Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Optimizations
2.2. Characterizations
2.2.1. Morphology
2.2.2. Microstructure
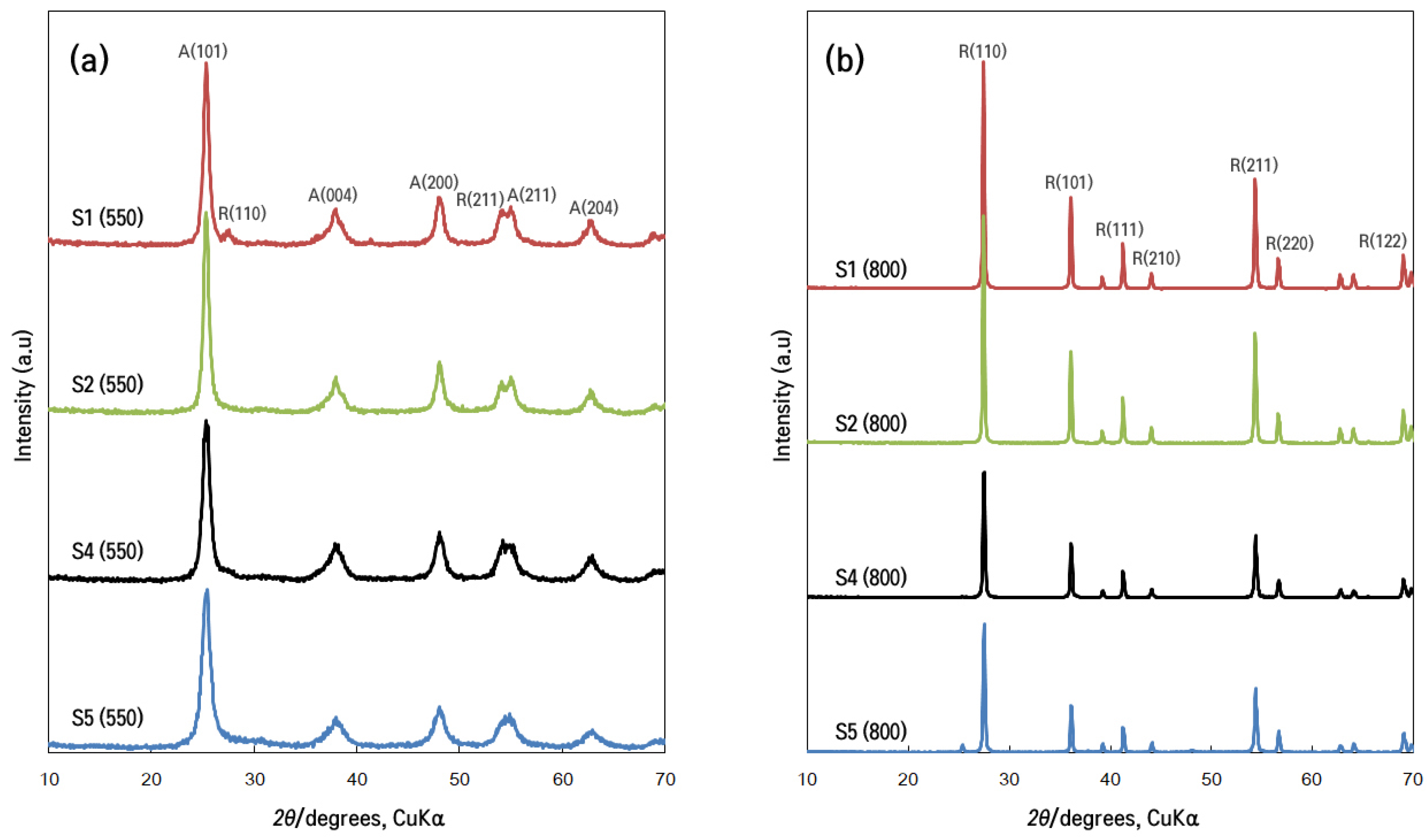
X-Ray Diffraction Analysis
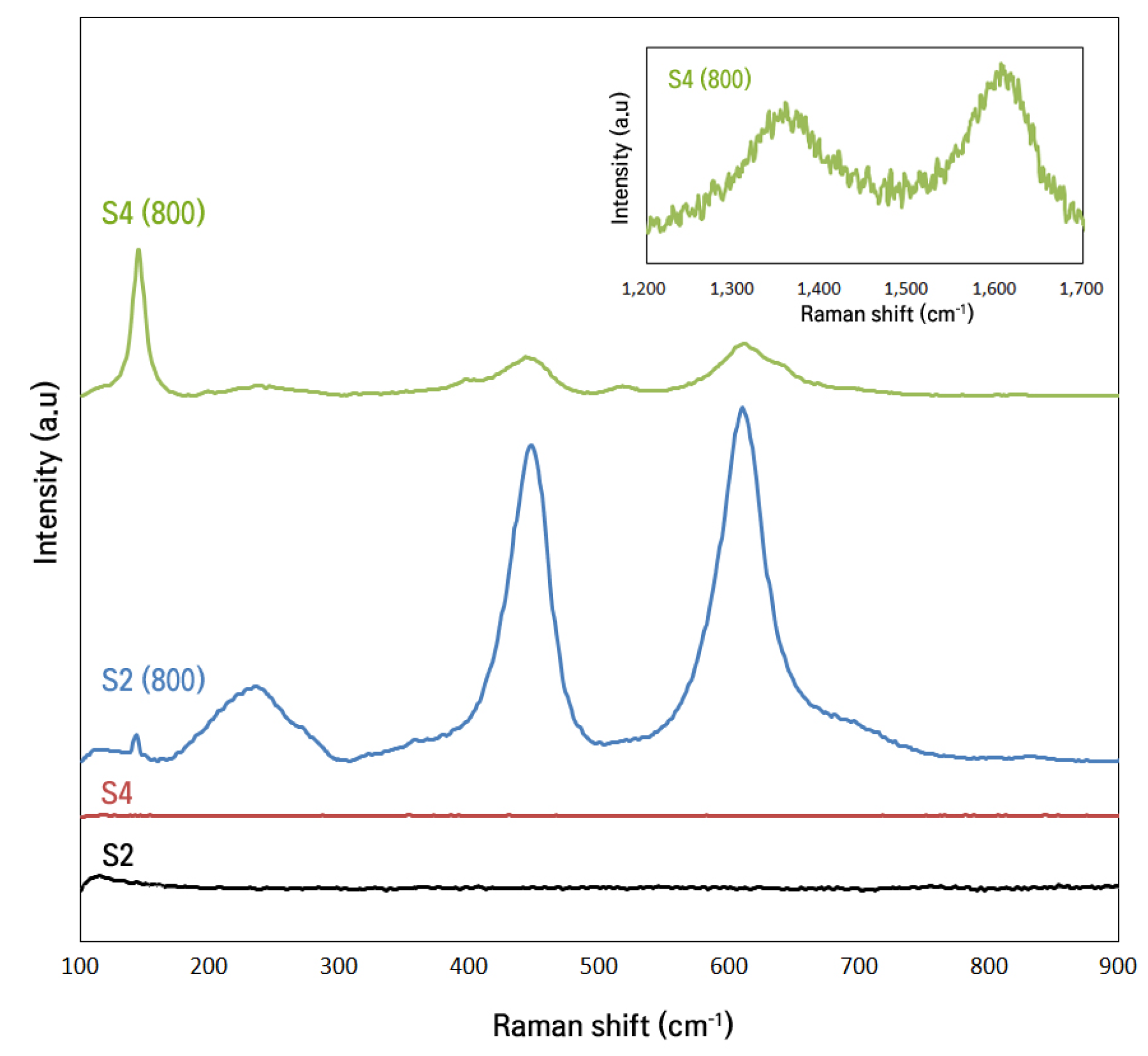
Raman Spectroscopy
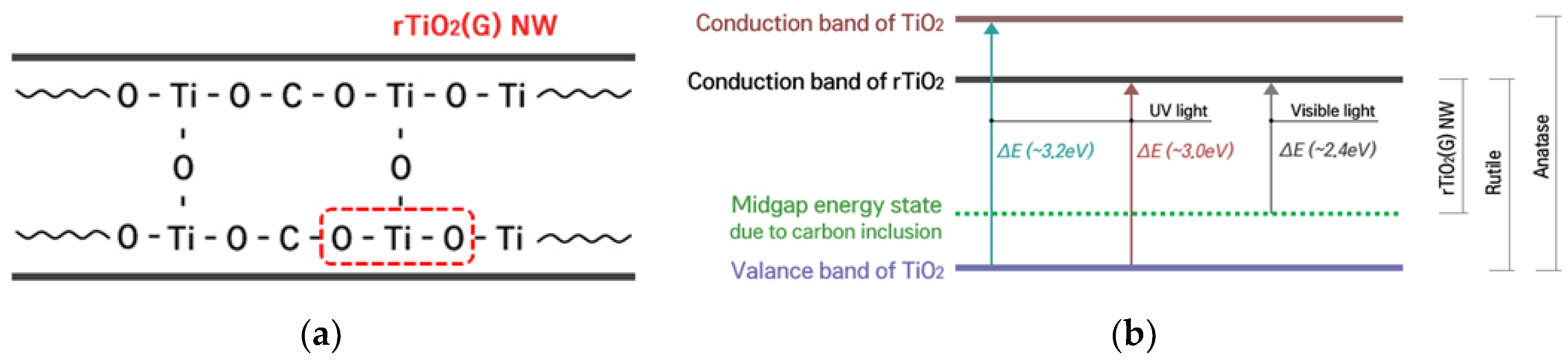
2.2.3. Optical Properties
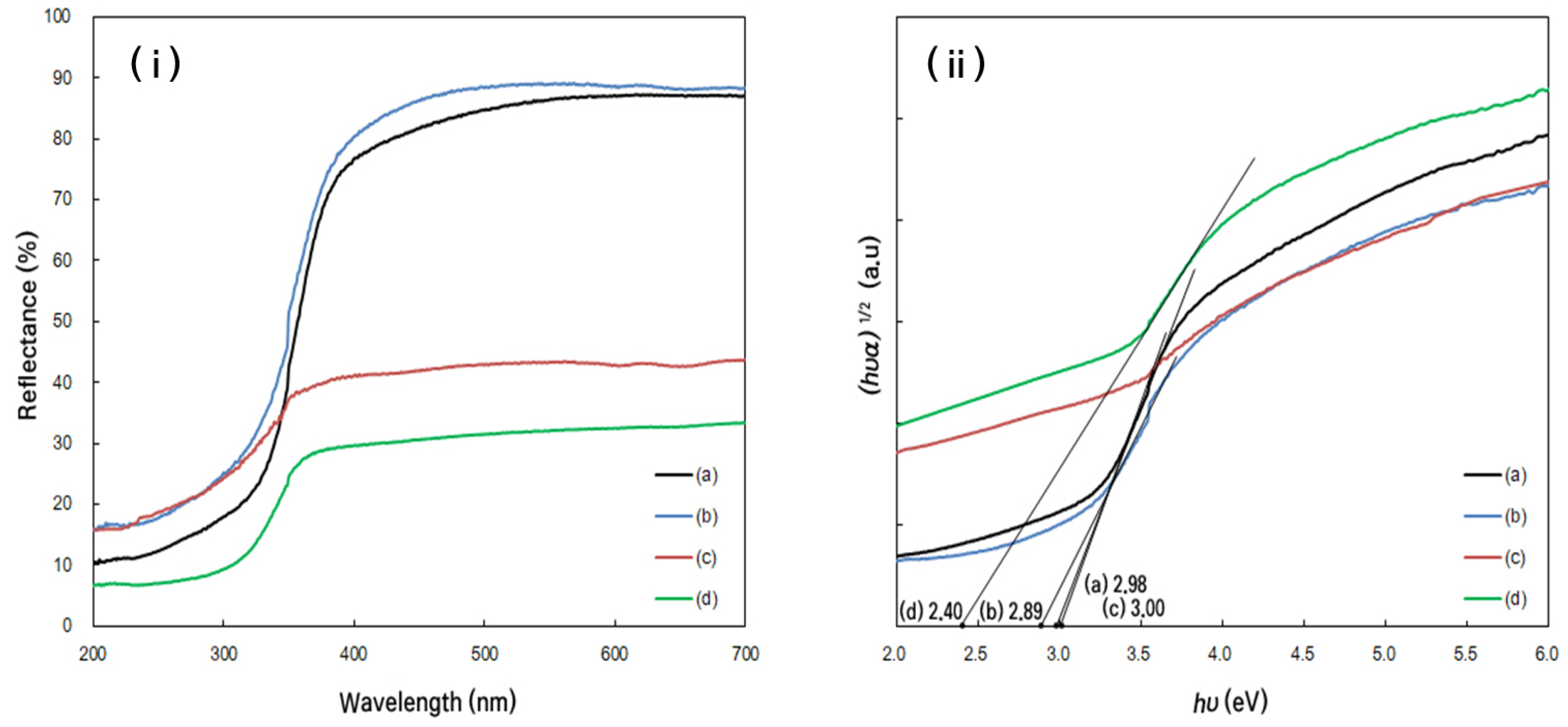
Diffuse Reflectance Spectroscopy
Photoluminescence
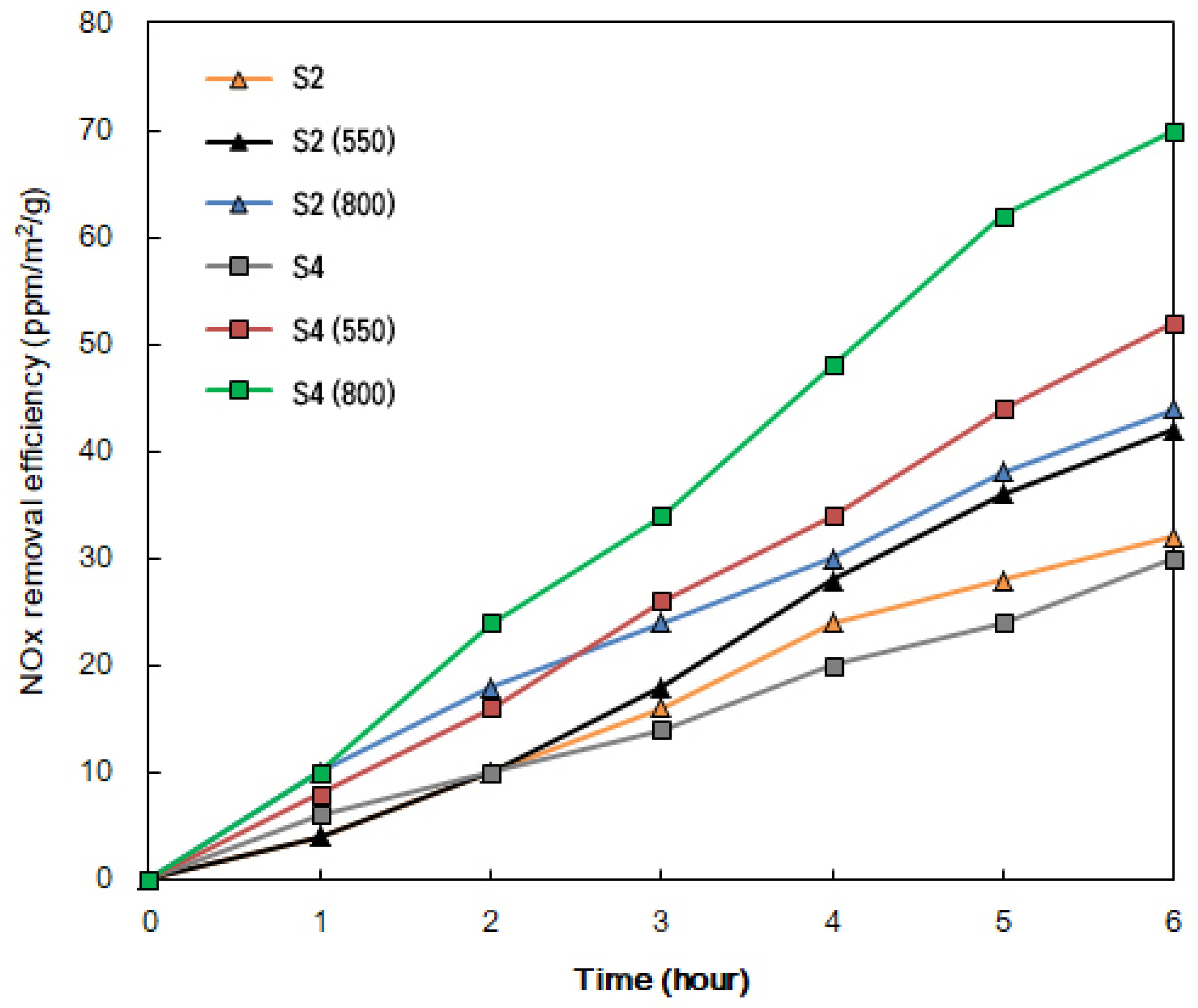
2.3. Photodegradation of NOX under Visible Light
3. Experimental
3.1. Materials and Methods
3.2. Characterization
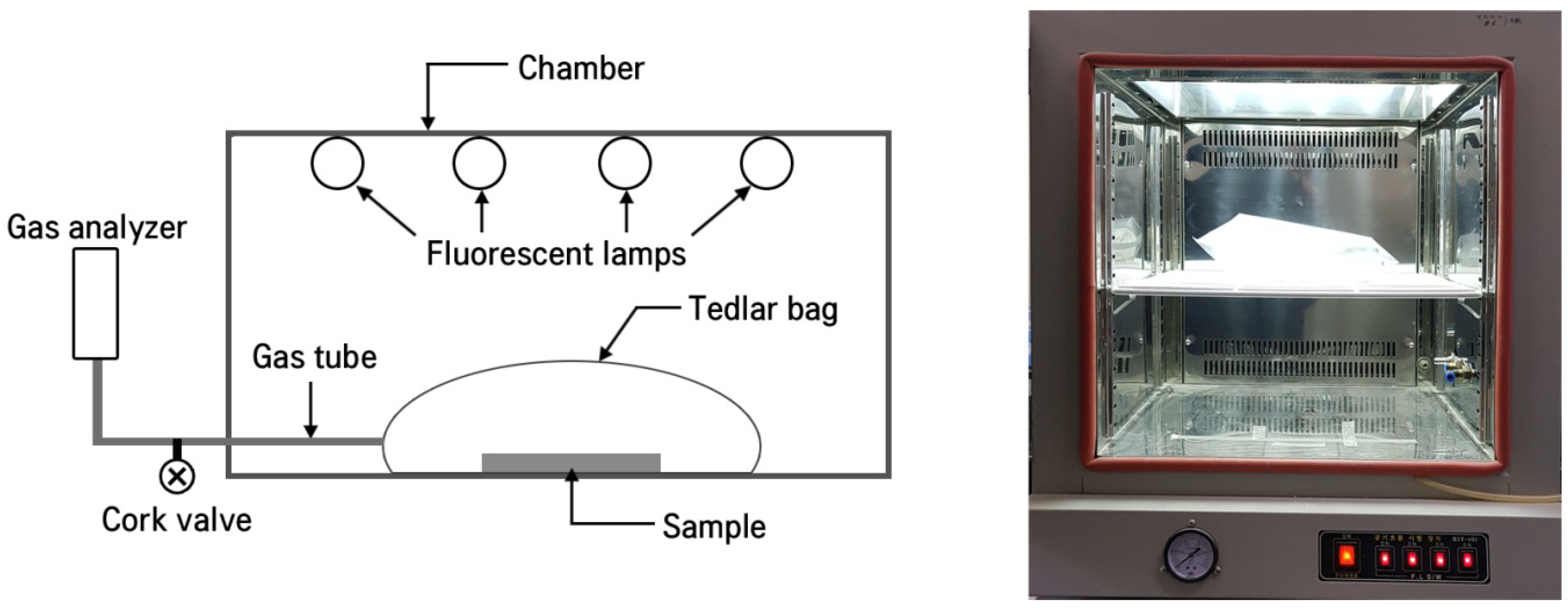
3.3. Visible LightPphotodegradation of NOx
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Navrotsky, A.; Jamieson, J.C.; Kleppa, O.J. Enthalpy of transformation of a high-pressure polymorph of titanium dioxide to the rutile modification. Science 1967, 158, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Lim, J.; Baek, M.; Choi, W.; Kim, W.; Yong, K. Investigating the unrevealed photocatalytic activity and stability of nanostructured brookite TiO2 film as an environmental photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 16252–16260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-G.; Ishigaki, T.; Sun, X. Anatase, brookite, and rutile nanocrystals via redox reactions under mild hydrothermal conditions: Phase-selective synthesis and physicochemical properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Martínez-de la Cruz, A. Enhanced Photocatalytic Activity of TiO2 Rutile by Coupling with Fly Ashes for the Removal of NO Gases. Ind. Eng. Chem. Res. 2016, 55, 11512–11519. [Google Scholar] [CrossRef]
