SBA-15 Anchored Metal Containing Catalysts in the Oxidative Desulfurization Process
Abstract
:1. Introduction
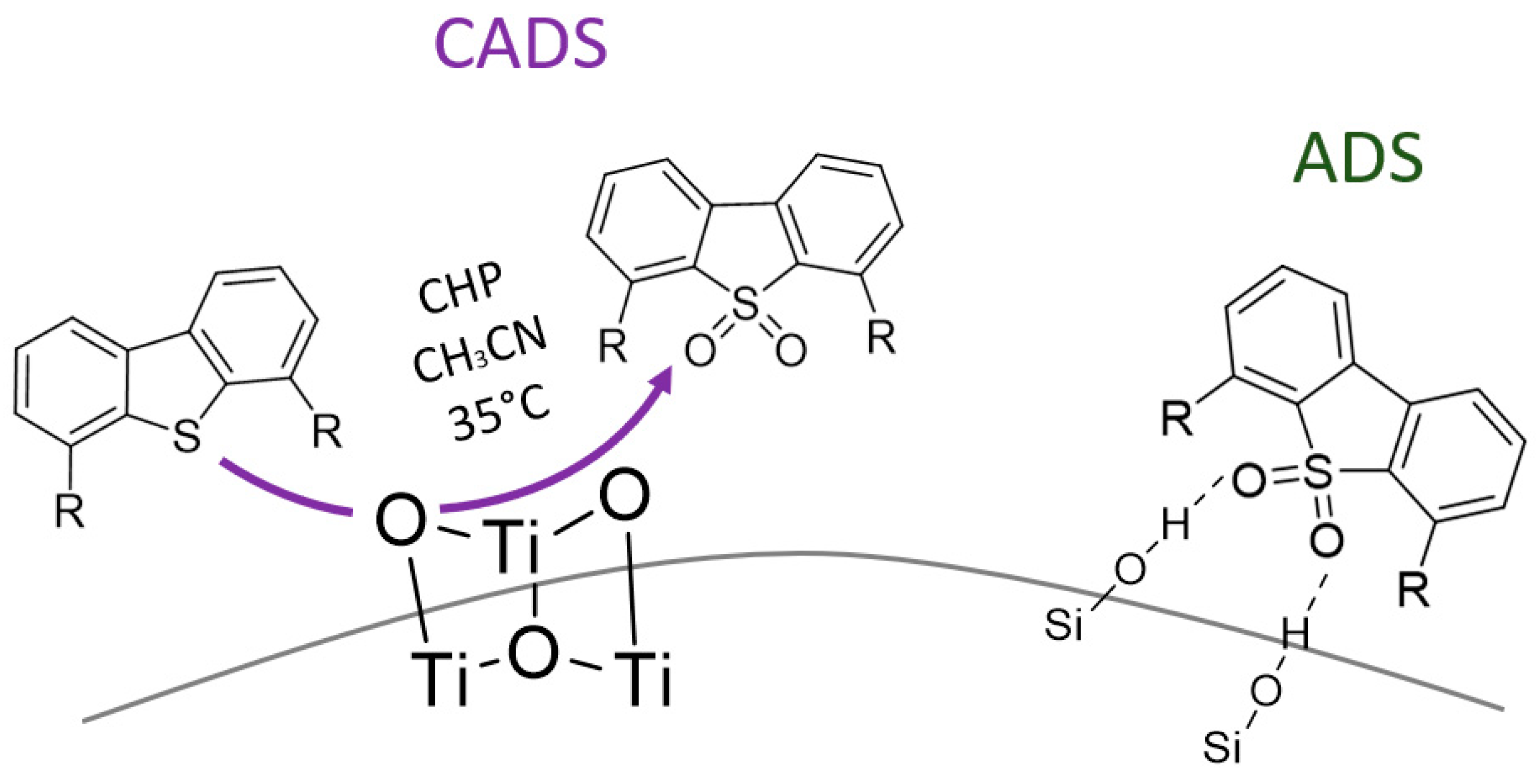
2. Titanium Oxides
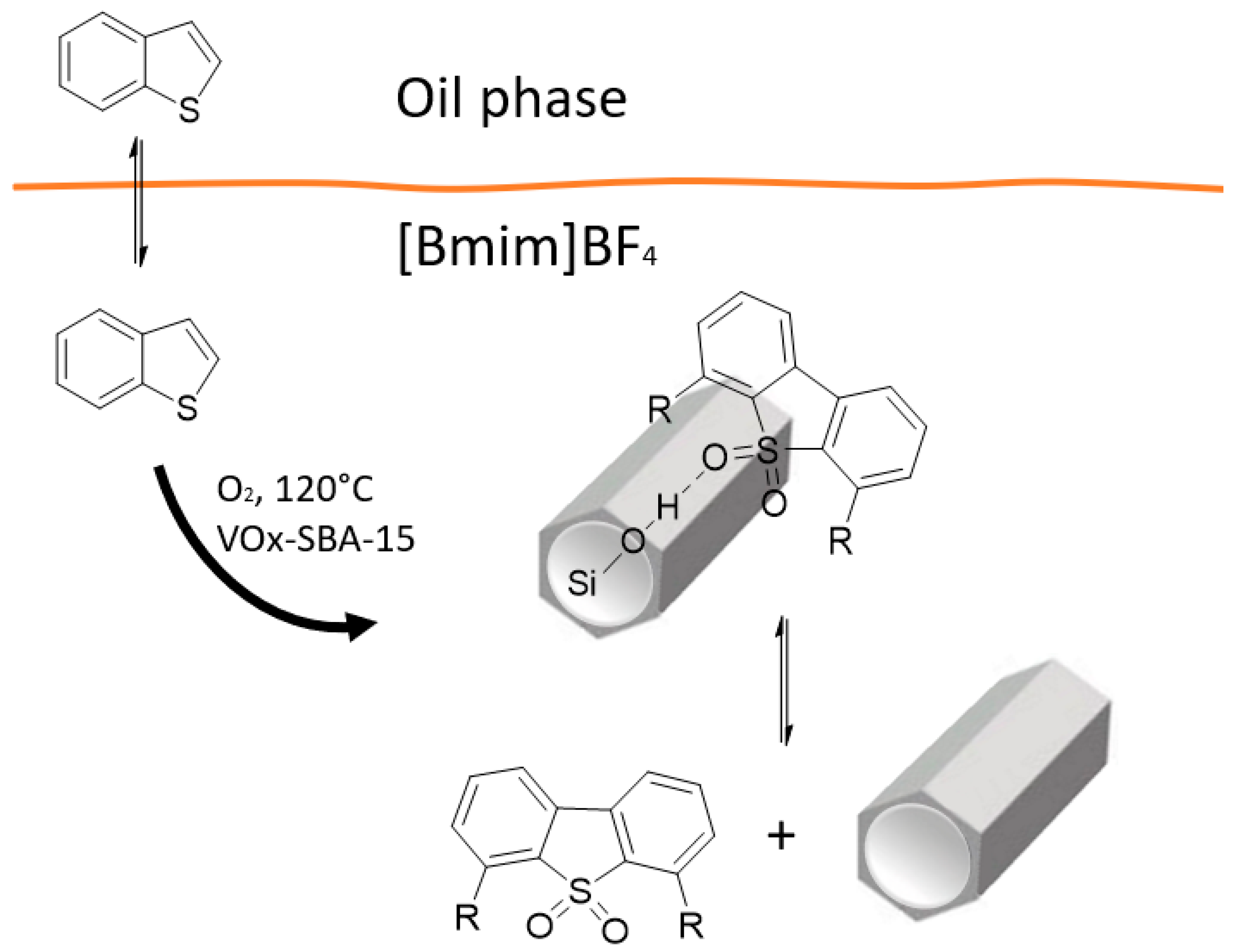
