Self-Immobilizing Biocatalysts Maximize Space–Time Yields in Flow Reactors
Abstract
:1. Introduction
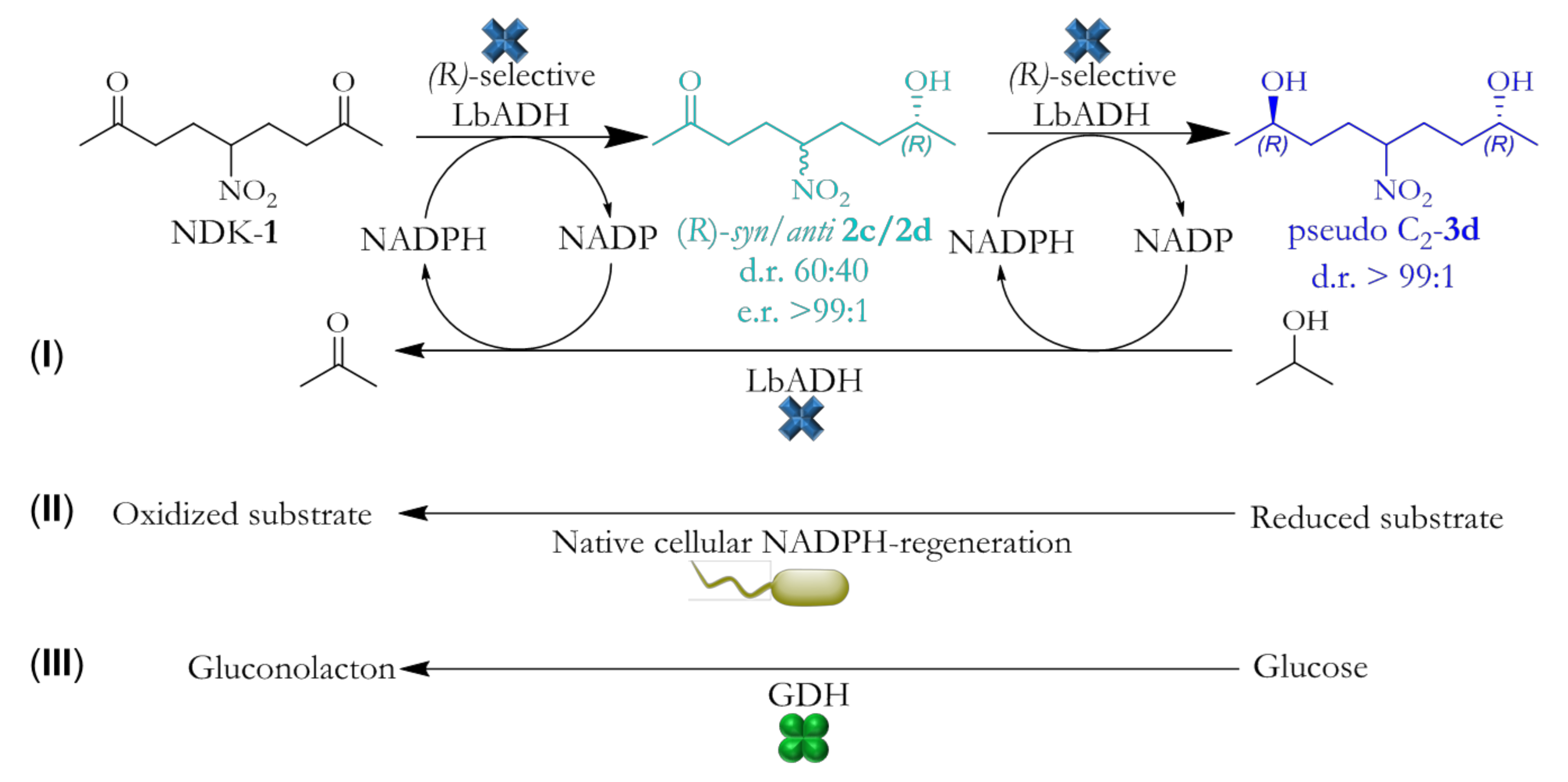
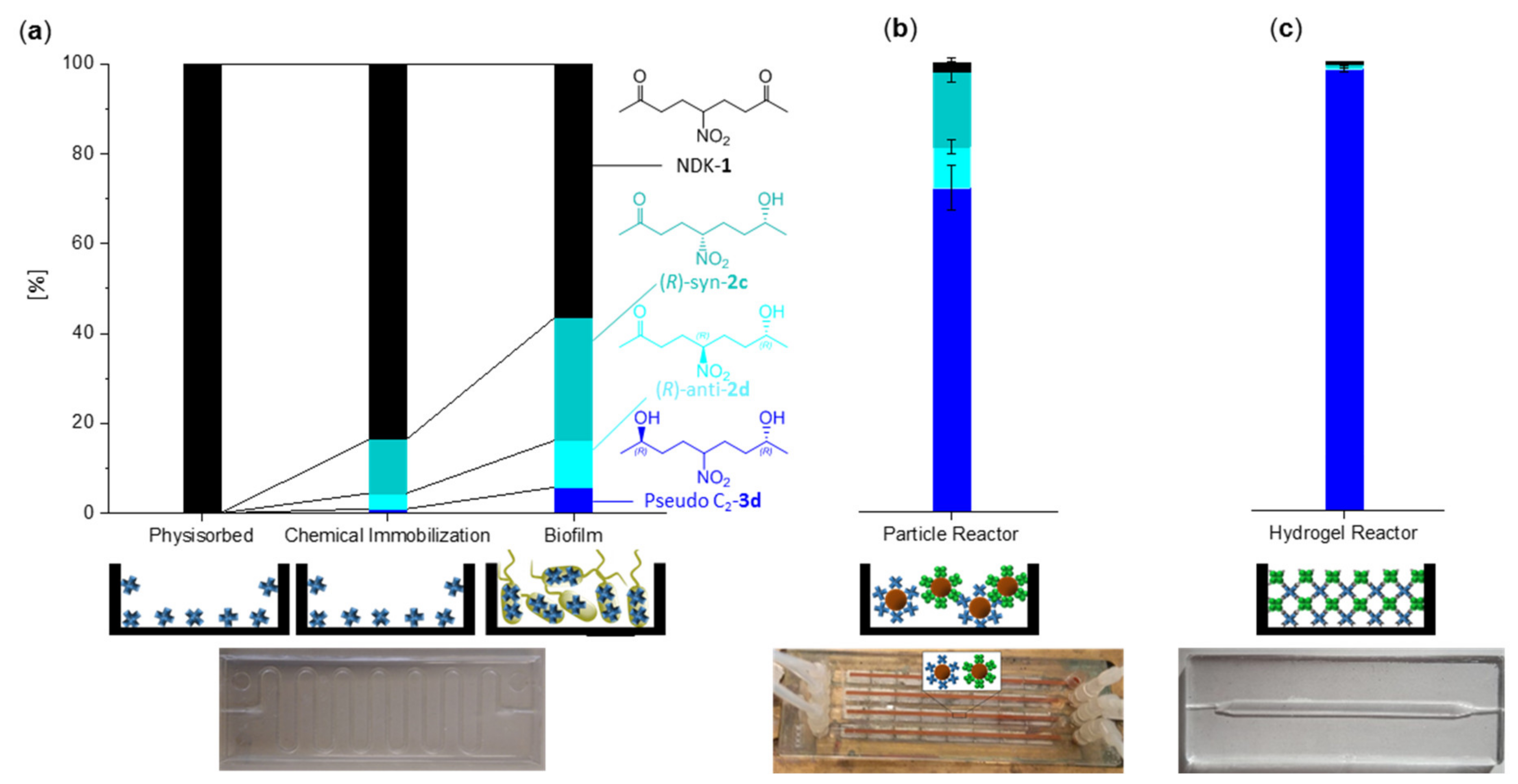
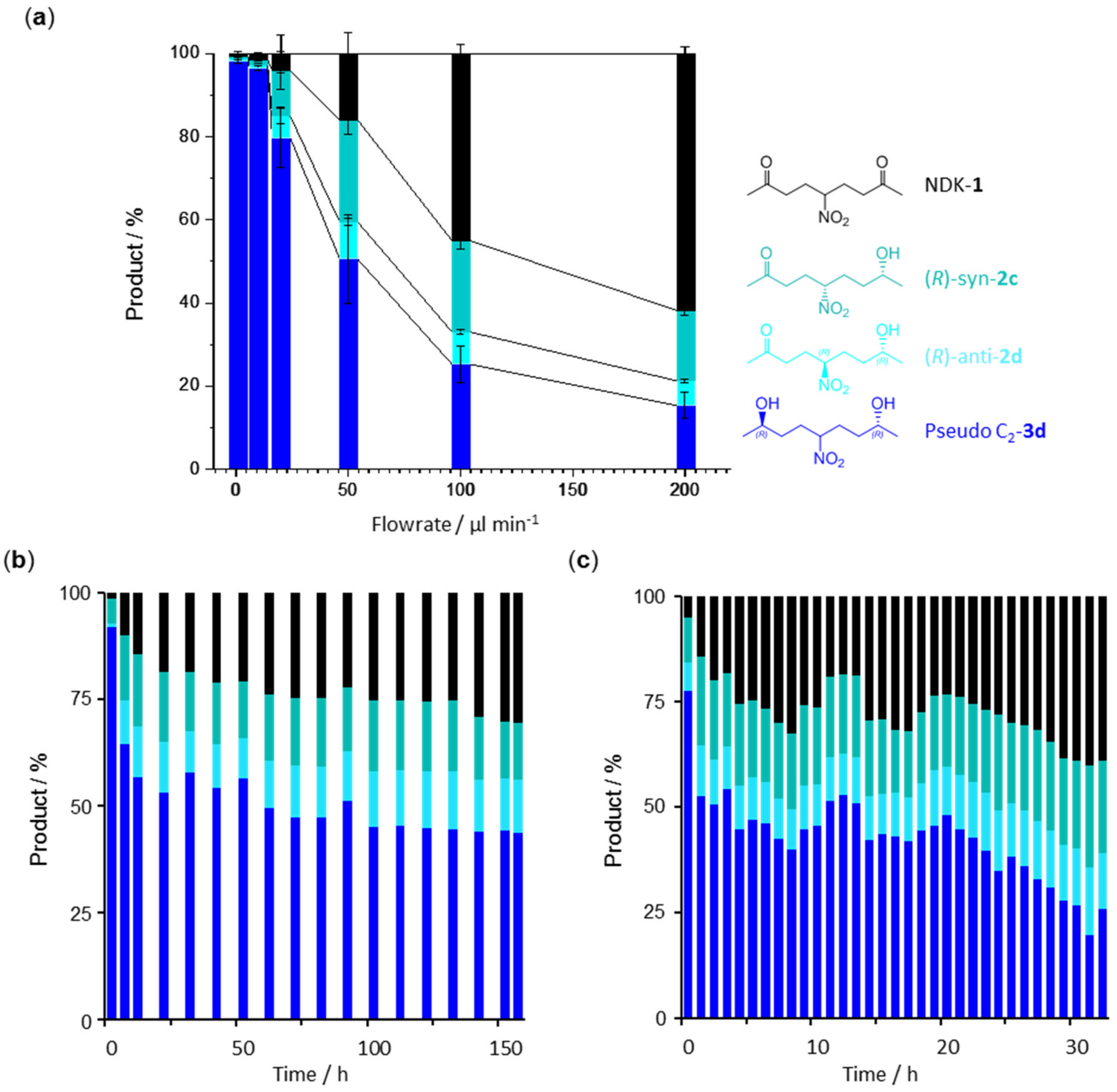
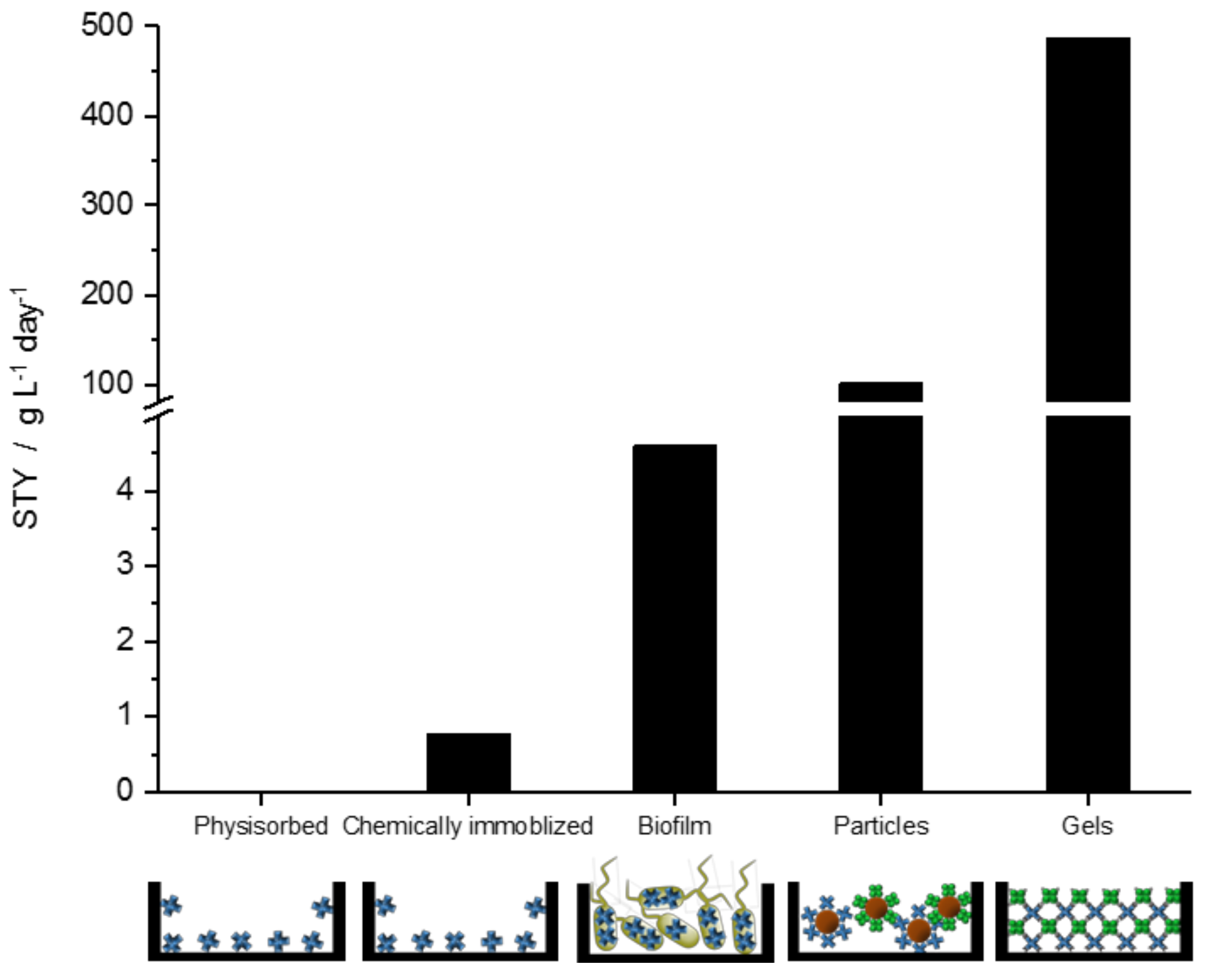
2. Results and Discussion
3. Materials and Methods
3.1. Cloning of Plasmids
3.2. Expression, Purification, and Characterization of Enzymes
3.3. Fabrication of the PDMS Chips
3.4. Preparation and Analysis of Reactor Modules Containing Physisorbed or Chemically Immobilized Enzymes
3.5. Preparation and Analysis of Catalytically Active E. coli Biofilm Reactor Modules
3.6. Preparation and Analysis of Microparticle Packed-Bed Modules Containing SBP-Tagged Enzymes
3.7. Preparation and Analysis of Hydrogel Reactor Modules
3.8. Chiral HPLC Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Straathof, A.J. Transformation of biomass into commodity chemicals using enzymes or cells. Chem. Rev. 2014, 114, 1871–1908. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2017, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lalonde, J.; Borup, B.; Mitchell, V.; Mundorff, E.; Trinh, N.; Kochrekar, D.A.; Cherat, R.N.; Pai, G.G. Development of a Biocatalytic Process as an Alternative to the (−)-DIP-Cl-Mediated Asymmetric Reduction of a Key Intermediate of Montelukast. Org. Process Res. Dev. 2010, 14, 193–198. [Google Scholar] [CrossRef]
- Ma, S.K.; Gruber, J.; Davis, C.; Newman, L.; Gray, D.; Wang, A.; Grate, J.; Huisman, G.W.; Sheldon, R.A. A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem. 2010, 12, 81–86. [Google Scholar] [CrossRef]