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Ozawa, K.; Yamamoto, S.; Yukawa, R.; Liu, R.-Y.; Terashima, N.; Natsui, Y.; Kato, H.; Mase, K.; Matsuda, I. Correlation between Photocatalytic Activity and Carrier Lifetime: Acetic Acid on Single-Crystal Surfaces of Anatase and Rutile TiO2. J. Phys. Chem. C 2018, 122, 9562–9569. [Google Scholar] [CrossRef]
- Pourjafari, D.; Reyes-Coronado, D.; Vega-Poot, A.; Escalante, R.; Kirkconnell-Reyes, D.; García-Rodríguez, R.; Anta, J.A.; Oskam, G. Brookite-Based Dye-Sensitized Solar Cells: Influence of Morphology and Surface Chemistry on Cell Performance. J. Phys. Chem. C 2018, 122, 14277–14288. [Google Scholar] [CrossRef]
- Maruska, H.P.; Ghosh, A.K. Photocatalytic decomposition of water at semiconductor electrodes. Solar Energy 1978, 20, 443–458. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, L.; Guo, J. Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl. Catal. B Environ. 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Wöll, C. Photocatalytic activity of bulk TiO2 anatase and rutile single crystals using infrared absorption spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, G.J.; Suja, S. TiO2 (rutile) embedded inulin—A versatile bio-nanocomposite for photocatalytic degradation of methylene blue. Carbohydr. Polym. 2016, 143, 51–60. [Google Scholar] [CrossRef]
