Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications
Abstract
:1. Introduction
2. Process Parameters Affecting the Performance of PMRs
3. Types of PMRs
3.1. PMRs with Immobilized Photocatalyst (PMRs with PMs)
3.2. Slurry PMRs
4. Photocatalytic Membrane Reactors (PMRs) in Organic Syntheses and Energy Storage
4.1. Photocatalytic Reductions
4.1.1. Applications of PMRs with PMs
Hydrogen Production
4.1.2. Applications of Slurry PMRs
Conversion of Acetophenone to Phenylethanol
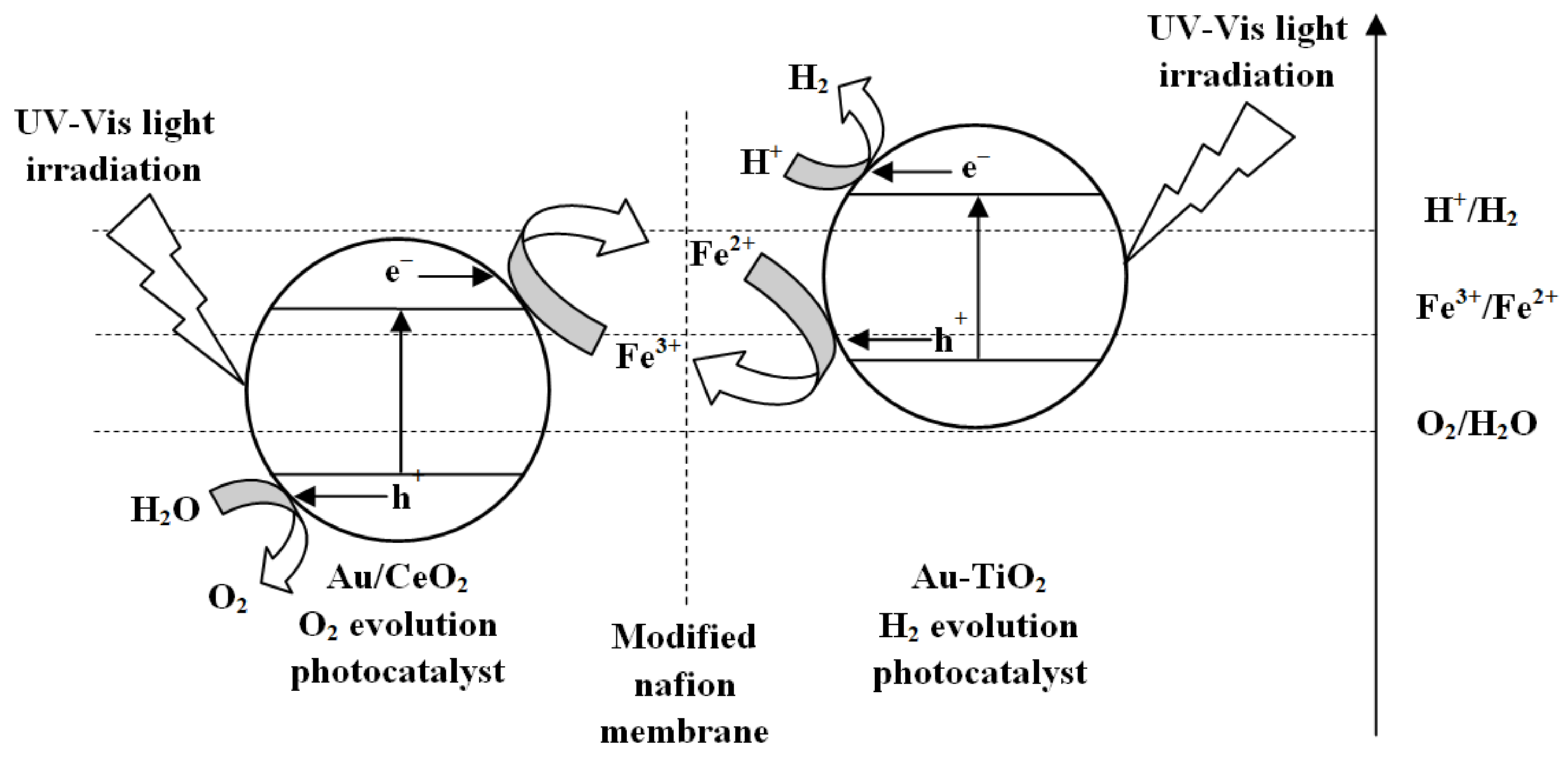
Hydrogen Production
4.2. Photocatalytic Oxidations
4.2.1. Applications of PMRs with PMs
4.2.2. Applications of Slurry PMRs
5. Photocatalytic Membrane Reactors (PMRs) in Environmental Applications
5.1. Applications of PMRs with PMs
5.2. PMRs with Photocatalyst in a Slurry: System Configurations and Applications
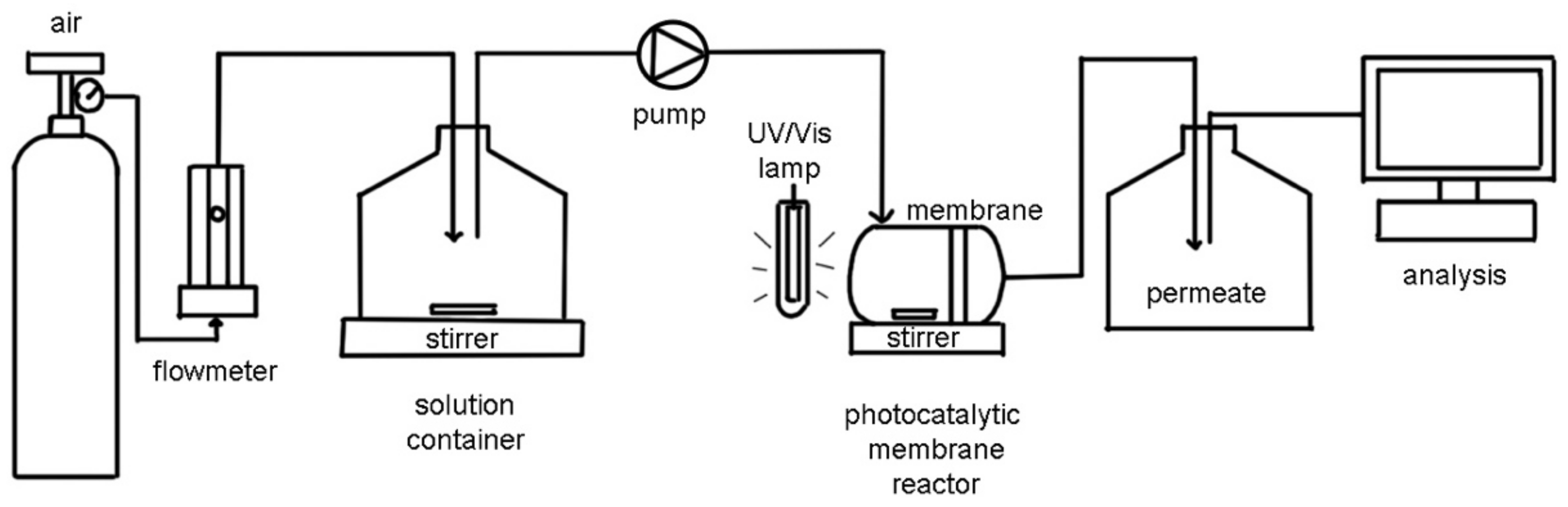
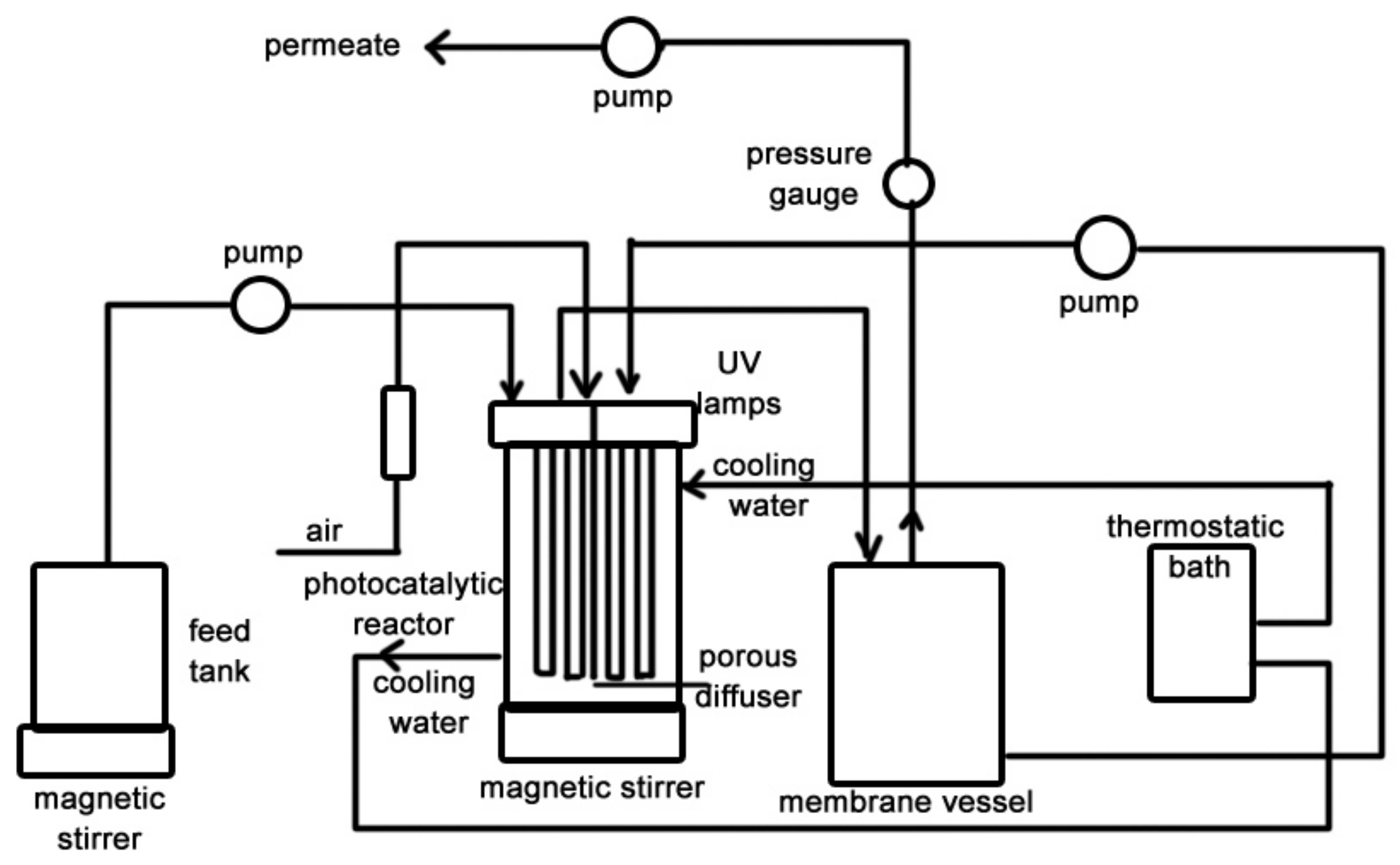
5.2.1. PMRs Utilizing Pressure-Driven Membrane Techniques
Depressurized PMRs: Systems with Submerged Membranes
Pressurized PMRs with Polymeric Membranes
Pressurized PMRs with Ceramic Membranes
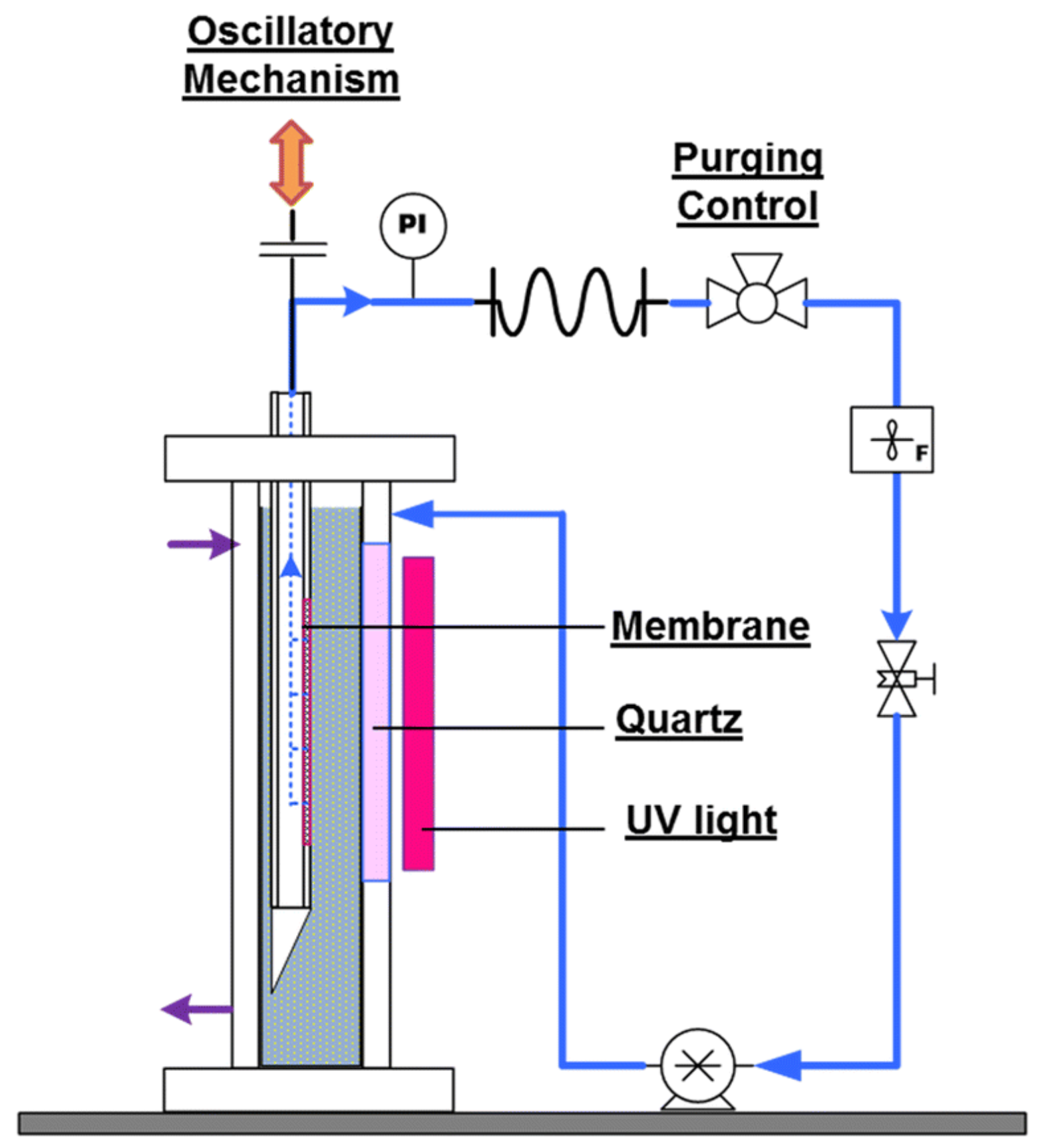
PMRs Utilizing Non-Pressure Driven Membrane Techniques
6. Summary and Future Perspective
Funding
Conflicts of Interest
Abbreviations
| AAS | atomic absorption spectroscopy |
| ALD | atomic layer deposition |
| AM | anion-exchange membrane |
| AOP | advanced oxidation process |
| AP | acetophenone |
| AROMA | Advanced Recovery and Oxidation Method for Aldehydes |
| BET | specific surface area |
| BPM | bipolar membrane |
| CA | cellulose actetate |
| CB | conduction band |
| CFV | cross flow velocity |
| CM | cation-exchange membrane |
| CNF | carbon nanofibre |
| CNT | carbon nanotube |
| COD | chemical oxygen demand |
| CR | congo red |
| DCF | diclofenac |
| DCMD | direct contact membrane distillation |
| DMF | N, N-dimethylformamide |
| DOC | dissolved organic carbon |
| e− | electron |
| Eg | band gap of energy |
| FAU | faujasite |
| FMBR | fungal membrane bioreactor |
| GO | graphene oxide |
| GQD | graphene quantum dot |
| GW | ground water |
| hn | energy |
| h+ | hole |
| HA | humic acid |
| HPLC | high performance liquid chromatography |
| HS | humic substances |
| IBU | ibuprofen |
| IR | infrared |
| KA oil | mixture of cyclohexanol (A) and cyclohexanone (K) |
| LBL | layer by layer |
| LPRO | low pressure reverse osmosis |
| MB | methylene blue |
| MD | membrane distillation |
| MDF | middle density fiberboard |
| MF | microfiltration |
| MOF | metal organic framework |
| MS | membrane separation |
| MV | methyl violet |
| MWCO | molecular weight cut off |
| NAP | naproxen |
| NF | nanofiltration |
| NMP | N-methyl-2-pyrrolidone |
| NP | photocatalyst nanoparticle |
| OTC | oxytetracycline |
| PAN | polyacrylonitrile |
| PC | photocatalysis |
| PDA | polydopamine |
| PE | phenyl ethanol |
| PE | primary effluent |
| PEBA | polyether-block-amide |
| PEG | poly(ethylene glycol) |
| PEI | polyethyleneimine |
| PES | polyethersulfone |
| PGS | progesterone |
| PM | photocatalytic membrane |
| PMR | photocatalytic membrane reactor |
| POMS | polyoctylmethylsiloxane |
| PP | polypropylene |
| PR | photoreactor |
| PSF | polysulfone |
| PU | polyurethane |
| PV | pervaporation |
| PVDF | poly(vinylidene fluoride) |
| PVP | polyvinylpyrrolidone |
| PVPR | pervaporation photocatalytic reactor |
| RhB | rhodamine B |
| RO | reactive orange |
| RO | reverse osmosis |
| ROS | reactive oxygen species |
| RTD | rapid thermal deposition |
| RW | real water |
| RWGS | reverse water-gas shift |
| SA | sodium alginate |
| SE | secondary effluent |
| SEM | scanning electron microscopy |
| SEOM | secondary effluent organic matter |
| SW | surface water |
| TBT | tetrabutyl titanate |
| TEM | transmission electron microscopy |
| TFC | thin film composite |
| THF | tetrahydrofuran |
| TMP | transmembrane pressure |
| TNA | titanium dioxide nanotube array |
| TOC | total organic carbon |
| TW | tap water |
| UF | ultrafiltration |
| UV | ultraviolet |
| UW | ultrapure water |
| VB | valence band |
| VIS | visible |
| VMD | vacuum membrane distillation |
References
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: State of the art and present applications. Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Molinari, R.; Marino, T.; Argurio, P. Photocatalytic membrane reactors for hydrogen production from water. Photocatalytic membrane reactors for hydrogen production from water. Int. J. Hydrogen Energy 2014, 39, 7247–7261. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Meng, L.; Shi, J.; Li, J.; Zhang, X.; Feng, M. Metal-organic frameworks/carbon-based materials for environmental remediation: A state-of-the-art mini-review. J. Environ. Manag. 2019, 232, 964–997. [Google Scholar] [CrossRef]
- Hwangbo, M.; Claycomb, E.C.; Liu, Y.; Alivio, T.E.G.; Banerjee, S.; Chu, K.-H. Effectiveness of zinc oxide-assisted photocatalysis for concerned constituents in reclaimed wastewater: 1,4-Dioxane, trihalomethanes, antibiotics, antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs). Sci. Total Environ. 2019, 649, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Z.; Zhang, Z. Pd-nanoparticle-decorated peanut-shaped BiVO4 with improved visible light-driven photocatalytic activity comparable to that of TiO2 under UV light. J. Catal. 2017, 356, 53–64. [Google Scholar] [CrossRef]