- Han, C.; Savage, S.; Al-Sayah, M.; Yajima, H.; Remarchuk, T.; Reents, R.; Wirz, B.; Iding, H.; Bachmann, S.; Fantasia, S.M.; et al. Asymmetric Synthesis of Akt Kinase Inhibitor Ipatasertib. Org. Lett. 2017, 19, 4806–4809. [Google Scholar] [CrossRef] [PubMed]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Silver, P.A. Designing biological compartmentalization. Trends Cell Biol. 2012, 22, 662–670. [Google Scholar] [CrossRef]
- Wheeldon, I.; Minteer, S.D.; Banta, S.; Barton, S.C.; Atanassov, P.; Sigman, M. Substrate channelling as an approach to cascade reactions. Nat. Chem. 2016, 8, 299–309. [Google Scholar]
- Kuchler, A.; Yoshimoto, M.; Luginbuhl, S.; Mavelli, F.; Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 2016, 11, 409–420. [Google Scholar]
- Chen, Z.; Zeng, A.P. Protein engineering approaches to chemical biotechnology. Curr. Opin. Biotechnol. 2016, 42, 198–205. [Google Scholar]
- Quin, M.B.; Wallin, K.K.; Zhang, G.; Schmidt-Dannert, C. Spatial organization of multi-enzyme biocatalytic cascades. Org. Biomol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- France, S.P.; Hepworth, L.J.; Turner, N.J.; Flitsch, S.L. Constructing Biocatalytic Cascades: In Vitro and in Vivo Approaches to de Novo Multi-Enzyme Pathways. ACS Catal. 2017, 7, 710–724. [Google Scholar] [CrossRef]
- Rabe, K.S.; Muller, J.; Skoupi, M.; Niemeyer, C.M. Cascades in Compartments: En Route to Machine-Assisted Biotechnology. Angew. Chem. Int. Ed. 2017, 56, 13574–13589. [Google Scholar] [CrossRef]