- Nair, R.V.; Jijith, M.; Gummaluri, V.S.; Vijayan, C. A novel and efficient surfactant-free synthesis of Rutile TiO2 microflowers with enhanced photocatalytic activity. Opt. Mater. 2016, 55, 38–43. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kato, H.; Kobayashi, M.; Kakihana, M. Hierarchical structures of rutile exposing high-index facets. J. Cryst. Growth 2015, 418, 86–91. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Lu, Z.; Xu, G.; Wang, X.; Qian, L.; Wang, H.; Zhang, E.; Xi, J.; Ji, Z. One-step synthesis of rutile nano-TiO2 with exposed {1 1 1} facets for high photocatalytic activity. J. Alloys Compd. 2015, 632, 133–139. [Google Scholar] [CrossRef]
- Maeda, K. Direct splitting of pure water into hydrogen and oxygen using rutile titania powder as a photocatalyst. Chem. Commun. 2013, 49, 8404–8406. [Google Scholar]
- Maeda, K. Photocatalytic properties of rutile TiO2 powder for overall water splitting. Catal. Sci. Technol. 2014, 4, 1949–1953. [Google Scholar] [CrossRef]
- Murakami, N.; Ono, A.; Nakamura, M.; Tsubota, T.; Ohno, T. Development of a visible-light-responsive rutile rod by site-selective modification of iron (III) ion on {1 1 1} exposed crystal faces. Appl. Catal. B Environ. 2010, 97, 115–119. [Google Scholar] [CrossRef]
- Sosnowchik, B.D.; Chiamori, H.C.; Ding, Y.; Ha, J.-Y.; Wang, Z.L.; Lin, L. Titanium dioxide nanoswords with highly reactive, photocatalytic facets. Nanotechnology 2010, 21, 485601. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Murakami, N.; Ohno, T. Dependence of activity of rutile titanium (IV) oxide powder for photocatalytic overall water splitting on structural properties. J. Phys. Chem. C 2014, 118, 9093–9100. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Cho, M.H. Electrically conductive polyaniline sensitized defective-TiO2 for improved visible light photocatalytic and photoelectrochemical performance: A synergistic effect. New J. Chem. 2015, 39, 8381–8388. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2009, 4, 380–386. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Zhou, Y.; Liu, L. Anatase TiO2 nanocrystals with exposed {001} facets on graphene sheets via molecular grafting for enhanced photocatalytic activity. Nanoscale 2012, 4, 613–620. [Google Scholar] [CrossRef]
- Long, R.; Casanova, D.; Fang, W.-H.; Prezhdo, O.V. Donor–acceptor interaction determines the mechanism of photoinduced electron injection from graphene quantum dots into TiO2: π-stacking supersedes covalent bonding. J. Am. Chem. Soc. 2017, 139, 2619–2629. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C. TiO2/graphene composite from thermal reaction of graphene oxide and its photocatalytic activity in visible light. J. Mater. Sci. 2011, 46, 2622–2626. [Google Scholar] [CrossRef]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Augugliaro, V.; Palmisano, L. Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J. Am. Chem. Soc. 2008, 130, 1568–1569. [Google Scholar] [CrossRef] [PubMed]
- Atitar, M.F.; Ismail, A.A.; Al-Sayari, S.; Bahnemann, D.; Afanasev, D.; Emeline, A. Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Seo, H.-K.; Kim, G.-S.; Ansari, S.; Kim, Y.-S.; Shin, H.-S.; Shim, K.-H.; Suh, E.-K. A study on the structure/phase transformation of titanate nanotubes synthesized at various hydrothermal temperatures. Solar Energy Mater. Solar Cells 2008, 92, 1533–1539. [Google Scholar] [CrossRef]