3. Vanadium Oxides
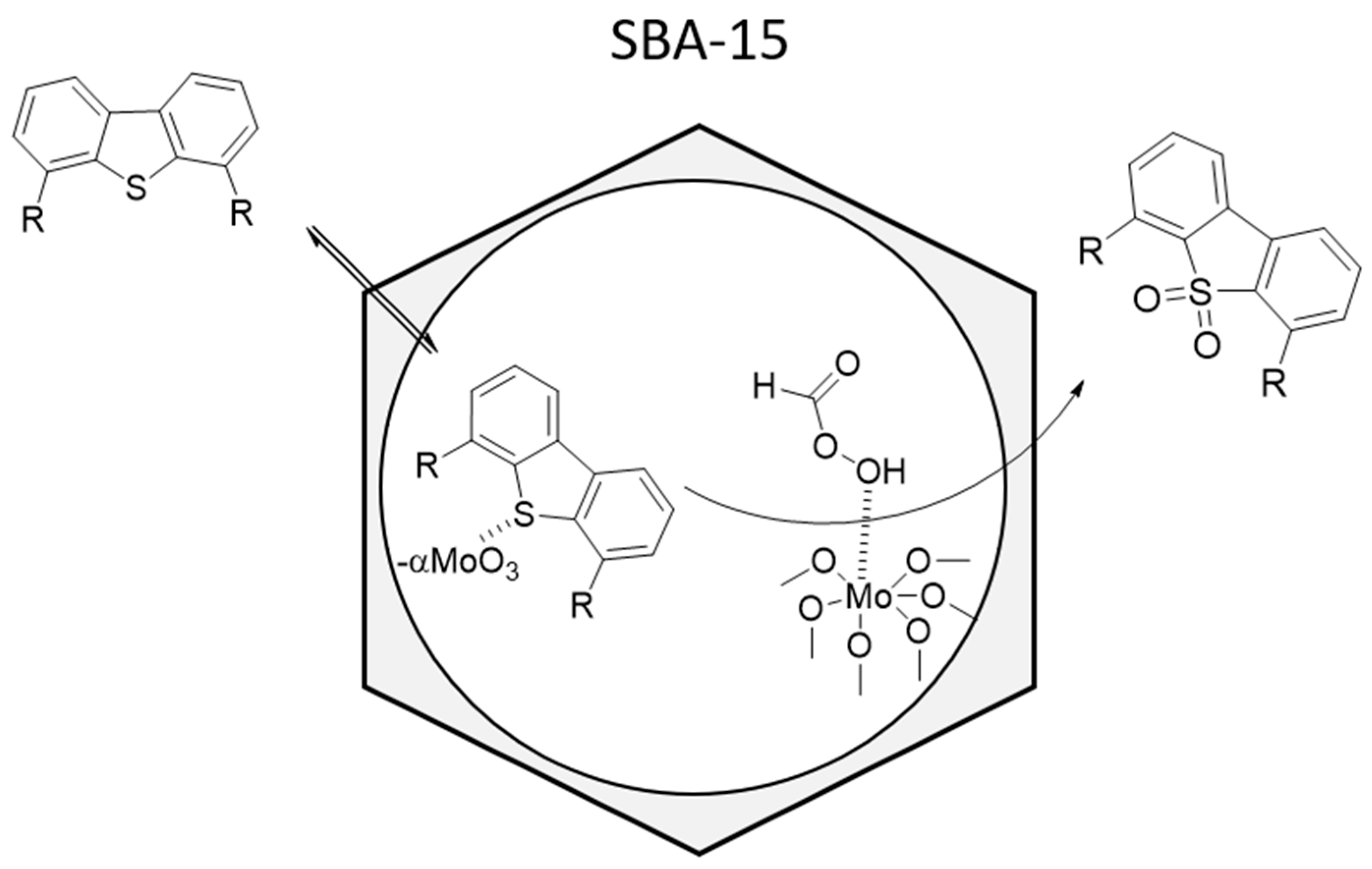
4. Molybdenum Oxides