- Ley, S.V.; Fitzpatrick, D.E.; Ingham, R.J.; Myers, R.M. Organic synthesis: march of the machines. Angew. Chem. Int. Ed. 2015, 54, 3449–3464. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ballmer, S.G.; Gillis, E.P.; Fujii, S.; Schmidt, M.J.; Palazzolo, A.M.; Lehmann, J.W.; Morehouse, G.F.; Burke, M.D. Synthesis of many different types of organic small molecules using one automated process. Science 2015, 347, 1221–1226. [Google Scholar] [CrossRef] [Green Version]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.C.; Myerson, A.S.; Revalor, E.M.; Snead, D.R. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry(II). Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Matosevic, S.; Szita, N.; Baganz, F. Fundamentals and applications of immobilized microfluidic enzymatic reactors. J. Chem. Technol. Biotechnol. 2011, 86, 325–334. [Google Scholar] [CrossRef]
- Wohlgemuth, R.; Plazl, I.; Znidarsic-Plazl, P.; Gernaey, K.V.; Woodley, J.M. Microscale technology and biocatalytic processes: opportunities and challenges for synthesis. Trends Biotechnol. 2015, 33, 302–314. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2017. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Radtke, C.P.; Hubbuch, J.; Niemeyer, C.M.; Rabe, K.S. On-Demand Production of Flow-Reactor Cartridges by 3D Printing of Thermostable Enzymes. Angew. Chem. Int. Ed. 2018, 57, 5539–5543. [Google Scholar] [CrossRef] [PubMed]
- Peschke, T.; Bitterwolf, P.; Gallus, S.; Hu, Y.; Oelschlaeger, C.; Willenbacher, N.; Rabe, K.S.; Niemeyer, C.M. Self-Assembling All-Enzyme Hydrogels for Flow Biocatalysis. Angew. Chem. Int. Ed. 2018, 57, 17028–17032. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.P.; Peñafiel, I.; Cosgrove, S.C.; Turner, N.J. Biocatalysis Using Immobilized Enzymes in Continuous Flow for the Synthesis of Fine Chemicals. Org. Process Res. Dev. 2018. [Google Scholar] [CrossRef]