- Biedermann, L.B.; Bolen, M.L.; Capano, M.A.; Zemlyanov, D.; Reifenberger, R.G. Insights into few-layer epitaxial graphene growth on 4 H-SiC (000 1¯) substrates from STM studies. Phys. Rev. B 2009, 79, 125411. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Gu, F.; Zhao, Y. Facile flame synthesis and photoluminescent properties of core/shell TiO2/SiO2 nanoparticles. J. Alloys Compd. 2007, 432, L5–L9. [Google Scholar] [CrossRef]
- Xu, L.; Garrett, M.P.; Hu, B. Doping effects on internally coupled seebeck coefficient, electrical, and thermal conductivities in aluminum-doped TiO2. J. Phys. Chem. C 2012, 116, 13020–13025. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Zhuang, Y.; Fu, X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Gopalan, A.; Lee, K.-P. Highly selective non-enzymatic electrochemical sensor based on a titanium dioxide nanowire–poly(3-aminophenyl boronic acid)–gold nanoparticle ternary nanocomposite. RSC Adv. 2018, 8, 2138–2147. [Google Scholar] [CrossRef]
- Nallal, M.; Anantha Iyengar, G.; Pill-Lee, K. New Titanium Dioxide-Based Heterojunction Nanohybrid for Highly Selective Photoelectrochemical–Electrochemical Dual-Mode Sensors. ACS Appl. Mater. Interfaces 2017, 9, 37166–37183. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Sai-Anand, G.; Gopalan, A.-I.; Lee, H.-G.; Yeo, H.K.; Kang, S.-W.; Lee, K.-P. Direct electrochemistry of cytochrome c with three-dimensional nanoarchitectured multicomponent composite electrode and nitrite biosensing. Sens. Actuators B Chem. 2016, 228, 737–747. [Google Scholar] [CrossRef]