- Zhi, Y.; Ma, S.; Xia, H.; Zhang, Y.; Shi, Z.; Mu, Y.; Liu, X. Construction of donor-acceptor type conjugated microporous polymers: A fascinating strategy for the development of efficient heterogeneous photocatalysts in organic synthesis. Appl. Catal. B Environ. 2019, 244, 36–44. [Google Scholar] [CrossRef]
- Parrino, F.; Bellardita, M.; García-López, E.I.; Marcì, G.; Loddo, V.; Palmisano, L. Heterogeneous Photocatalysis for Selective Formation of High-Value-Added Molecules: Some Chemical and Engineering Aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Megna, B.; Parrino, F.; Palmisano, L. Photocatalytic oxidation of trans-ferulic acid to vanillin on TiO2 and WO3-loaded TiO2 catalysts. Catal. Today 2015, 252, 195–200. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Lavorato, C. Review on reduction and partial oxidation of organics in Photocatalytic (Membrane) Reactors. Curr. Org. Chem. 2013, 17, 2516–2537. [Google Scholar] [CrossRef]
- Palmisano, G.; Augugliaro, V.; Pagliaro, M.; Palmisano, L. Photocatalysis: A promising route for 21st century organic chemistry. Chem. Commun. 2007, 33, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P.; Bellardita, M.; Palmisano, L. Photocatalytic Processes in Membrane Reactors. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; Volume 3, pp. 101–138. [Google Scholar] [CrossRef]
- König, B. Photocatalysis in Organic Synthesis—Past, Present, and Future. Eur. J. Org. Chem. 2017, 15, 1979–1981. [Google Scholar] [CrossRef]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Heterogeneous Photocatalysis: A Promising Advanced Oxidation Process. In Current Trends and Future Developments on (Bio-)Membranes—Photocatalytic Membranes and Photocatalytic Membrane Reactors; Basile, A., Mozia, S., Molinari, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–43. [Google Scholar]
- Molinari, R.; Argurio, P. PMRs in Photocatalytic Synthesis of Organic Compounds. In Current Trends and Future Developments on (Bio-)Membranes—Photocatalytic Membranes and Photocatalytic Membrane Reactors; Basile, A., Mozia, S., Molinari, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 209–231. [Google Scholar]
- Meng, X.; Zhang, Z.; Li, X. Synergetic photoelectrocatalytic reactors for environmental remediation: A review. J. Photochem. Photobiol. C 2015, 24, 83–101. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-Y. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Q.; Chen, L.; Wang, J.; Cheng, R. Photocatalytic membrane reactor (PMR) for virus removal in water: Performance and mechanisms. Chem. Eng. J. 2015, 277, 124–129. [Google Scholar] [CrossRef]
- Jiang, L.; Choo, K. Photocatalytic mineralization of secondary effluent organic matter with mitigating fouling propensity in a submerged membrane photoreactor. Chem. Eng. J. 2016, 288, 798–805. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Wróbel, R.; Morawski, A.W. A study on the stability of polyethersulfone ultrafiltration membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2015, 495, 176–186. [Google Scholar] [CrossRef]
- Choo, K.; Chang, D.; Park, K.; Kim, M. Use of an integrated photocatalysis/hollow fiber microfiltration system for the removal of trichloroethylene in water. J. Hazard. Mater. 2008, 152, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Caruso, A.; Argurio, P.; Poerio, T. Degradation of the drugs Gemfibrozil and Tamoxifen in pressurized and de-pressurized membrane photoreactors using suspended polycrystalline TiO2 as catalyst. J. Membr. Sci. 2008, 319, 54–63. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Rokicka, P.; Wang, K.; Endo, M.; Morawski, A.W.; Kowalska, E. Photocatalytic water disinfection under solar irradiation by D-glucose-modified titania. Catalysts 2018, 8, 316. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Mohammad, A.W.; Kadhum, A.A.H. Effect of various zinc oxide nanoparticles in membrane photocatalytic reactor for Congo red dye treatment. Sep. Purif. Technol. 2014, 137, 74–81. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S. Performance of an indigenous integrated slurry photocatalytic membrane reactor (PMR) on the removal of aqueous phenanthrene (PHE). Water Sci. Technol. 2018, 77, 2642–2656. [Google Scholar] [CrossRef] [PubMed]
- Damodar, R.A.; You, S.-J.; Ou, S.-H. Coupling of membrane separation with photocatalytic slurry reactor for advanced dye wastewater treatment. Sep. Purif. Technol. 2010, 76, 64–71. [Google Scholar] [CrossRef]
- Sopajaree, K.; Qasim, S.A.; Basak, S.; Rajeshwar, K. An integrated flow reactor-membrane filtration system for heterogeneous photocatalysis. Part II: Experiments on the ultrafiltration unit and combined operation. J. Appl. Electrochem. 1999, 29, 1111–1118. [Google Scholar] [CrossRef]