- Schmid-Dannert, C.; Lopez-Gallego, F. Advances and opportunities for the design of self-sufficient and spatially organized cell-free biocatalytic systems. Curr. Opin. Chem. Biol. 2018, 49, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gandomkar, S.; Żądło-Dobrowolska, A.; Kroutil, W. Extending Designed Linear Biocatalytic Cascades for Organic Synthesis. ChemCatChem 2019, 11, 225–243. [Google Scholar] [CrossRef]
- Buchholz, K.; Kasche, V.; Bornscheuer, U.T. Biocatalysts and Enzyme Technology-2nd Edition; Wiley-Blackwell: Weinheim, Germany, 2012; 444p. [Google Scholar]
- Jonkheijm, P.; Weinrich, D.; Schroeder, H.; Niemeyer, C.M.; Waldmann, H. Chemical Strategies for Generating Protein Biochips. Angew. Chem. Int. Ed. 2008, 47, 9618–9647. [Google Scholar] [CrossRef]
- Britton, J.; Dyer, R.P.; Majumdar, S.; Raston, C.L.; Weiss, G.A. Ten-Minute Protein Purification and Surface Tethering for Continuous-Flow Biocatalysis. Angew. Chem. Int. Ed. 2017, 56, 2296–2301. [Google Scholar] [CrossRef] [Green Version]
- Peschke, T.; Skoupi, M.; Burgahn, T.; Gallus, S.; Ahmed, I.; Rabe, K.S.; Niemeyer, C.M. Self-Immobilizing Fusion Enzymes for Compartmentalized Biocatalysis. ACS Catal. 2017, 7, 7866–7872. [Google Scholar] [CrossRef]
- Contente, M.L.; Paradisi, F. Self-sustaining closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions. Nat. Catal. 2018, 1, 452–459. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface 2015, 12, 20140891. [Google Scholar] [CrossRef]