- Lee, K.-P.; Gopalan, A.-I.; Lee, C.-J.; Jeon, K.-Y.; Kim, W.-J. Characterization and Photocatalytic Activity of Heat Treated Zinc Doped Titanium Dioxide. Adv. Porous Mater. 2013, 1, 279–285. [Google Scholar] [CrossRef]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Lee, B.-C.; Kang, S.-W.; Lee, K.-P. Facile synthesis of functionalized graphene-palladium nanoparticle incorporated multicomponent TiO2 composite nanofibers. Mater. Chem. Phys. 2015, 154, 125–136. [Google Scholar] [CrossRef]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Kang, S.-W.; Lee, K.-P. New Heterojunction Titanium Dioxide Nanowire as Photocatalyst. J. Nanosci. Nanotechnol. 2015, 15, 7421–7425. [Google Scholar] [CrossRef] [PubMed]
- Santara, B.; Giri, P.; Imakita, K.; Fujii, M. Microscopic origin of lattice contraction and expansion in undoped rutile TiO2 nanostructures. J. Phys. D Appl. Phys. 2014, 47, 215302. [Google Scholar] [CrossRef]
- Murphy, A. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Solar Energy Mater. Solar Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Lin, H.; Weng, S.; Zheng, Z.; Hou, Y.; Liu, P. Facile synthesis of defect-mediated TiO2−x with enhanced visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 10099–10102. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 nanorod spheres and arrays compatible with flexible applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Eder, D.; Motta, M.S.; Windle, A.H. Nanoengineering with residual catalyst from CNT templates. Acta Mater. 2010, 58, 4406–4413. [Google Scholar] [CrossRef]
- Naumenko, D.; Snitka, V.; Snopok, B.; Arpiainen, S.; Lipsanen, H. Graphene-enhanced Raman imaging of TiO2 nanoparticles. Nanotechnology 2012, 23, 465703. [Google Scholar] [CrossRef] [PubMed]