- Molinari, R.; Caruso, A.; Poerio, T. Direct benzene conversion to phenol in a hybrid photocatalytic membrane reactor. Catal. Today 2009, 144, 81–86. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Photocatalytic reduction of acetophenone in membrane reactors under UV and visible light using TiO2 and Pd/TiO2 catalysts. Chem. Eng. J. 2015, 274, 307–316. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Bekkouche, S.; Bouhelassa, M.; Hadj Salah, N.; Meghlaoui, F.Z. Study of adsorption of phenol on titanium oxide (TiO2). Desalination 2004, 166, 355–362. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Cardillo, A.; Loddo, V.; Palmisano, L.; Parrino, F. Improvement of membrane performances to enhance the yield of vanillin in a pervaporation reactor. Membranes 2014, 4, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Emeline, A.V.; Ryabchuk, V.; Serpone, N. Factors affecting the efficiency of a photocatalyzed process aqueous metal-oxide dispersions: Prospect of distinguishing between two kinetic models. J. Photochem. Photobiol. Chem. 2000, 133, 89–97. [Google Scholar] [CrossRef]
- Brosillon, S.; Lhomme, L.; Vallet, C.; Bouzaza, A.; Wolbert, D. Gas phase photocatalysis and liquid phase photocatalysis: Interdependence and influence of substrate concentration and photon flow on degradation reaction kinetics. Appl. Catal. B Environ. 2008, 78, 232–241. [Google Scholar] [CrossRef]
- Palmisano, G.; Yurdakal, S.; Augugliaro, V.; Loddo, V.; Palmisano, L. Photocatalytic selective oxidation of 4-methoxybenzyl alcohol to aldehyde in aqueous suspension of home-prepared titanium dioxide catalyst. Adv. Synth. Catal. 2007, 349, 964–970. [Google Scholar] [CrossRef]
- Zhang, T.; You, L.; Zhang, Y. Photocatalytic reduction of p-chloronitrobenzene on illuminated nano-titanium dioxide particles. Dyes Pigments 2006, 68, 95–100. [Google Scholar] [CrossRef]
- Du, X.; Qu, F.-S.; Liang, H.; Li, K.; Bai, L.-M.; Li, G.-B. Control of submerged hollow fiber membrane fouling caused by fine particles in photocatalytic membrane reactors using bubbly flow: Shear stress and particle forces analysis. Sep. Purif. Technol. 2017, 172, 130–139. [Google Scholar] [CrossRef]
- Szymański, K.; Mozia, S.; Morawski, A.W. Humic acids removal in a photocatalytic membrane reactor with a ceramic UF membrane. Chem. Eng. J. 2016, 305, 19–27. [Google Scholar] [CrossRef]
- Mozia, S.; Szymański, K.; Michalkiewicz, B.; Tryba, B.; Toyoda, M.; Morawski, A.W. Effect of process parameters on fouling and stability of MF/UF TiO2 membranes in a photocatalytic membrane reactor. Sep. Purif. Technol. 2015, 142, 137–148. [Google Scholar] [CrossRef]
- Li, Q.; Jia, R.; Shao, J.; He, Y. Photocatalytic degradation of amoxicillin via TiO2 nanoparticle coupling with a novel submerged porous ceramic membrane reactor. J. Clean. Prod. 2019, 209, 755–761. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Orecki, A.; Wróbel, R.; Wilpiszewska, K.; Morawski, A.W. Microscopic studies on TiO2 fouling of MF/UF polyethersulfone membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2014, 470, 356–368. [Google Scholar] [CrossRef]
- Sabouni, R.; Gomaa, H. Photocatalytic degradation of pharmaceutical micro-pollutants using ZnO. Environ. Sci. Pollut. Res. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Wang, W.Y.; Irawan, A.; Ku, Y. Photocatalytic degradation of Acid Red 4 using a titanium dioxide membrane supported on a porous ceramic tube. Water Res. 2008, 42, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Stability study of PVDF/TiO2 dual layer hollow fibre membranes under long-term UV irradiation exposure. J. Water Process Eng. 2017, 15, 78–82. [Google Scholar] [CrossRef]
- Kim, J.H.; Joshi, M.K.; Lee, J.; Park, C.H.; Kim, C.S. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiong, S.; Wang, Y. Atomic layer deposition of hybrid metal oxides on carbon nanotube membranes for photodegradation of dyes. Comput. Commun. 2019, 12, 39–46. [Google Scholar] [CrossRef]
- De Filpo, G.; Pantuso, E.; Armentano, K.; Formoso, P.; Di Profio, G.; Poerio, T.; Fontananova, E.; Meringolo, C.; Mashin, A.I.; Nicoletta, F.P. Chemical Vapor Deposition of Photocatalyst Nanoparticles on PVDF Membranes for Advanced Oxidation Processes. Membranes 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Yadav, V.S.K.; Purkait, M.K. Cu2O photocatalyst modified antifouling polysulfone mixed matrix membrane for ultrafiltration of protein and visible light driven photocatalytic pharmaceutical removal. Sep. Purif. Technol. 2019, 212, 191–204. [Google Scholar] [CrossRef]
- Rao, G.; Zhang, Q.; Zhao, H.; Chen, J.; Li, Y. Novel titanium dioxide/iron (III) oxide/graphene oxide photocatalytic membrane for enhanced humic acid removal from water. Chem. Eng. J. 2016, 302, 633–640. [Google Scholar] [CrossRef]
- Gao, B.; Chen, W.; Liu, J.; An, J.; Wang, L.; Zhu, Y.; Sillanpää, M. Continuous removal of tetracycline in a photocatalytic membrane reactor(PMR) with ZnIn2S4 as adsorption and photocatalytic coating layer on PVDF membrane. J. Photochem. Photobiol. A 2018, 364, 732–739. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L. Evaluation of the antifouling and photocatalytic properties of novel poly(vinylidene fluoride) membranes with a reduced graphene oxide–Bi2WO6 active layer. J. Appl. Polym. Sci. 2017, 42, 45426. [Google Scholar] [CrossRef]
- Yu, Z.; Min, X.; Li, F.; Yin, D.; Peng, Y.; Zeng, G. A mussel-inspired method to fabricate a novel reduced graphene oxide/Bi12O17Cl2 composites membrane for catalytic degradation and oil/water separation. Polym. Adv. Technol. 2019, 30, 101–109. [Google Scholar] [CrossRef]