- Gkantzou, E.; Patila, M.; Stamatis, H. Magnetic Microreactors with Immobilized Enzymes-From Assemblage to Contemporary Applications. Catalysts 2018, 8, 282. [Google Scholar] [CrossRef]
- Qureshi, N.; Annous, B.A.; Ezeji, T.C.; Karcher, P.; Maddox, I.S. Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb. Cell Fact. 2005, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Leuchs, S.; Greiner, L. Alcohol Dehydrogenase from Lactobacillus brevis: A Versatile Robust Catalyst for Enantioselective Transformations. Chem. Biochemi. Engin. Q. 2011, 25, 267–281. [Google Scholar]
- Skoupi, M.; Vaxelaire, C.; Strohmann, C.; Christmann, M.; Niemeyer, C.M. Enantiogroup-Differentiating Biocatalytic Reductions of Prochiral Cs-Symmetrical Dicarbonyl Compounds to meso Compounds. Chem. Eur. J. 2015, 21, 8701–8705. [Google Scholar] [CrossRef]
- Keefe, A.D.; Wilson, D.S.; Seelig, B.; Szostak, J.W. One-step purification of recombinant proteins using a nanomolar-affinity streptavidin-binding peptide, the SBP-Tag. Protein Expr. Purif. 2001, 23, 440–446. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Liang, S. Several affinity tags commonly used in chromatographic purification. J. Anal. Methods Chem. 2013, 2013, 581093. [Google Scholar] [CrossRef]
- Hansen, S.H.; Kabbeck, T.; Radtke, C.P.; Krause, S.; Krolitzki, E.; Peschke, T.; Gasmi, J.; Rabe, K.S.; Wagner, M.; Horn, H.; et al. Machine-assisted cultivation and analysis of biofilms. bioRxiv 2017. [Google Scholar] [CrossRef]
