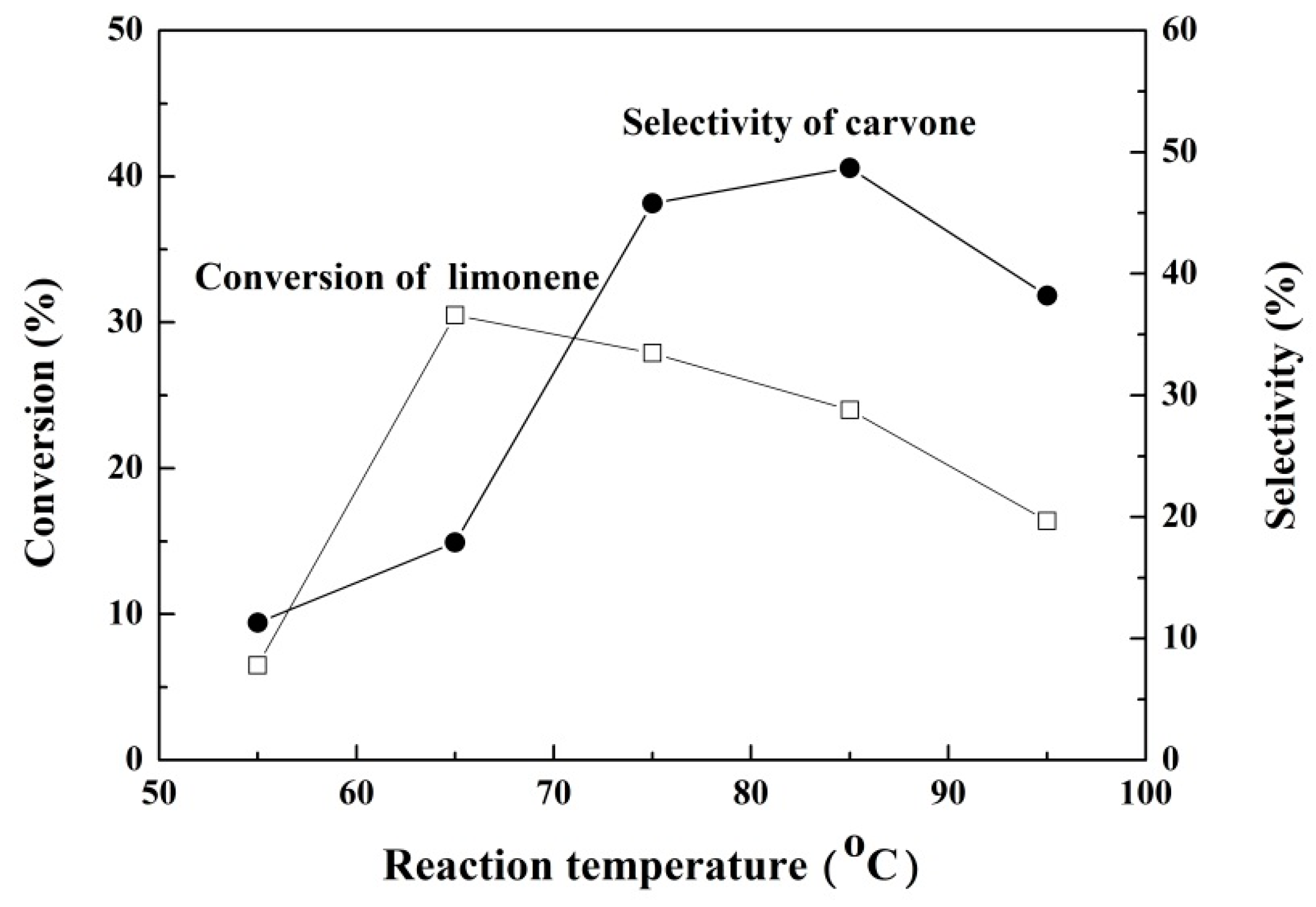
2.2.6. The Effect of the Temperature on the Reaction
2.2.7. The Effect of the Reaction Time on the Reaction
2.2.8. Study of the Catalyst Stability
3. Materials and Methods
3.1. Materials and Solvents
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, E.F.; Mallat, T.; Baiker, A. Allylic oxofunctionalization of cyclic olefins with homogeneous and heterogeneous catalysts. Catal. Today 2000, 31, 115–126. [Google Scholar] [CrossRef]
- Sakthivel, A.; Dapurkar, S.E.; Selvam, P. Allylic oxidation of cyclohexene over chromium containing mesoporous molecular sieves. Appl. Catal. A-Gen. 2003, 246, 283–293. [Google Scholar] [CrossRef]
- Goncalves, J.C.; Oliveira, F.S.; Benedito, R.B.; Sousa, D.P.; de Almeida, R.N.; de Araújo, D.A. Antinociceptive Activity of (-)-Carvone: Evidence of Association with Decreased Peripheral Nerve Excitability. Biol. Pharm. Bull. 2008, 31, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar]
- Li, N.; Wang, F.; Li, M. The Chemical Modification of L(-)-Carvone and its Biological Activity to Three Fungal Pathogens of Plant. J. Mt. Agric. Biol. 2010. [Google Scholar] [CrossRef]
- Linder, S.M.; Greenspan, F.P. Reactions of Limonene Monoxide. The Synthesis of Carvone. J. Org. Chem. 1957, 22, 949–951. [Google Scholar] [CrossRef]
- Robles-Dutenhefner, P.A.; Brandão, B.B.N.S.; de Sousa, L.F.; Gusevskaya, E.V. Solvent-free chromium catalyzed aerobic oxidation of biomass-based alkenes as a route to valuable fragrance compounds. Appl. Catal. A Gen. 2011, 399, 172–178. [Google Scholar] [CrossRef]
- Caovilla, M.; Caovilla, A.; Pergher, S.B.C.; Esmelindro, M.C.; Fernandes, C.; Dariva, C.; Bernardo-Gusmão, K.; Oestreicher, E.G.; Antunes, O.A.C. Catalytic oxidation of limonene, α-pinene and β-pinene by the complex [FeIII(BPMP)Cl(μ-O)FeIIICl3] biomimetic to MMO enzyme. Catal. Today 2008, 133, 695–698. [Google Scholar]
- Młodzik, J.; Wróblewska, A.; Makuch, E.; Wróbel, R.J.; Michalkiewicz, B. Fe/EuroPh catalysts for limonene oxidation to 1,2-epoxylimonene, its diol, carveol, carvone and perillyl alcohol. Catal. Today 2015, 268, 111–120. [Google Scholar] [CrossRef]
- Wróblewska, A.; Makuch, E.; Miądlicki, P. The oxidation of limonene at raised pressure and over the various titanium-silicate catalysts. Pol. J. Chem. Technol. 2015, 17, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Zi, G.; Yao, Z.; Luo, Z.; Wang, Y.; Xue, D.; Wang, B.; Wang, J. Synthesis, characterizations and catalytic allylic oxidation of limonene to carvone of cobalt doped mesoporous silica templated by reed leaves. Catal. Commun. 2015, 59, 233–237. [Google Scholar] [CrossRef]