- Baig, U.; Matin, A.; Gondal, M.A.; Zubaira, S.M. Facile fabrication of superhydrophobic, superoleophilic photocatalytic membrane for efficient oil-water separation and removal of hazardous organic pollutants. J. Clean. Prod. 2019, 208, 904–915. [Google Scholar] [CrossRef]
- Alias, S.S.; Harun, Z.; Suyana, I.; Latif, A. Characterization and performance of porous photocatalytic ceramic membranes coated with TiO2 via different dip-coating routes. J. Mater. Sci. 2018, 53, 11534–11552. [Google Scholar] [CrossRef]
- Salim, N.E.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Qtaishat, M.R.; Matsuura, T.; Othman, M.H.D.; Rahman, M.A.; Aziza, F.; Yusof, N. Effects of hydrophilic surface macromolecule modifier loading on PES/O-gC3N4 hybrid photocatalytic membrane for phenol removal. Appl. Surf. Sci. 2019, 465, 180–191. [Google Scholar] [CrossRef]
- Dong, P.; Huan, Z.; Nie, X.; Cheng, X.; Jin, Z.; Zhang, X. Plasma enhanced decoration of nc-TiO2 on electrospun PVDF fibers for photocatalytic application. Mater. Res. Bull. 2019, 111, 102–112. [Google Scholar] [CrossRef]
- Zhang, G.; Sheng, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hierarchical Titanium Dioxide Nanowire/Metal–Organic Framework/Carbon Nanofiber Membranes for Highly Efficient Photocatalytic Degradation of Hydrogen Sulfide. Chem. Eur. J. 2018, 24, 15019–15025. [Google Scholar] [CrossRef] [PubMed]
- Kovács, I.; Beszédes, S.; Kertész, S.; Veréb, G.; Hodúr, C.; Papp, I.Z.; Kukovecz, Á.; László, Z. Investigation of titanium-dioxide coatings on membrane filtration properties. Studia Universitatis Babes-Bolyai Chemia 2017, 62, 249–259. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal Nitrogen-Doped TiO2 Photocatalytic Coatings for Sunlight-Activated Membranes. Adv. Sustain. Syst. 2017, 1, 1600041. [Google Scholar] [CrossRef]
- Yang, H.-C.; Waldman, R.Z.; Chen, Z.; Darling, S.B. Atomic layer deposition for membrane interface engineering. Nanoscale 2018, 10, 20505. [Google Scholar] [CrossRef] [PubMed]
- Camera-Roda, G.; Loddo, V.; Palmisano, L.; Parrino, F. PMRs Utilizing None Pressure-Driven Membrane Techniques. In Current Trends and Future Developments on (Bio-)Membranes—Photocatalytic Membranes and Photocatalytic Membrane Reactors; Basile, A., Mozia, S., Molinari, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 129–167. [Google Scholar]
- Vankelecom, I.F.J. Polymeric Membranes in Catalytic Reactors. Chem. Rev. 2002, 102, 3779–3810. [Google Scholar] [CrossRef] [PubMed]
- Dioos, B.M.L.; Vankelecom, I.F.J.; Jacobs, P.A. Aspects of Immobilisation of Catalysts on Polymeric Supports. Adv. Synth. Catal. 2006, 348, 1413–1446. [Google Scholar] [CrossRef]
- Pomilla, F.R.; Brunetti, A.; Marcì, G.; Garcıá-Loṕez, E.I.; Fontananova, E.; Palmisano, L.; Barbieri, G. CO2 to Liquid Fuels: Photocatalytic Conversion in a Continuous Membrane Reactor. ACS Sustain. Chem. Eng. 2018, 6, 8743–8753. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yu, J.C.-C.; Nguyen, V.-H.; Wu, J.C.-S.; Wang, W.-H.; Kocí, K. Reactor Design for CO2 Photo-Hydrogenation toward Solar Fuels under Ambient Temperature and Pressure. Catalysts 2017, 7, 63. [Google Scholar] [CrossRef]
- Lunde, P.J.; Kester, F.L. Rates of methane formation from carbon dioxide and hydrogen over a ruthenium catalyst. J. Catal. 1973, 30, 423–429. [Google Scholar] [CrossRef]
- Thampi, K.R.; Kiwi, J.; Gratzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508. [Google Scholar] [CrossRef]
- Lin, W.; Han, H.; Frei, H. CO2 splitting by H2O to CO and O2 under UV light in TiMCM-41 silicate sieve. J. Phys. Chem. B 2004, 108, 18269–18273. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, Y.; Ke, X.; Tong, X.; Sun, Z.; Liang, M.; Xue, S. Photocatalytic reduction of carbon dioxide to formic acid, formaldehyde, and methanol using dye-sensitized TiO2 film. Appl. Catal. B Environ. 2013, 129, 599–605. [Google Scholar] [CrossRef]
- Slamet, H.; Nasution, H.W.; Purnama, E.; Kosela, S.; Lazuardi, G. Photocatalytic reduction of CO2 on copper-doped titania catalysts prepared by improved-impregnation method. Catal. Commun. 2005, 6, 313–319. [Google Scholar] [CrossRef]
- Mele, G.; Annese, C.; De Riccardis, A.; Fusco, C.; Palmisano, L.; Vasapollo, G.; D’Accolti, L. Turning lipophilic phthalocyanines/TiO2 composites into efficient photocatalysts for the conversion of CO2 into formic acid under UV–vis light irradiation. Appl. Catal. A Chem 2014, 481, 169–172. [Google Scholar] [CrossRef]
- Ichikawa, S.; Doi, R. Hydrogen production from water and conversion of carbon dioxide to useful chemicals by room temperature photoelectrocatalysis. Catal. Today 1996, 27, 271–277. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of Material Design and Reactor Engineering on TiO2 Photocatalysis for CO2 Reduction. J. Photochem. Photobiol. C 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Maina, J.W.; Schütz, J.A.; Grundy, L.; Des Ligneris, E.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumée, L.F. Inorganic Nanoparticles/Metal Organic Framework Hybrid Membrane Reactors for Efficient Photocatalytic Conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schutz, J.; Hill, M.; Dumee, L.F. Metal Organic Framework Based Catalysts for CO2 Conversion. Mater. Horiz. 2017, 4, 345–361. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-Based Mofs for Photocatalytic CO2 reduction: Role of Coordination Unsaturated Sites and Dual Excitation Pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal−Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Ye, J. Metal-Organic Frameworks for Photocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 7563–7572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, X.; Xu, R.; Li, J.; Gao, S.; Cao, R. CdS/NH2-UiO-66 hybrid membrane reactors for the efficient photocatalytic conversion of CO2. J. Mater. Chem. A 2018, 6, 20152–20160. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-Doped g-C3N4 with Enhanced Photocatalytic CO2-Reduction Performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Mao, J.; Peng, T.; Zhang, X.; Li, K.; Ye, L.; Zan, L. Effect of Graphitic Carbon Nitride Microstructures on the Activity and Selectivity of Photocatalytic CO2 Reduction Under Visible Light. Catal. Sci. Technol. 2013, 3, 1253–1260. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, L. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Liu, L.; Yue, M.; Lu, J.; Hu, J.; Liang, Y.; Cui, W. The Enrichment of Photo-Catalysis Via Self-Assembly Perylenetetracarboxylic Acid Diimide Polymer Nanostructures Incorporating TiO2 Nano-Particles. Appl. Surf. Sci. 2018, 456, 645–656. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Wu, M.; Du, Y.; Fan, X.; Wang, M.; Zhang, L.; Kong, Q.; Shi, J. Mesostructured CeO2/g-C3N4 nanocomposites: Remarkably Enhanced Photocatalytic Activity for CO2 Reduction by Mutual Component Activations. Nano Energy 2016, 19, 145–155. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, S.; Ren, H.; Hou, Y.; Wang, X. Photocatalytic Reduction of CO2 by Graphitic Carbon Nitride Polymers Derived from Urea and Barbituric Acid. Appl. Catal. B Environ. 2015, 179, 1–8. [Google Scholar] [CrossRef]
- Xiao, J.; Han, Q.; Xie, Y.; Yang, J.; Su, Q.; Chen, Y.; Cao, H. Is C3N4 Chemically Stable toward Reactive Oxygen Species in Sunlight-Driven Water Treatment? Environ. Sci. Technol. 2017, 51, 13380–13387. [Google Scholar] [CrossRef] [PubMed]
- Pomilla, F.R.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Molinari, R.; Barbieri, G.; Marcì, G.; Palmisano, L.; Sharma, P.K.; Brown, A.; Byrne, J.A. An Investigation into the Stability of Graphitic C3N4 as a Photocatalyst for CO2 Reduction. J. Phys. Chem. C 2018, 122, 28727–28738. [Google Scholar] [CrossRef]
- Liu, X.; Jian, X.; Yang, H.; Song, X.; Liang, Z. A photocatalytic graphene quantum dots–Cu2O/bipolar membrane as a separator for water splitting. New J. Chem. 2016, 40, 3075–3079. [Google Scholar] [CrossRef]
- Vargas-Barbosa, N.M.; Geise, G.M.; Hickner, M.A.; Mallouk, T.E. Assessing the utility of bipolar membranes for use in photoelectrochemical water-splitting cells. ChemSusChem 2014, 7, 3017–3020. [Google Scholar] [CrossRef] [PubMed]
- Readi, O.M.K.; Kuenen, H.J.; Zwijnenberg, H.J.; Nijmeijer, K. Novel membrane concept for internal pH control in electrodialysis of amino acids using a segmented bipolar membrane (sBPM). J. Membr. Sci. 2013, 443, 219–226. [Google Scholar] [CrossRef]
- Rajesh, A.M.; Kumar, M.; Shahi, V.K. Functionalized biopolymer based bipolar membrane with poly ethylene glycol interfacial layer for improved water splitting. J. Membr. Sci. 2011, 372, 249–257. [Google Scholar] [CrossRef]
- Atienzar, P.; Primo, A.; Lavorato, C.; Molinari, R.; García, H. Preparation of Graphene Quantum Dots from Pyrolyzed Alginate. Langmuir 2013, 29, 6141–6146. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Forni, L.; Selli, E. Photocatalytic hydrogen production by liquid-and gas-phase reforming of CH3OH over flame-made TiO2 and Au/TiO2. Catal. Today 2009, 144, 69–74. [Google Scholar] [CrossRef]
- Hattori, M.; Noda, K. All electrochemical fabrication of a bilayer membrane composed of nanotubular photocatalyst and palladium toward high-purity hydrogen production. Appl. Surf. Sci. 2015, 357, 214–220. [Google Scholar] [CrossRef]
- Su, C.-Y.; Wang, L.-C.; Liu, W.-S.; Wang, C.-C.; Perng, T.-P. Photocatalysis and Hydrogen Evolution of Al- and Zn-Doped TiO2 Nanotubes Fabricated by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 33287–33295. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Wang, C.C.; Hsueh, Y.C.; Gurylev, V.; Kei, C.C.; Perng, T.P. Enabling High Solubility of ZnO in TiO2 by Nanolamination of Atomic Layer Deposition. Nanoscale 2015, 7, 19222–19230. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Wang, C.C.; Hsueh, Y.C.; Gurylev, V.; Kei, C.C.; Perng, T.P. Fabrication of Highly Homogeneous Al-doped TiO2 Nanotubes by Nanolamination of Atomic Layer Deposition. J. Am. Ceram. Soc. 2017, 100, 4988–4993. [Google Scholar] [CrossRef]
- Lavorato, C.; Argurio, P.; Mastropietro, T.F.; Pirri, G.; Poerio, T.; Molinari, R. Pd/TiO2 doped faujasite photocatalysts for acetophenone transfer hydrogenation in a photocatalytic membrane reactor. J. Catal. 2017, 353, 152–161. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Wen, F.; Li, C. A visible-light-driven transfer hydrogenation on CdS nanoparticles combined with iridium complexes. Chem. Commun. 2011, 47, 7080–7082. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M.; Duchamp, C.; Karkmaz, M.; Thu Hoai, B.; Lachheb, H.; Puzenat, E.; Guillard, C. Environmental green chemistry as defined by photocatalysis. J. Hazard. Mater. 2007, 146, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M. Utilization of TiO2 photocatalysts in green chemistry. Pure Appl. Chem. 2000, 72, 1265–1270. [Google Scholar] [CrossRef]