- Peschke, T.; Rabe, K.S.; Niemeyer, C.M. Orthogonal Surface Tags for Whole-Cell Biocatalysis. Angew. Chem. Int. Ed. 2017, 56, 2183–2186. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, P. Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnol. Adv. 2007, 25, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sanchez, J.; France, S.P.; Montgomery, S.L.; Aleku, G.A.; Man, H.; Sharma, M.; Ramsden, J.I.; Grogan, G.; Turner, N.J. Imine reductases (IREDs). Curr. Opin. Chem. Biol. 2017, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Berglund, P. Transaminase biocatalysis: optimization and application. Green Chem. 2017, 19, 333–360. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Nidetzky, B. Microfluidic reactor for continuous flow biotransformations with immobilized enzymes: The example of lactose hydrolysis by a hyperthermophilic beta-glycoside hydrolase. Eng. Life Sci. 2008, 8, 40–48. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peschke, T.; Bitterwolf, P.; Hansen, S.; Gasmi, J.; Rabe, K.S.; Niemeyer, C.M. Self-Immobilizing Biocatalysts Maximize Space–Time Yields in Flow Reactors. Catalysts 2019, 9, 164. https://doi.org/10.3390/catal9020164
Peschke T, Bitterwolf P, Hansen S, Gasmi J, Rabe KS, Niemeyer CM. Self-Immobilizing Biocatalysts Maximize Space–Time Yields in Flow Reactors. Catalysts. 2019; 9(2):164. https://doi.org/10.3390/catal9020164
Chicago/Turabian StylePeschke, Theo, Patrick Bitterwolf, Silla Hansen, Jannis Gasmi, Kersten S. Rabe, and Christof M. Niemeyer. 2019. "Self-Immobilizing Biocatalysts Maximize Space–Time Yields in Flow Reactors" Catalysts 9, no. 2: 164. https://doi.org/10.3390/catal9020164