- Toma, F.; Bertrand, G.; Klein, D.; Coddet, C. Photocatalytic removal of nitrogen oxides via titanium dioxide. Environ. Chem. Lett. 2004, 2, 117–121. [Google Scholar] [CrossRef]
- Khan, S.U.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Theory of carbon doping of titanium dioxide. Chem. Mater. 2005, 17, 6656–6665. [Google Scholar] [CrossRef]
- Long, M.; Qin, Y.; Chen, C.; Guo, X.; Tan, B.; Cai, W. Origin of visible light photoactivity of reduced graphene oxide/TiO2 by in situ hydrothermal growth of undergrown TiO2 with graphene oxide. J. Phys. Chem. C 2013, 117, 16734–16741. [Google Scholar] [CrossRef]
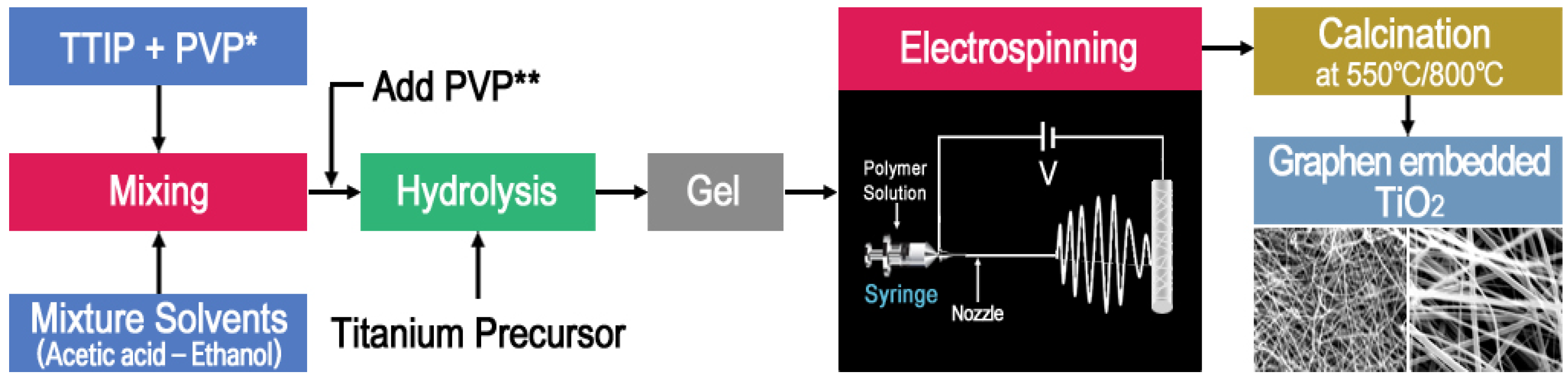
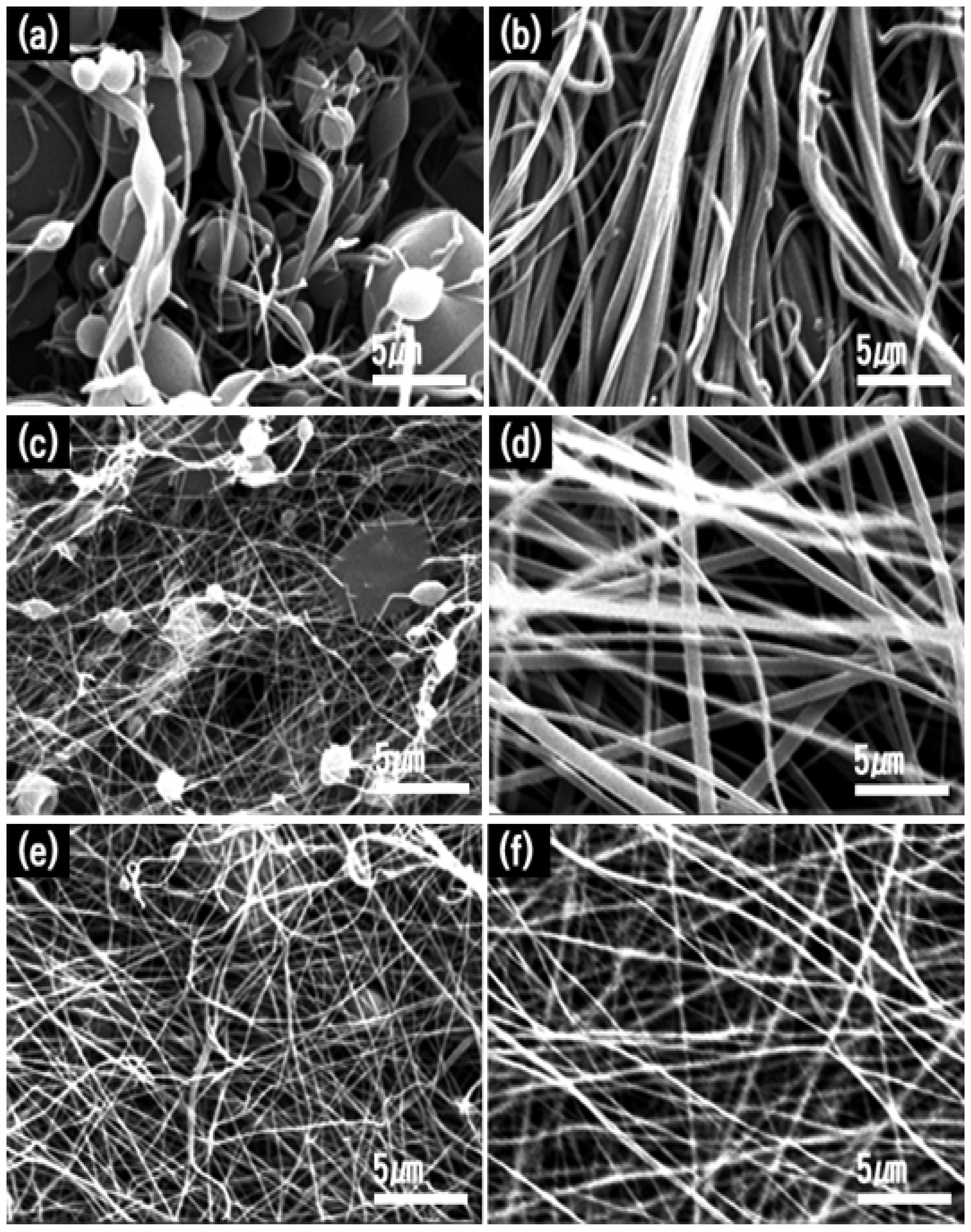
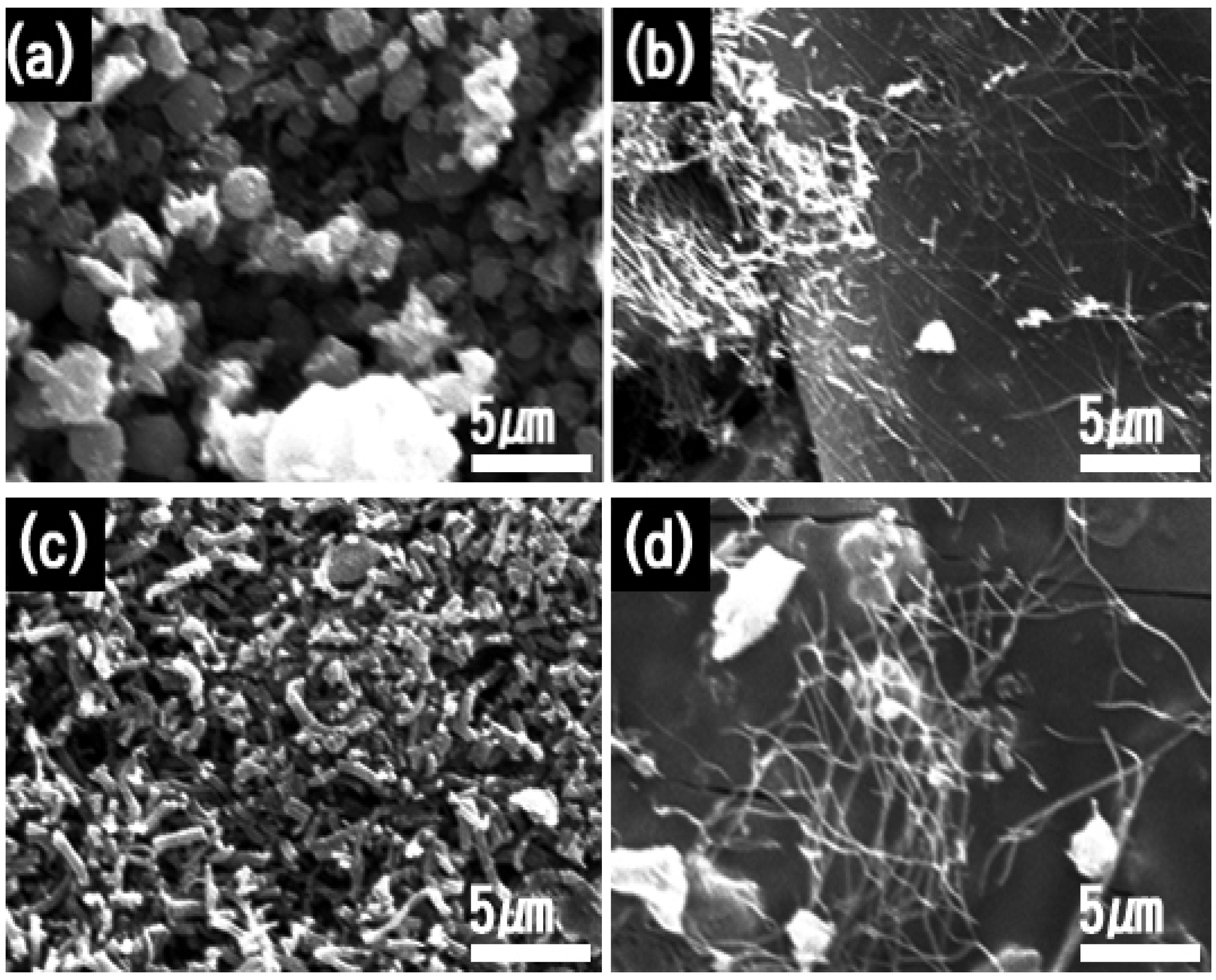
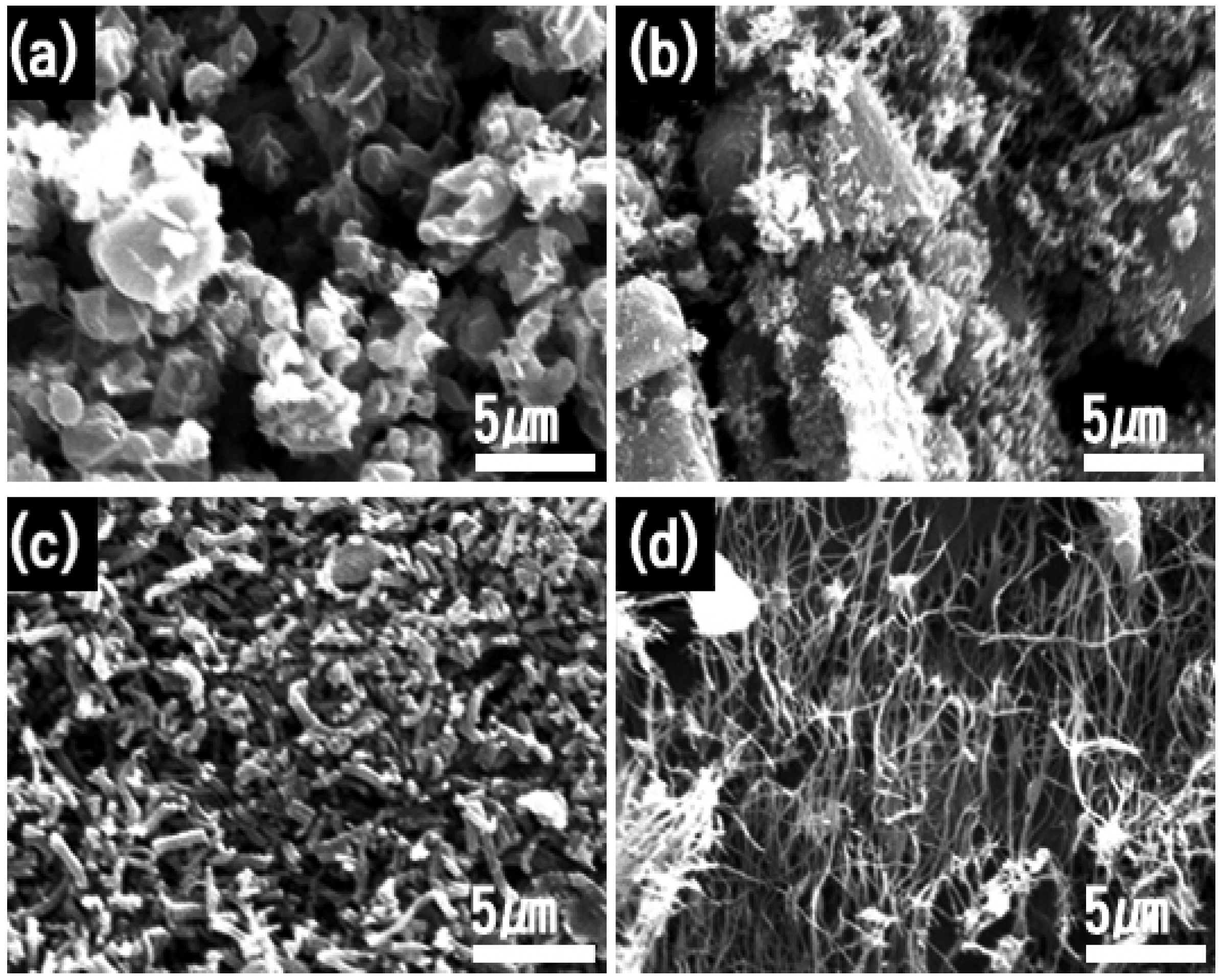
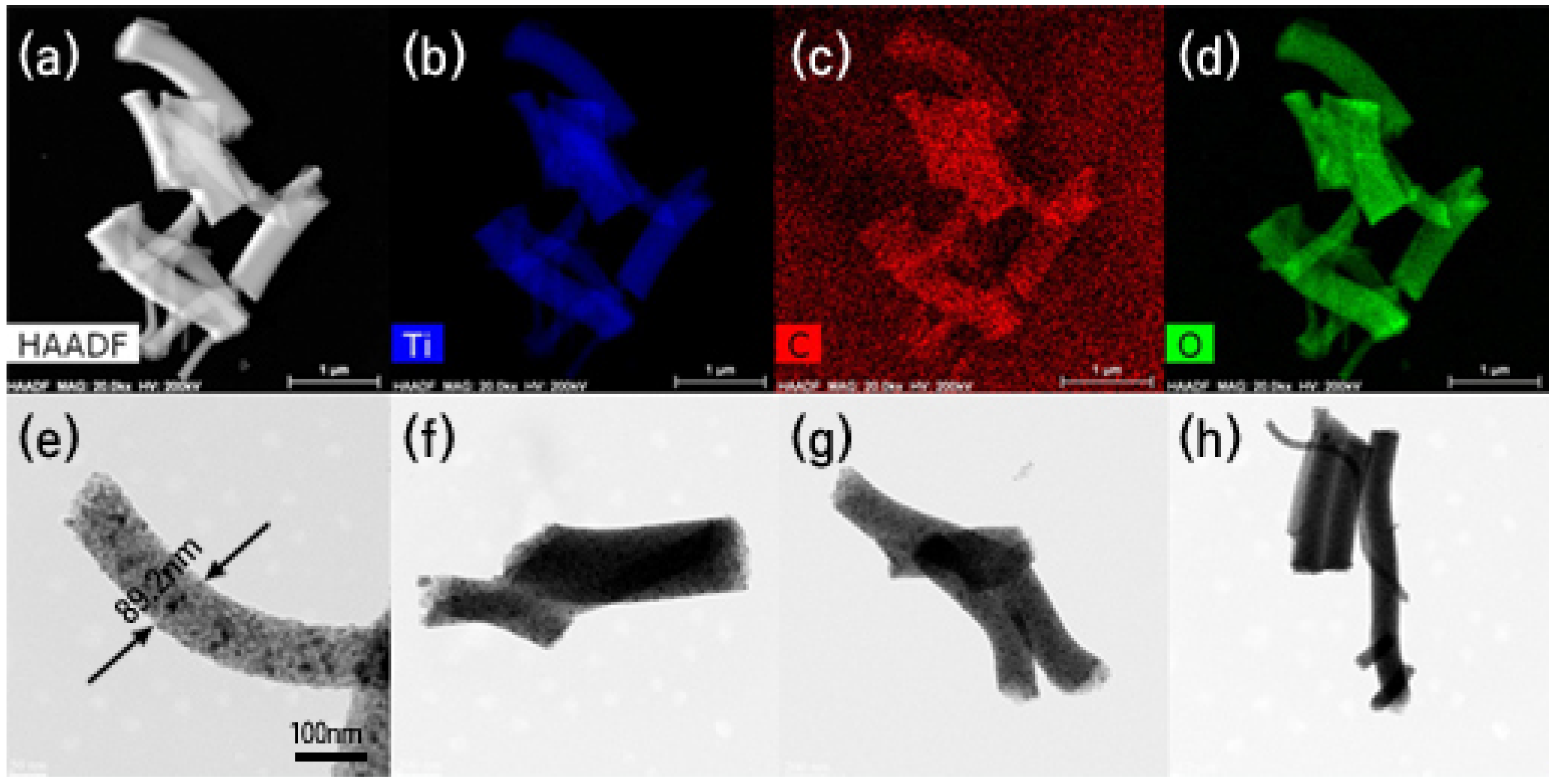
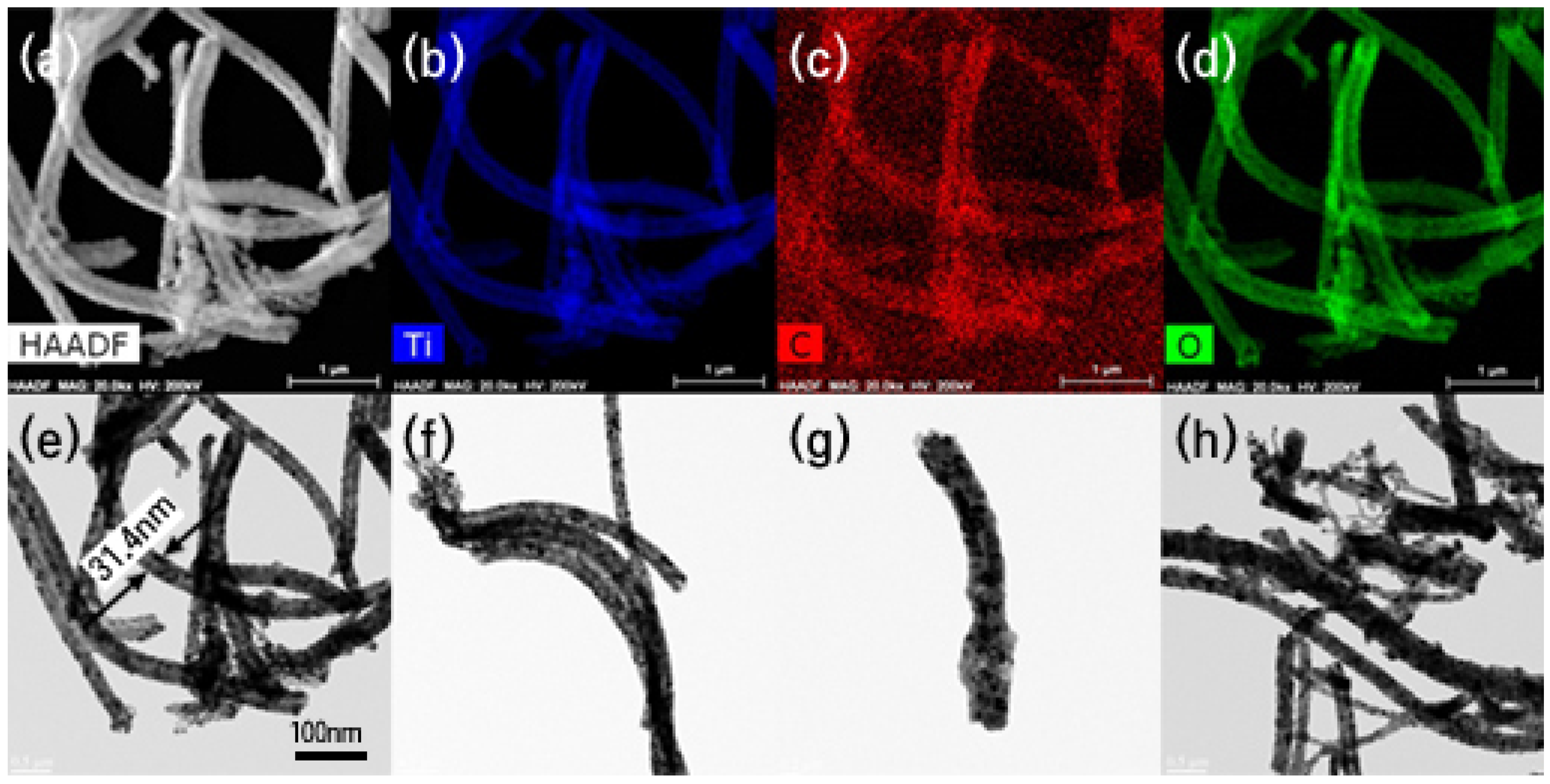
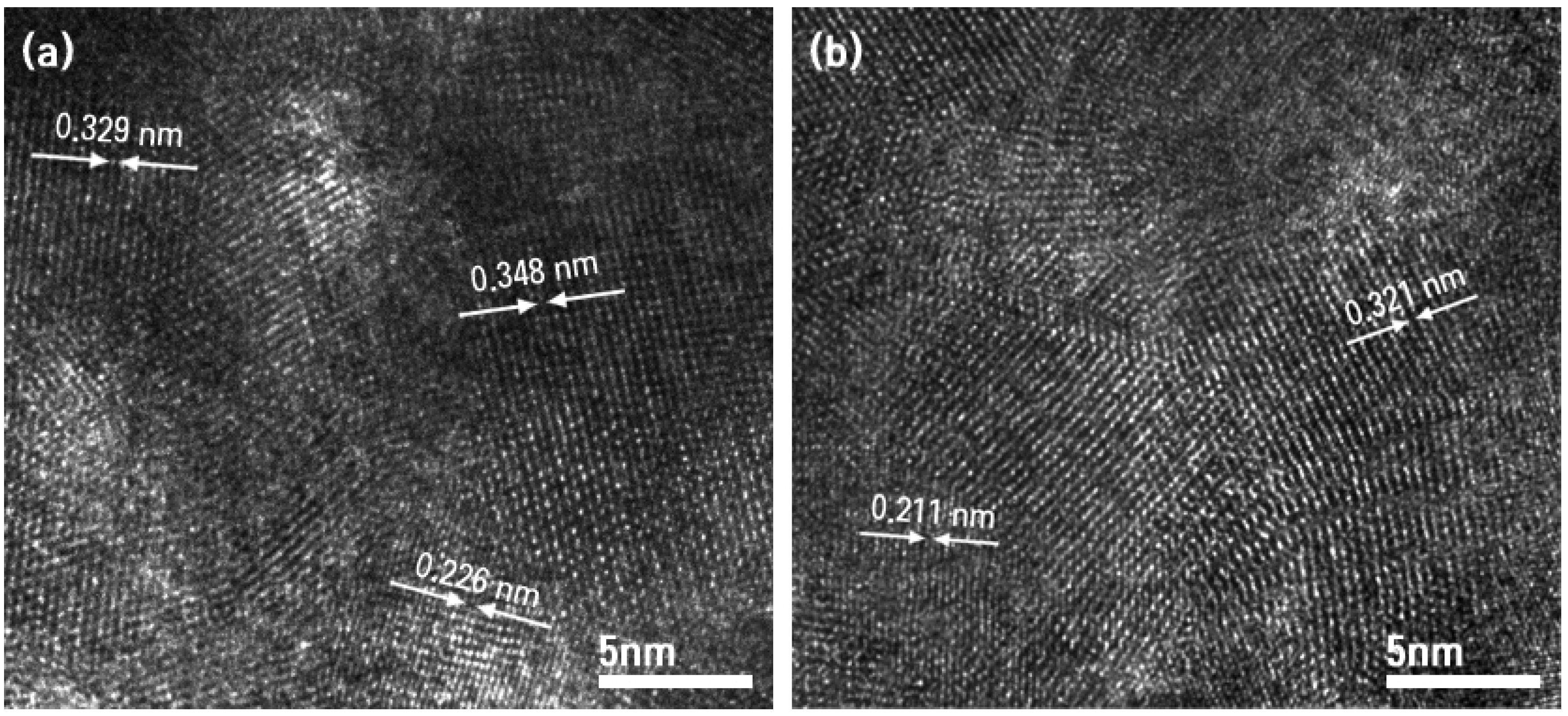

| TIIP (g) | Electrospun Products | PVP (g) | Graphene Precursor(g) | Designation of Calcined Samples Based on Temperature of Calcination | ||
|---|---|---|---|---|---|---|
| Designation | Molecular Weight (40,000) | Molecular Weight (1,300,000) | 550 °C | 800 °C | ||
| 1.5 | S1 | 2.4 | - | - | S1 (550) | S1 (800) |
| S2 | - | 0.4 | - | S2 (550) | S2 (800) | |
| S3 | - | 0.4 | 0.080 | S3 (550) | S3 (800) | |
| S4 | 2.4 | 0.4 | 0.080 | S4 (550) | S4 (800) | |
| S5 | 2.4 | 0.4 | 0.120 | S5 (550) | S5 (880) | |
| S6 | 2.4 | 0.4 | 0.040 | S6 (550) | S6 (800) | |
| Sample | Lattice Parameter | Unit Cell Volume (A3) | Crystallite Size (t)(nm) | Mass Fraction (%) | ||||