- Dubey, N.; Rayalu, S.S.; Labhsetwar, N.K.; Naidu, R.R.; Chatti, R.V.; Devotta, S. Photocatalytic properties of zeolite-based materials for the photoreduction of methyl orange. Appl. Catal. A Gen. 2006, 303, 152–157. [Google Scholar] [CrossRef]
- Marino, T.; Figoli, A.; Molino, A.; Argurio, P.; Molinari, R. Hydrogen and Oxygen Evolution in a Membrane Photoreactor Using Suspended Nanosized Au/TiO2 and Au/CeO2. ChemEngineering 2019, 3, 5. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Qiu, P.; Wen, P.; Wang, H.; Xu, G.; Han, Y. C-doped Cr2O3/NaY composite membrane supported on stainless steel mesh with enhanced photocatalytic activity for cyclohexane oxidation. J. Mater. Sci. 2018, 53, 6552–6561. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Hirai, T. Visible light-induced partial oxidation of cyclohexane on WO3 loaded with Pt nanoparticles. Catal. Sci. Technol. 2012, 2, 400–405. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Parrino, F.; Puma, M.A. Synthesis of vanillin in water by TiO2 photocatalysis. Appl. Catal. B Environ. 2012, 111, 555–561. [Google Scholar] [CrossRef]
- Pickett-Baker, J.; Ozaki, R. Pro-Environmental Products: Marketing Influence on Consumer Purchase Decision. J. Consum. Mark. 2008, 25, 281–293. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Cardillo, A.; Loddo, V.; Palmisano, G.; Palmisano, L. A pervaporation photocatalytic reactor for the green synthesis of vanillin. Chem. Eng. J. 2013, 224, 136–143. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Santarelli, F.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Photocatalytic process intensification by coupling with pervaporation. Catal. Today 2011, 161, 209–213. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Santarelli, F. Design of a Pervaporation Photocatalytic Reactor for Process Intensification. Chem. Eng. Technol. 2012, 35, 1221–1228. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Bengston, G.; Pingel, H.; Dozel, S. Pervaporation of high boilers using heated membranes. Desalination 1993, 90, 249–257. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Gatfield, I.L.; Jähnig, J.; Schorm, C. Pervaporation at the vapor pressure limit: Vanillin. J. Membr. Sci. 1997, 137, 155–158. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Loddo, V.; Palmisano, L. Pervaporation membrane reactors. In Handbook of Membrane Reactors: Reactor Types and Industrial Applications; Basile, A., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 2, pp. 107–151. [Google Scholar]
- Brazhina, C.; Barbosa, B.; Crespo, G.J. Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step. Green Chem. 2011, 13, 2197–2203. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Production of Aldehydes by Oxidation in Aqueous Medium with Recovery of the Product by Means of Pervaporation. U.S. Patent 20130123546A1, 10 June 2011. [Google Scholar]
- Molinari, R.; Lavorato, C.; Poerio, T. Performance of vanadium based catalyst in a membrane contactor for the benzene hydroxylation to phenol. Appl. Catal. A Gen. 2012, 417, 87–92. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Direct oxidation of benzene to phenol by molecular oxygen over catalytic systems containing Pd(OAc)2 and heteropolyacid immobilized on HMS or PIM. J. Mol. Catal. A Chem. 2006, 256, 247–255. [Google Scholar] [CrossRef]
- Shimizu, K.; Akahane, H.; Kodama, T.; Kitayama, Y. Selective photo-oxidation of benzene over transition metal-exchanged BEA zeolite. Appl. Catal. A Gen. 2004, 269, 75–80. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Vanadyl acetylacetonate filled PVDF membranes as the core of a liquid phase continuous process for pure phenol production from benzene. J. Membr. Sci. 2015, 476, 490–499. [Google Scholar] [CrossRef]
- Molinari, R.; Palmisano, L.; Loddo, V.; Mozia, S.; Morawski, A.W. Photocatalytic membrane reactors: Configurations, performance and applications in water treatment and chemical production. In Handbook of Membrane Reactors: Reactor Types and Industrial Applications; Basile, A., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 2, pp. 808–845. [Google Scholar]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.H. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Mesones, S.; Marugán, J. Hybrid UV-C/microfiltration process in membrane photoreactorfor wastewater disinfection. Environ. Sci. Pollut. Res. 2018, 1–8. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Luster, E.; Lozzi, L.; Luxbacher, T.; Mamane, H. MS2 bacteriophage inactivation using a N-doped TiO2-coated photocatalytic membrane reactor: Influence of water-quality parameters. Chem. Eng. J. 2018, 354, 995–1006. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; Mesones, S.; Casado, C.; van Grieken, R. Bacterial inactivation and degradation of organic molecules by titanium dioxide supported on porous stainless steel photocatalytic membranes. Chem. Eng. J. 2017, 318, 29–38. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Cho, M.; Lee, S.S.; Zhang, F.; Biswas, P.; Fortner, J.D. In Situ Photocatalytic Synthesis of Ag Nanoparticles (nAg) by Crumpled Graphene Oxide Composite Membranes for Filtration and Disinfection Applications. Environ. Sci. Technol. 2016, 50, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate. J. Hazard. Mater. 2018, 344, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef]
