Overcoming Water Insolubility in Flow: Enantioselective Hydrolysis of Naproxen Ester
Abstract
:1. Introduction
2. Results
2.1. NSAID Hydrolysis
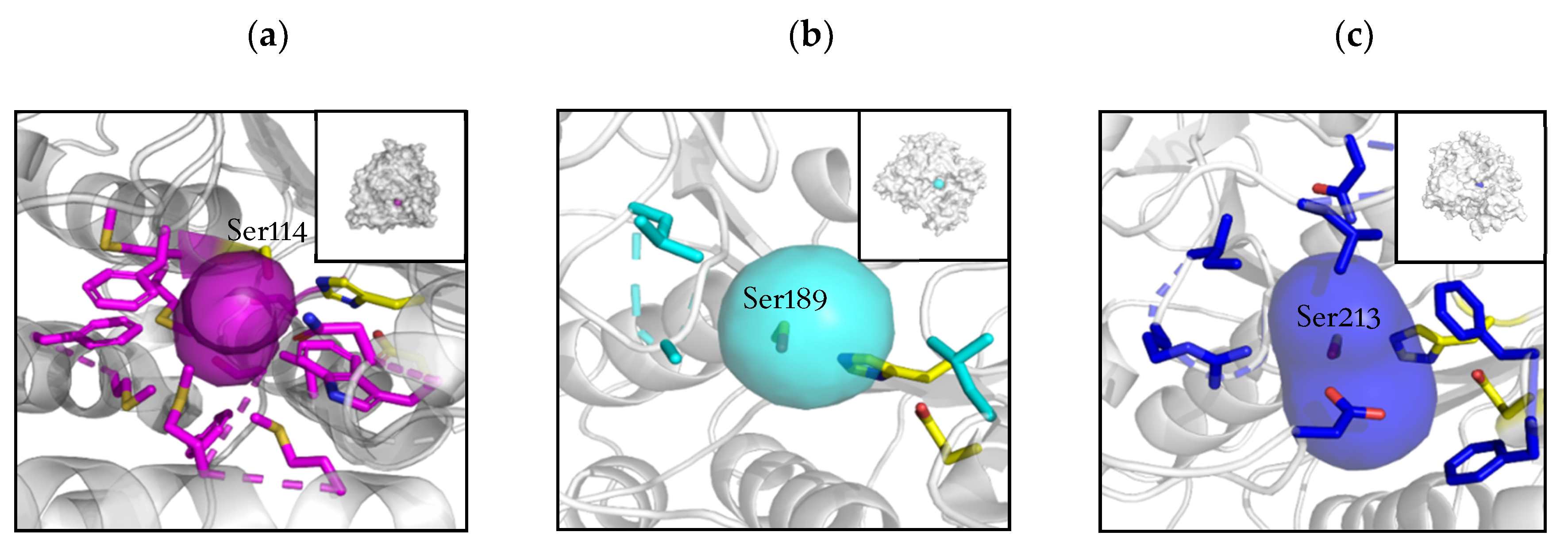
2.2. Active Site and Tunnel Architecture
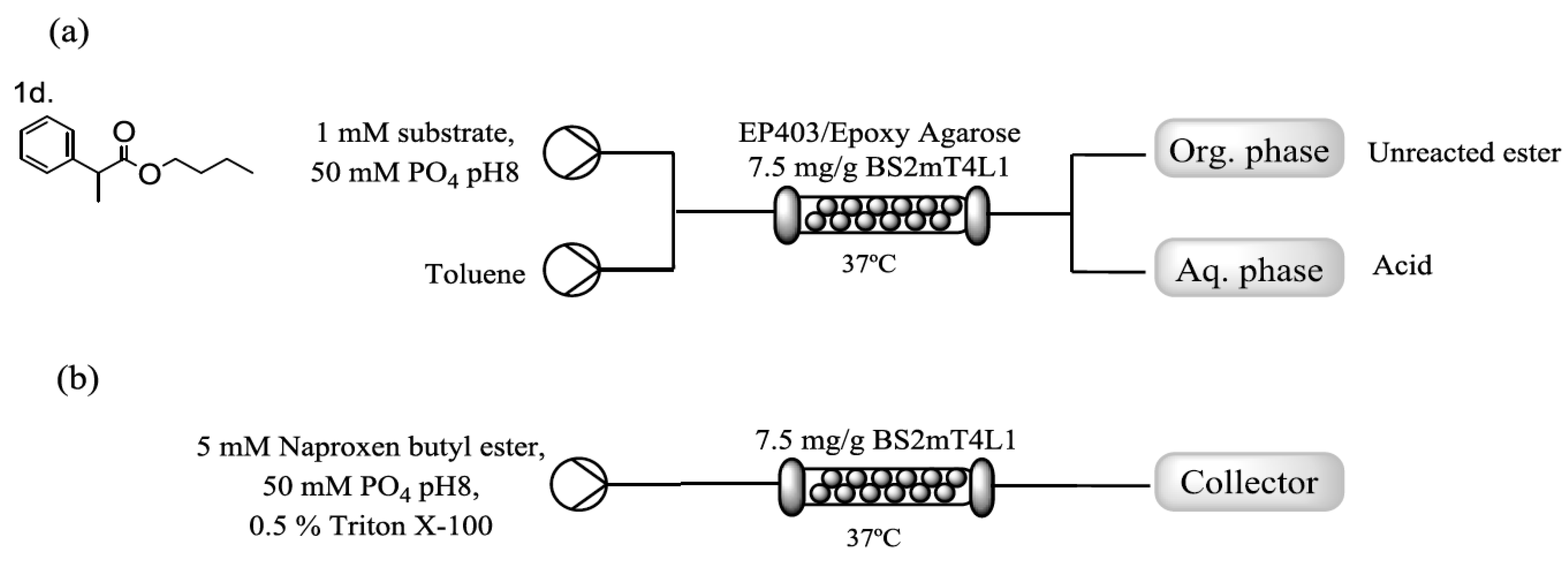
2.3. Immobilization of BS2mT4L1 and Flow Assisted Hydrolysis of Naproxen Butyl Ester
3. Discussion
4. Materials and Methods
4.1. Halomonas elongata (HeE) Cloning
4.2. BS2m, HeE and BCE Production
4.3. Esterase Activity Assay
4.4. Butyl-Ester NSAID’s Synthesis
4.5. Batch Biotransformations with Free Enzymes
4.6. HPLC Analysis to Determine the Enantioselectivity
4.7. Computational Analysis of Tunnels
4.8. Preparation of Agarose with Epoxide Groups
4.9. Immobilization of BS2mT4L1 in Epoxy-Agarose
4.10. Flow Reactions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayball, P.J.; Hayball, D.P. Chirality and Nonsteroidal Anti-Inflammatory Drugs. Drugs 1996, 52, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Wynne, S.; Djakiew, D. NSAID Inhibition of Prostate Cancer Cell Migration Is Mediated by Nag-1 Induction via the p38 MAPK-p75NTR Pathway. Mol. Cancer Res. 2010, 8, 1656–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, I.T.; Lewis, B.; Nelson, P.; Rooks, W.; Roszkowski, A.; Tomolonis, A.; Fried, J.H. Nonsteroidal antiinflammatory agents. I. 6-Substituted 2-naphthylacetic acids. J. Med. Chem. 1970, 13, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Arpigny, J.L.; Jaeger, K.-E. Bacterial lipolytic enzymes: classification and properties. Biochem. J. 1999, 343, 177. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, N.; Hotelier, T.; Velluet, E.; Bourne, Y.; Marchot, P.; Chatonnet, A. ESTHER, the database of the α/β-hydrolase fold superfamily of proteins: tools to explore diversity of functions. Nucleic Acids Res. 2012, 41, D423–D429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-S.; Hsu, C.-S. Lipase-catalyzed enantioselective esterification of (S)-naproxen hydroxyalkyl ester in organic media. Biotechnol. Lett. 2003, 25, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.; Kamaruddin, A.; Bhatia, S.; Long, W.; Lim, S.; Kumari, R. Performance of free Candida antarctica lipase B in the enantioselective esterification of (R)-ketoprofen. Enzyme Microb. Technol. 2006, 39, 924–929. [Google Scholar] [CrossRef]