|---|---|---|---|---|---|---|---|---|
| d-Spacing | a(Å ) | b(Å) | c(Å) | Anatase XA | Rutile XR | |||
| S1 (550) | 3.515 | 3.782 | 3.782 | 9.499 | 136.78 | 20.95 | 90.14 | 9.86 |
| S2 (550) | 3.518 | 3.781 | 3.781 | 9.495 | 136.43 | 15.64 | 93.76 | 6.24 |
| S4 (550) | 3.488 | 3.776 | 3.776 | 9.486 | 135.25 | 20.95 | 87.82 | 12.18 |
| S5 (550) | 3.493 | 3.771 | 3.771 | 9.430 | 134.10 | 31.86 | 97.72 | 2.28 |
| S1 (800) | 3.250 | 4.594 | 4.594 | 2.959 | 62.450 | 49.52 | 1.00 | 99.0 |
| S2 (800) | 3.509 | 4.593 | 4.593 | 2.957 | 62.12 | 31.85 | 1.01 | 99.0 |
| S4 (800) | 3.507 | 4.593 | 4.593 | 2.959 | 62.42 | 31.85 | Trace | 100 |
| S5 (800) | 3.513 | 4.592 | 4.592 | 2.958 | 62.37 | 38.64 | Trace | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-C.; Gopalan, A.-I.; Sai-Anand, G.; Lee, K.-P.; Kim, W.-J. Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal. Catalysts 2019, 9, 170. https://doi.org/10.3390/catal9020170
Lee J-C, Gopalan A-I, Sai-Anand G, Lee K-P, Kim W-J. Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal. Catalysts. 2019; 9(2):170. https://doi.org/10.3390/catal9020170
Chicago/Turabian StyleLee, Jun-Cheol, Anantha-Iyengar Gopalan, Gopalan Sai-Anand, Kwang-Pill Lee, and Wha-Jung Kim. 2019. "Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal" Catalysts 9, no. 2: 170. https://doi.org/10.3390/catal9020170
APA StyleLee, J.-C., Gopalan, A.-I., Sai-Anand, G., Lee, K.-P., & Kim, W.-J. (2019). Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal. Catalysts, 9(2), 170. https://doi.org/10.3390/catal9020170