- Roso, M.; Boaretti, C.; Bonora, R.; Modesti, M.; Lorenzetti, A. Nanostructured Active Media for Volatile Organic Compounds Abatement: The Synergy of Graphene Oxide and Semiconductor Coupling. Ind. Eng. Chem. Res. 2018, 57, 16635–16644. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, Y.; Jiang, Y. Photocatalytic self-cleaning carbon nitride nanotube intercalated reduced graphene oxide membranes for enhanced water purification. Chem. Eng. J. 2019, 356, 915–925. [Google Scholar] [CrossRef]
- Sanches, S.; Nunes, C.; Passarinho, P.C.; Ferreira, F.C.; Pereira, V.J.; Crespo, J.G. Development of photocatalytic titanium dioxide membranes for degradation of recalcitrant compounds. Chem. Technol. Biotechnol. 2017, 92, 1727–1737. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 2019, 240, 9–18. [Google Scholar] [CrossRef]
- Guo, B.; Pasco, E.V.; Xagoraraki, I.; Tarabara, V.V. Virus removal and inactivation in a hybrid microfiltration-UV process with a photocatalytic membrane. Sep. Purif. Technol. 2015, 149, 245–254. [Google Scholar] [CrossRef]
- Song, L.; Zhu, B.; Jegatheesan, V.; Gray, S.; Duke, M.; Muthukumaran, S. Treatment of secondary effluent by sequential combination of photocatalytic oxidation with ceramic membrane filtration. Environ. Sci. Pollut. Res. 2018, 25, 5191–5202. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.F.; Abdulrahman, A.A.; Hamza, N.H. Hydrodynamic and kinetic study of a hybrid detoxification process with zero liquid discharge system in an industrial wastewater treatment. J. Environ. Health Sci. Eng. 2014, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Yatmaz, H.C.; Dizge, N.; Kurt, M.S. Combination of photocatalytic and membrane distillation hybrid processes for reactive dyes treatment. Environ. Technol. 2017, 38, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Patsios, S.I.; Sarasidis, V.C.; Karabelas, A.J. A hybrid photocatalysis-UF separation process for water purification: Humic acids and polysaccharides degradation. In Proceedings of the International Desalination Association World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. [Google Scholar]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2–ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Darowna, D.; Wróbel, R.; Morawski, A.W.; Mozia, S. The influence of feed composition on fouling and stability of a polyethersulfone ultrafiltration membrane in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 310, 360–367. [Google Scholar] [CrossRef]
- Fernández López, R.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (MPR). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar] [CrossRef]
- Sarasidis, V.C.; Plakas, K.V.; Patsios, S.I.; Karabelas, A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014, 239, 299–311. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Szymański, K.; Cristóvã, R.O.; Mendes, A.; Vilar, V.J.P.; Mozia, S. Performance of hybrid systems coupling advanced oxidation processes and ultrafiltration for oxytetracycline removal. Catal. Today 2018. [Google Scholar] [CrossRef]
- Vatanpour, V.; Karami, A.; Sheydaei, M. Improved visible photocatalytic activity of TiO2 nanoparticles to use in submerged membrane photoreactor for organic pollutant degradation. Int. J. Environ. Sci. Technol. 2018, 1–10. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Matahum, J.; Tayo, L.; You, S.-J. Photodegradation of Reactive Black 5 in a ZnO/UV slurry membrane reactor. J. Taiwan Inst. Chem. Eng. 2015, 49, 136–141. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, X.; Peng, T. Removal of methylene blue using photocatalytic slurry reactor coupled with ceramic membrane cross-flow filtration process. Mater. Res. Innov. 2014, 18, 191–195. [Google Scholar] [CrossRef]
- O’Neal Stancl, H.; Hristovski, K.; Westerhoff, P. Hexavalent Chromium Removal Using UV-TiO2/Ceramic Membrane Reactor. Environ. Eng. Sci. 2015, 32, 676–683. [Google Scholar] [CrossRef]
- Doruk, N.; Yatmaz, H.C.; Dizge, N. Degradation Efficiency of Textile and Wood Processing Industry Wastewater by Photocatalytic Process Using In Situ Ultrafiltration Membrane. CSAWAC 2016, 44, 219–324. [Google Scholar] [CrossRef]
- Deveci, E.Ü.; Dizge, N.; Yatmaz, H.C.; Aytepe, Y. Integrated process of fungal membrane bioreactor and photocatalytic membrane reactor for the treatment of industrial textile wastewater. Biochem. Eng. J. 2016, 105, 420–427. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Szymański, K.; Grondzewska, S.; Borchert, K.; Wróbel, R.; Morawski, A.W. Performance of two photocatalytic membrane reactors for treatment of primary and secondary effluents. Catal. Today 2014, 236A, 135–145. [Google Scholar] [CrossRef]
- Szymański, K.; Morawski, A.W.; Mozia, S. Effectiveness of treatment of secondary effluent from a municipal wastewater treatment plant in a photocatalytic membrane reactor and hybrid UV/H2O2—Ultrafiltration system. Chem. Eng. Process 2018, 125, 318–324. [Google Scholar] [CrossRef]