- Chen, J.-C.; Tsai, S.-W. Enantioselective Synthesis of (S)-Ibuprofen Ester Prodrug in Cyclohexane by Candida rugosa Lipase Immobilized on Accurel MP1000. Biotechnol. Progress 2000, 16, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Mohammadi, M.; Habibi, Z. Enantioselective resolution of racemic ibuprofen esters using different lipases immobilized on octyl sepharose. J. Mol. Catal. B Enzym. 2014, 104, 87–94. [Google Scholar] [CrossRef]
- Tamborini, L.; Romano, D.; Pinto, A.; Bertolani, A.; Molinari, F.; Conti, P. An efficient method for the lipase-catalysed resolution and in-line purification of racemic flurbiprofen in a continuous-flow reactor. J. Mol. Catal. B Enzym. 2012, 84, 78–82. [Google Scholar] [CrossRef]
- Quax, W.J.; Broekhuizen, C.P. Development of a newBacillus carboxyl esterase for use in the resolution of chiral drugs. Appl. Microbiol. Biotechnol. 1994, 41, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Won, H.S.; Ro, H.-S.; Ryu, Y.-W.; Chung, B.H. Preparation of enantiomerically pure (S)-flurbiprofen by an esterase from Pseudomonas sp. KCTC 10122BP. J. Mol. Catal. B Enzym. 2003, 26, 149–156. [Google Scholar] [CrossRef]
- Steenkamp, L.; Brady, D. Screening of commercial enzymes for the enantioselective hydrolysis of R,S-naproxen ester. Enzyme Microb. Technol. 2003, 32, 472–477. [Google Scholar] [CrossRef]
- A Sousa, H.; Rodrigues, C.; Klein, E.; Afonso, C.A.M.; Crespo, J.G. Immobilisation of pig liver esterase in hollow fibre membranes. Enzyme Microb. Technol. 2001, 29, 625–634. [Google Scholar] [CrossRef]
- Fini, A. Solubility and solubilization properties of non-steroidal anti-inflammatory drugs. Int. J. Pharm. 1995, 126, 95–102. [Google Scholar] [CrossRef]
- Hopkin, M.D.; Baxendale, I.R.; Ley, S.V. An expeditious synthesis of imatinib and analogues utilising flow chemistry methods. Org. Biomol. Chem. 2013, 11, 1822–1839. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, I.R. The integration of flow reactors into synthetic organic chemistry. J. Chem. Technol. Biotechnol. 2013, 88, 519–552. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Henke, E.; Heinze, B.; Kourist, R.; Hidalgo, A.; Bornscheuer, U.T. A versatile esterase fromBacillus subtilis: Cloning, expression, characterization, and its application in biocatalysis. Biotechnol. J. 2007, 2, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Hackenschmidt, S.; Moldenhauer, E.J.; Behrens, G.A.; Gand, M.; Pavlidis, I.V.; Bornscheuer, U.T. Enhancement of Promiscuous Amidase Activity of a Bacillus subtilis Esterase by Formation of a π-π Network. ChemCatChem 2013, 6, 1015–1020. [Google Scholar] [CrossRef]
- De Vitis, V.; Nakhnoukh, C.; Pinto, A.; Contente, M.L.; Barbiroli, A.; Milani, M.; Bolognesi, M.; Molinari, F.; Gourlay, L.J.; Romano, D. A stereospecific carboxyl esterase from Bacillus coagulans hosting nonlipase activity within a lipase-like fold. FEBS J. 2018, 285, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Planchestainer, M.; Padrosa, D.R.; Contente, M.; Paradisi, F. Genetically Fused T4L Acts as a Shield in Covalent Enzyme Immobilisation Enhancing the Rescued Activity. Catalysts 2018, 8, 40. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Atkins, J.D.; Salehe, B.R.; Shuid, A.N.; Roche, D.B.; Pérez-Berná, A.J.; Marion, S.; Chichón, F.J.; Fernández, J.J.; Winkler, D.C.; et al. IntFOLD: An integrated server for modelling protein structures and functions from amino acid sequences. Nucleic Acids Res. 2015, 43, W169–W173. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.J.; Lill, M.A. Substrate tunnels in enzymes: Structure-function relationships and computational methodology. Proteins 2015, 83, 599–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavelka, A.; Sebestova, E.; Kozlikova, B.; Brezovský, J.; Sochor, J.; Damborsky, J. CAVER: Algorithms for Analyzing Dynamics of Tunnels in Macromolecules. IEEE/ACM Trans. Comput. Biol. Bioinf. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Gora, A.; Brezovsky, J.; Damborsky, J. Gates of enzymes. Chem. Rev. 2013, 113, 5871–5923. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Ren, H.; Xing, Z.; Yang, J.; Jiang, W.; Zhang, G.; Tang, J.; Li, Q. Construction of an Immobilized Thermophilic Esterase on Epoxy Support for Poly(ε-caprolactone) Synthesis. Molecules 2016, 21, 796. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Bolivar, J.M.; Godoy, C.A.; Rocha-Martín, J.; Pessela, B.C.; Curiel, J.A.; Muñoz, R.; Guisán, J.M.; Fernández-Lorente, G. Improvement of Enzyme Properties with a Two-Step Immobilizaton Process on Novel Heterofunctional Supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar, S.; Bernal, C.; Mesa, M. Kinetic study of the colloidal and enzymatic stability of β-galactosidase, for designing its encapsulation route through sol–gel route assisted by Triton X-100 surfactant. Biochem. Eng. J. 2013, 75, 32–38. [Google Scholar] [CrossRef]
- Perna, R.F.; Tiosso, P.C.; Sgobi, L.M.; Vieira, A.M.; Tardioli, P.W.; Soares, C.M.; Zanin, G.M. Effects of Triton X-100 and PEG on the Catalytic Properties and Thermal Stability of Lipase from Free and Immobilized on Glyoxyl-Agarose. TOBIOCJ 2017, 11, 66–76. [Google Scholar] [CrossRef] [PubMed]

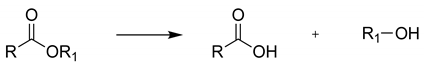
| Substrate | BCE 1 | HeE 2 | BS2m 2 | |||||
|---|---|---|---|---|---|---|---|---|
| m.c. | e.e. | m.c. | e.e. | m.c. | e.e. | |||
| 1a. | 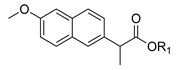 | Naproxen Ester | <5% (48 h) | n.d. | 35% (48 h) | <5% | 55% (48 h) | 80% (R) |
| 1b. | 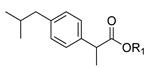 | Ibuprofen ester | 22% (24 h) | >97% (S) | >95% (8 h) | <5% | >95% (8 h) | <5% |
| 1c. | 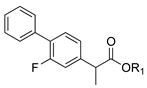 | Flurbiprofen ester | 36% (24 h) | >97% (S) | 88% (24 h) | <5% | 81% (24 h) | <5% |
| Naproxen Butyl Ester Concentration (mM) | Residence Time (minutes) | Molar Conversion (%) | e.e. (%) |
|---|---|---|---|
| 1 mM | 30 | >99% | <5% |
| 5 mM | 30 | >99% | <5% |
| 10 | 65% | 40% | |
| 6 | 24% | 80% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roura Padrosa, D.; De Vitis, V.; Contente, M.L.; Molinari, F.; Paradisi, F. Overcoming Water Insolubility in Flow: Enantioselective Hydrolysis of Naproxen Ester. Catalysts 2019, 9, 232. https://doi.org/10.3390/catal9030232
Roura Padrosa D, De Vitis V, Contente ML, Molinari F, Paradisi F. Overcoming Water Insolubility in Flow: Enantioselective Hydrolysis of Naproxen Ester. Catalysts. 2019; 9(3):232. https://doi.org/10.3390/catal9030232
Chicago/Turabian StyleRoura Padrosa, David, Valerio De Vitis, Martina Letizia Contente, Francesco Molinari, and Francesca Paradisi. 2019. "Overcoming Water Insolubility in Flow: Enantioselective Hydrolysis of Naproxen Ester" Catalysts 9, no. 3: 232. https://doi.org/10.3390/catal9030232