- Drake, T.; Ji, P.; Lin, W. Site Isolation in Metal–Organic Frameworks Enables Novel Transition Metal Catalysis. Acc. Chem. Res. 2018, 51, 2129–2138. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Ghosh, S.K. Potential of metal–organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures. Coord. Chem. Rev. 2018, 367, 82–126. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.F.; Corraza, M.L.; Cardozo-Filho, L.; Márquez-Alvarez, H.; Antuntes, O.A.C. Oxidation of limonene catalyzed by Metal(Salen) complexes. Braz. J. Chem. Eng. 2006, 23. [Google Scholar] [CrossRef]
- Raj, N.K.K.; Puranik, V.G.; Gopinathan, C.; Ramaswarmy, A.V. Selective oxidation of limonene over sodium salt of cobalt containing sandwich-type polyoxotungstate [WCo3(H2O)2{W9CoO34}2]10−. Appl. Catal. A-Gen. 2003, 256, 265–273. [Google Scholar]
- Jiang, D.; Mallat, T.; Meier, D.M.; Urakawa, A.; Baiker, A. Copper metal–organic framework: Structure and activity in the allylic oxidation of cyclohexene with molecular oxygen. J. Catal. 2010, 270, 26–33. [Google Scholar] [CrossRef]
- Yang, H.; He, X.-W.; Wang, F.; Kang, Y.; Zhang, J. Doping copper into ZIF-67 for enhancing gas uptake capacity and visible-light-driven photocatalytic degradation of organic dye. J. Mater Chem. 2012, 22, 21849–21851. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Jose, T.; Babu, R.; Hwang, G.-Y.; Kathalikkattil, A.M.; Kim, D.-W.; Park, D.-W. A room temperature synthesizable and environmental friendly heterogeneous ZIF-67 catalyst for the solvent less and co-catalyst free synthesis of cyclic carbonates. Appl. Catal. B-Environ. 2016, 182, 562–569. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.; Sun, M.; Gao, C. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate. Catal. Commun. 2014, 54, 86–90. [Google Scholar] [CrossRef]
- Zhu, M.; Srinivas, D.; Bhogeswararao, S.; Ratnasamy, P.; Carreon, M.A. Catalytic activity of ZIF-8 in the synthesis of styrene carbonate from CO2 and styrene oxide. Catal. Commun. 2013, 32, 36–40. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.; Diao, G.; Sun, M.; Cheng, G.; Chen, S. Zeolitic imidazolate framework-68 as an efficient heterogeneous catalyst for chemical fixation of carbon dioxide. J. Mol. Catal. A-Chem. 2014, 392, 278–283. [Google Scholar] [CrossRef]
- Jose, T.; Hwang, Y.; Kim, D.-W.; Kim, M.-I.; Park, D.-W. Functionalized zeolitic imidazolate framework F-ZIF-90 as efficient catalyst for the cycloaddition of carbon dioxide to allyl glycidyl ether. Catal. Today 2015, 245, 61–67. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.W.; Zeng, H.P.; Li, J. Zeolitic imidazolate frameworks for kinetic separation of propane and propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/Co ZIF Particles Derived from Core-Shell ZIF-67@ZIF-8 as Selective Catalyst for the Semi-Hydrogenation of Acetylene. Angew. Chem. Int. Ed. Engl. 2015, 127, 11039–11043. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Tang, Y.; Chou, L.-Y.; Sneed, B.T.; Brodsky, C.N.; Zhao, Z.; Tsung, C.K. Yolk–shell nanocrystal@ZIF-8 nanostructures for gas-phase heterogeneous catalysis with selectivity control. J. Am. Chem. Soc. 2012, 134, 14345–14348. [Google Scholar] [CrossRef]
- Wee, L.H.; Lescouet, T.; Ethiraj, J.; Bonino, F.; Vidruk, R.; Garrier, E.; Packet, D.; Bordiga, S.; Farrusseng, D.; Herskowitz, M.; et al. Hierarchical zeolitic imidazolate framework-8 catalyst for monoglyceride synthesis. ChemCatChem 2013, 5, 3562–3566. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal− organic framework: acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, Z.; Song, Z.; Li, J.; Dong, J. Synthesis of ZIF-8 and ZIF-67 by Steam-Assisted Conversion and an Investigation of Their Tribological Behaviors. Angew. Chem. Int. Ed. Engl. 2011, 50, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Sun, F.; Qin, L.; Li, J.; Dong, J. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]







| Catalysts | Conversion (%) | Selectivity (%) |
|---|---|---|
| MIL-101(Fe) | 50.8 | 20.3 |
| ZIF-8(Zn) | 16.5 | 9.2 |
| ZIF-67(Co) | 35.7 | 32.6 |
| MIL-101(Cr) | 44.1 | 26.6 |
| HKUST-1(Cu) | 20.4 | 7.9 |
| MIL-125(Ti) | 18.2 | 10.1 |
| No catalyst | 26.7 | 0.05 |
| Oxidant | Conversion (%) | Selectivity (%) |
|---|---|---|
| Air | 11.3 | 30.9 |
| 30% H2O2 | 6.8 | 9.9 |
| 70% t-BHP | 28.1 | 36.7 |
| Solvent | Conversion (%) | Selectivity (%) |
|---|---|---|
| Acetic acid | 25.9 | 33.3 |
| Acetic anhydride | 38.1 | 15.9 |
| Ethyl acetate | 42.6 | 23.1 |
| Benzene | 20.3 | 49.4 |
| Times | Conversion (%) | Selectivity (%) | TON |
|---|---|---|---|
| First round | 29.8 | 55.4 | 18.1 |
| Second round | 33.5 | 41.6 | 13 |
| Third round | 32.5 | 28.0 | 8.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, Y.; Chen, D.; Luo, Z.; Wang, W.; Ao, Y.; Zhang, L.; Yan, Z.; Wang, J. Liquid-Phase Catalytic Oxidation of Limonene to Carvone over ZIF-67(Co). Catalysts 2019, 9, 374. https://doi.org/10.3390/catal9040374
Li Y, Yang Y, Chen D, Luo Z, Wang W, Ao Y, Zhang L, Yan Z, Wang J. Liquid-Phase Catalytic Oxidation of Limonene to Carvone over ZIF-67(Co). Catalysts. 2019; 9(4):374. https://doi.org/10.3390/catal9040374
Chicago/Turabian StyleLi, Yizhou, Yepeng Yang, Daomei Chen, Zhifang Luo, Wei Wang, Yali Ao, Lin Zhang, Zhiying Yan, and Jiaqiang Wang. 2019. "Liquid-Phase Catalytic Oxidation of Limonene to Carvone over ZIF-67(Co)" Catalysts 9, no. 4: 374. https://doi.org/10.3390/catal9040374