- Darowna, D.; Grondzewska, S.; Morawski, A.W.; Mozia, S. Removal of non-steroidal anti-inflammatory drugs from primary and secondary effluents in a photocatalytic membrane reactor. J. Chem. Technol. Biotechnol. 2014, 89, 1265–1273. [Google Scholar] [CrossRef]
- Szymański, K.; Morawski, A.W.; Mozia, S. Surface water treatment in hybrid systems coupling advanced oxidation processes and ultrafiltration using ceramic membrane. Desalin. Water Treat. 2017, 64, 302–306. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Choo, K.H. Submerged microfiltration-catalysis hybrid reactor treatment: Photocatalytic inactivation of bacteria in secondary wastewater effluent. Sep. Purif. Technol. 2018, 198, 87–92. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Yuan, Y.; Chen, H.; Wang, H.; Hou, D. A novel microwave assisted photo-catalytic membrane distillation process for treating the organic wastewater containing inorganic ions. J. Water Process Eng. 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]





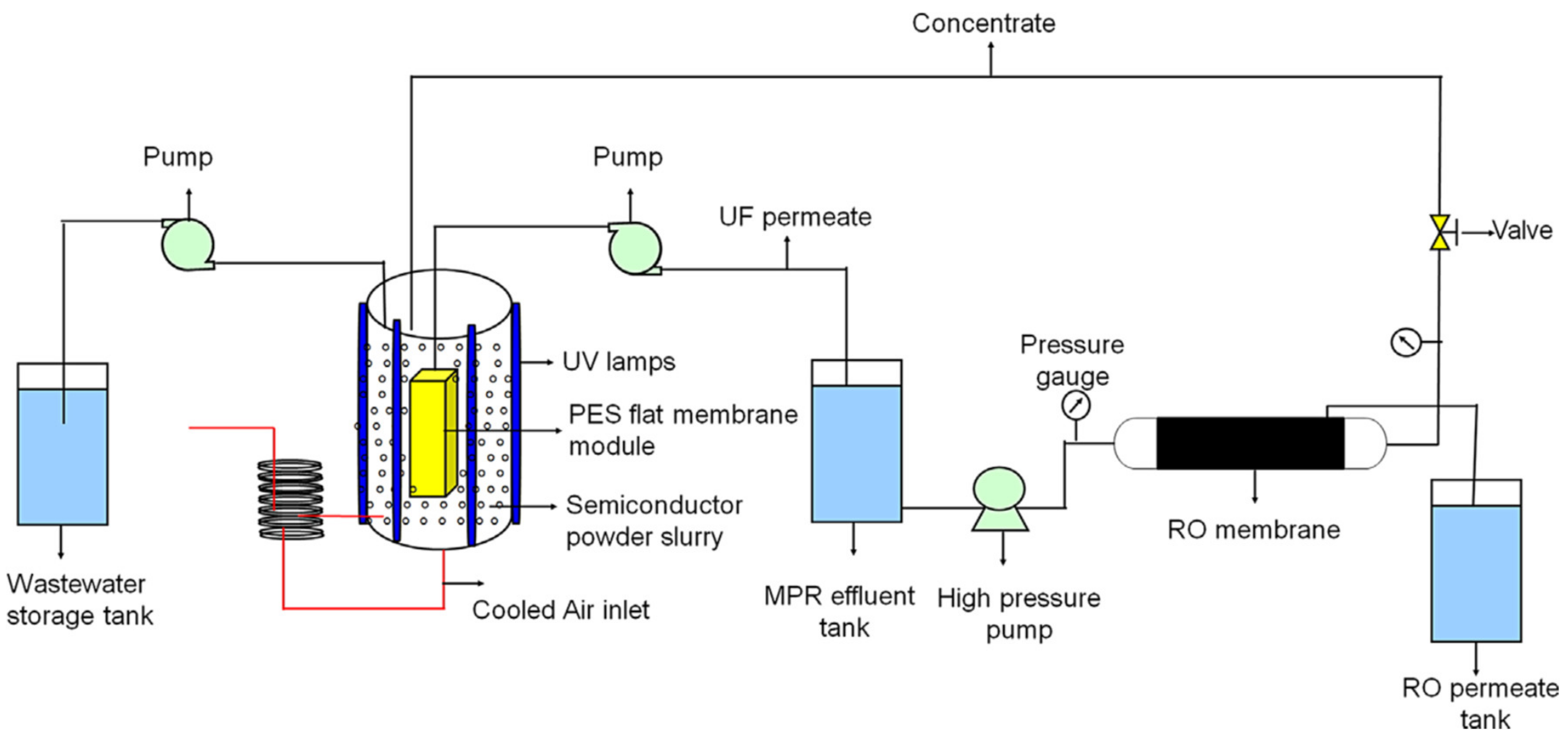
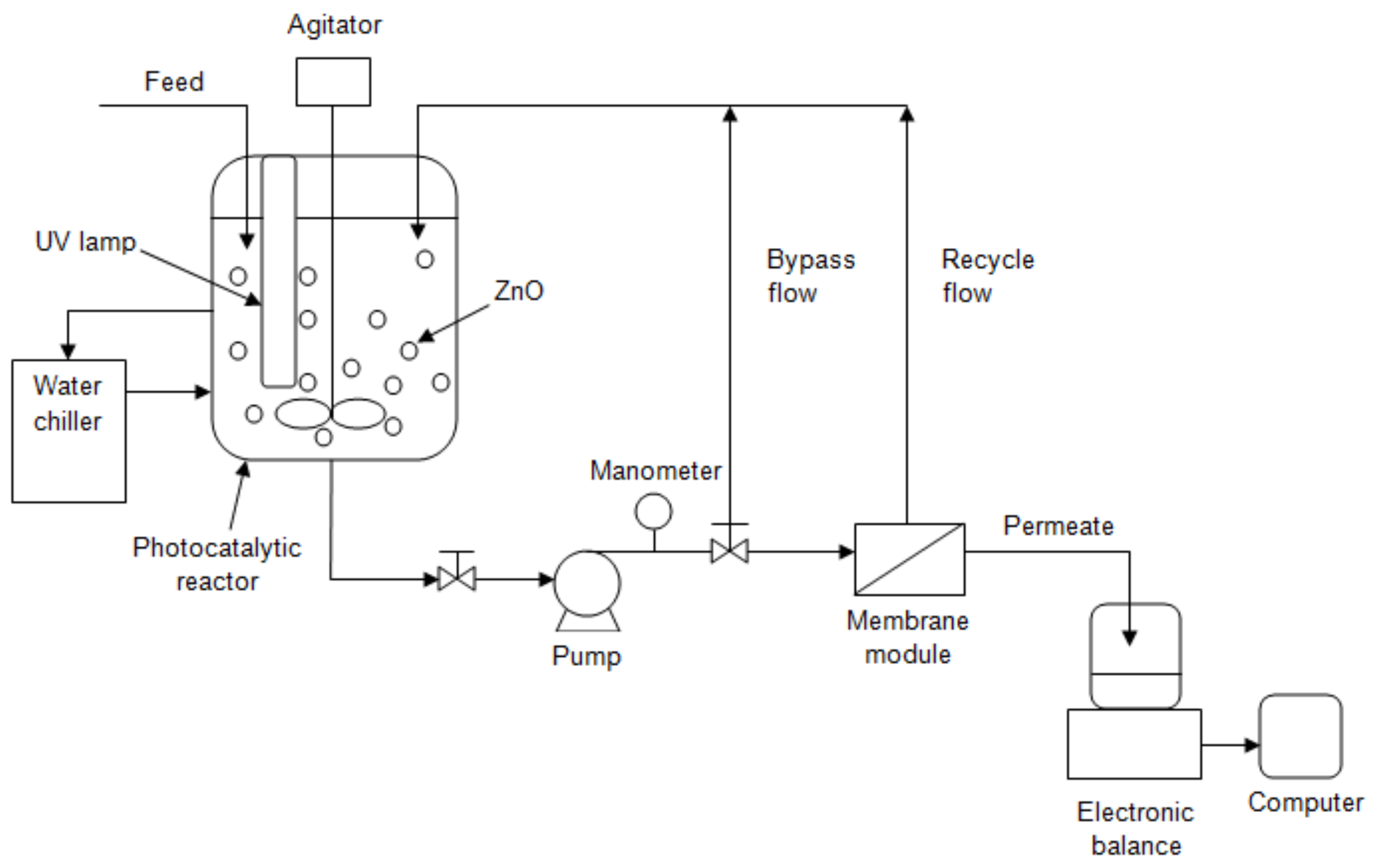

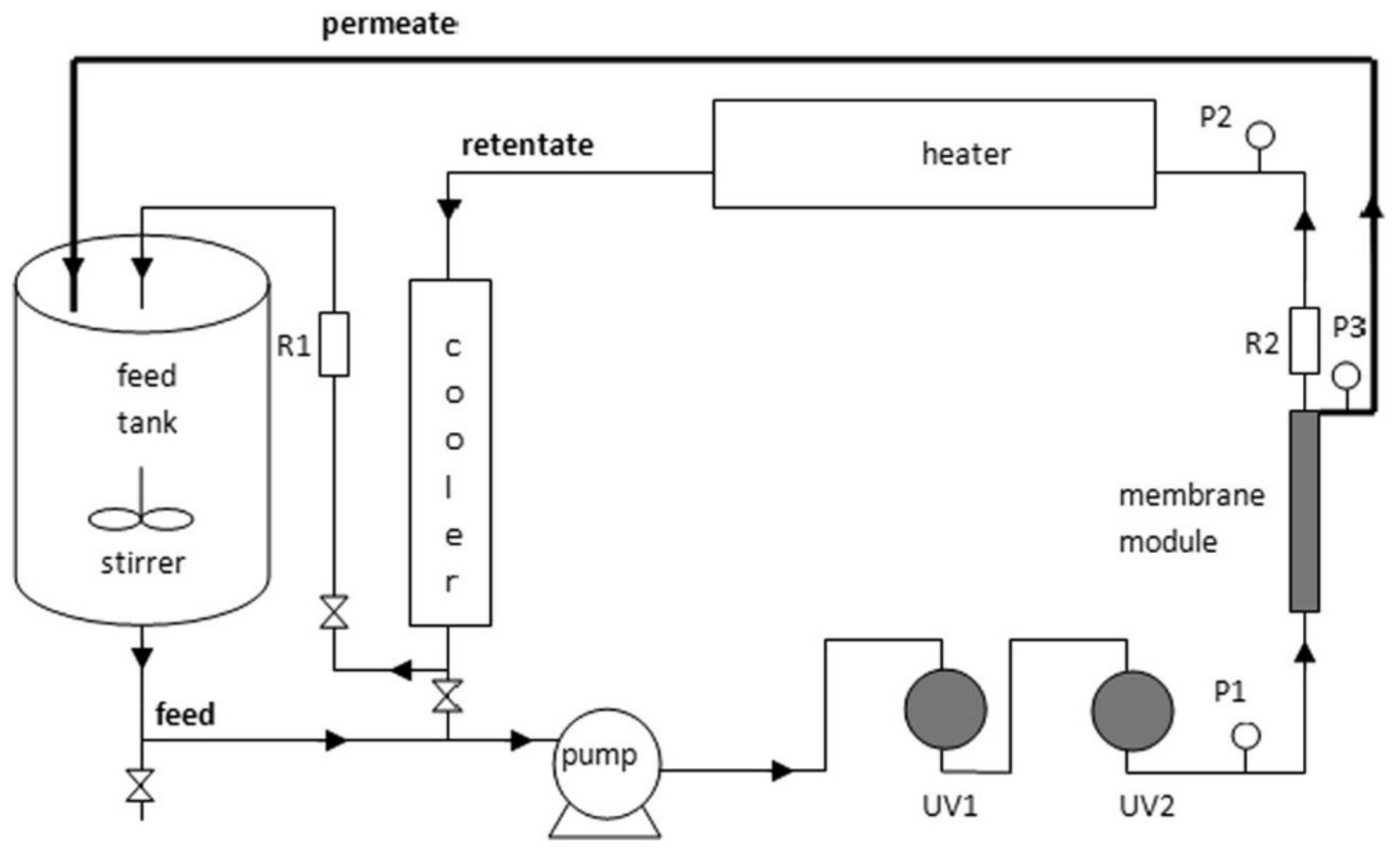

| Operation Factors | Effects | Ref. |
|---|---|---|
| Type of membrane process | PMRs utilizing pressure driven membrane processes are more prone to fouling than systems with other driving forces (e.g., pervaporation, membrane distillation, dialysis). | [24] |
| Operating mode | PMRs utilizing pressure driven membrane techniques:
| [47] [45] |
| Operating conditions | Aeration: improvement of the process performance (fouling mitigation; increase of photodecomposition efficiency); however, at high aeration rates the bubble cloud can attenuate the UV/VIS light transmission in the photoreactor. | [45] |
| Back-flushing: alleviation of membrane fouling caused by photocatalyst particles or in case of membranes with large pores no positive results. | [48] [47] | |
| Cross flow velocity (CFV): enhancement of membrane performance with increasing CFV as a result of higher shear rates prevents the deposition of photocatalyst cake layer. | [49] | |
Transmembrane pressure (pressure driven membrane techniques):
| [49] [47] | |
| Photocatalyst | Under unfavorable process conditions, membrane fouling due to the presence of photocatalyst particles, especially in case of pressurized systems. | [47] |
| Fouling mitigation due to decomposition of organic contaminants in case of both PMs and slurry PMRs. | [50] | |
| Higher photocatalyst loading can cause increase of permeate flux; however, at too high concentration the screening effect may attenuate the UV/VIS radiation transmission in the photoreactor. | [50] | |
| Feed composition | Chemical nature of contaminants strictly affects their adsorption on photocatalyst particles thus influencing their removal efficiency and fouling severity. | [33] [46] |
| Less severe membrane fouling at neutral and acidic pH compared to alkaline conditions. |
| Pollutant | Membrane | Parameters | Photodegradation Efficiency | Ref. |
|---|---|---|---|---|
| Methylene blue | atomic-layer-deposited carbon nanotube ZnO-TiO2; | C0 = 20 mg L−1, UV irradiation | 94% in 100 min | [57] |
| hydrophobic CeO2 coated stainless steel membrane; | C0 = 10 mg L−1 in oil-water mixture, UV irradiation | >99% in 80 min | [64] | |
| PVDF/GO/ZnO nanocomposites membrane | C0 = 10 mg L−1, xenon light irradiation | 86.84% in 100 min | [143] | |
| Rhodamine B | g-C3N4 NT/rGO NF membrane | C0 = 10 mg L−1, VIS irradiation | >98% in 300 min | [144] |
| Eosin yellow | PSF/N,Pd co-doped TiO2 composite membrane | C0 = 100 mg L−1, VIS irradiation | >97% in 4 h | [30] |
| Ibuprofen | Cu2O modified PSF UF membrane | C0 = 10 mg L−1, VIS irradiation | up to 86% | [59] |
| Diclofenac | ZnO and TiO2 sputtered membranes | C0 = 9.3×10−5 M, UV irradiation | almost complete in 6 h | [58] |
| Carbamazepine | N-doped TiO2 coated PM | C0 = 1 g L−1, UV and VIS light | 90% higher reaction rate | [53] |
| Tetracycline | ZnIn2S4 coated PVDF membrane | C0 = 100 µg L−1, VIS irradiation | 92% in 36 h | [61] |
| Phenol | PES/O-g-C3N4 hybrid membrane | C0 = 10 mg L−1, UV and VIS irradiation | 35.8% in 300 min | [66] |
| Humic acids | TiO2 nanowires/Fe2O3 nanoparticles/GO sheets membrane; | C0 = 25 mg L−1, solar radiation | 92% in 12 h | [60] |
| ceramic membranes coated with TiO2 | C0 = 0.2 g L−1, UV irradiation | 98.56% removal | [65] | |
| Diuron Chlorfenvinphos | magnetron sputtered TiO2 on ceramic membrane | C0 = 1 mg L−1, | 75% in 3 h | [145] |
| solar radiation | 78% in 3 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, R.; Lavorato, C.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications. Catalysts 2019, 9, 239. https://doi.org/10.3390/catal9030239
Molinari R, Lavorato C, Argurio P, Szymański K, Darowna D, Mozia S. Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications. Catalysts. 2019; 9(3):239. https://doi.org/10.3390/catal9030239
Chicago/Turabian StyleMolinari, Raffaele, Cristina Lavorato, Pietro Argurio, Kacper Szymański, Dominika Darowna, and Sylwia Mozia. 2019. "Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications" Catalysts 9, no. 3: 239. https://doi.org/10.3390/catal9030239