5. Iron Oxides
6. Tungsten Oxides
7. Silver Oxides
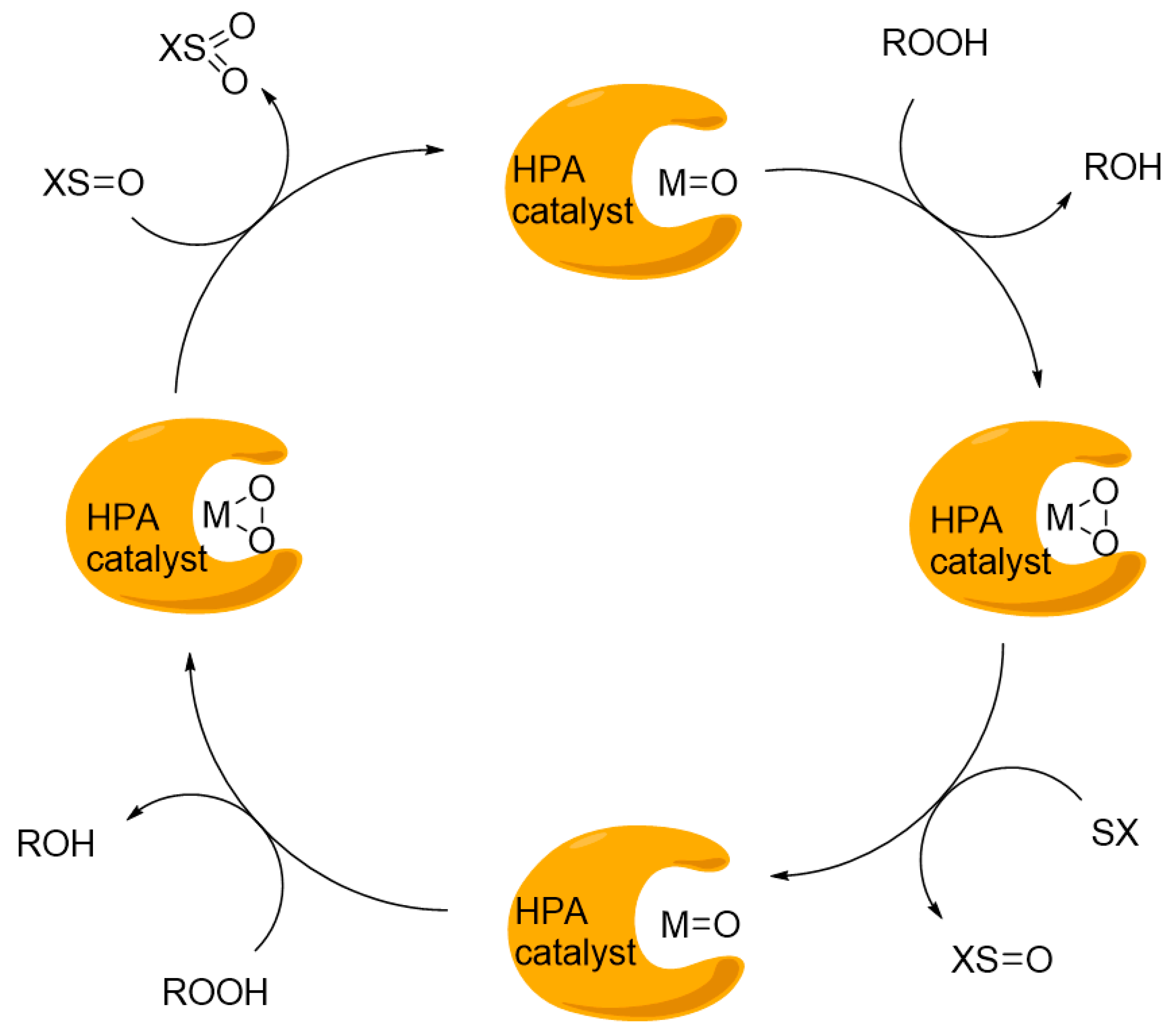
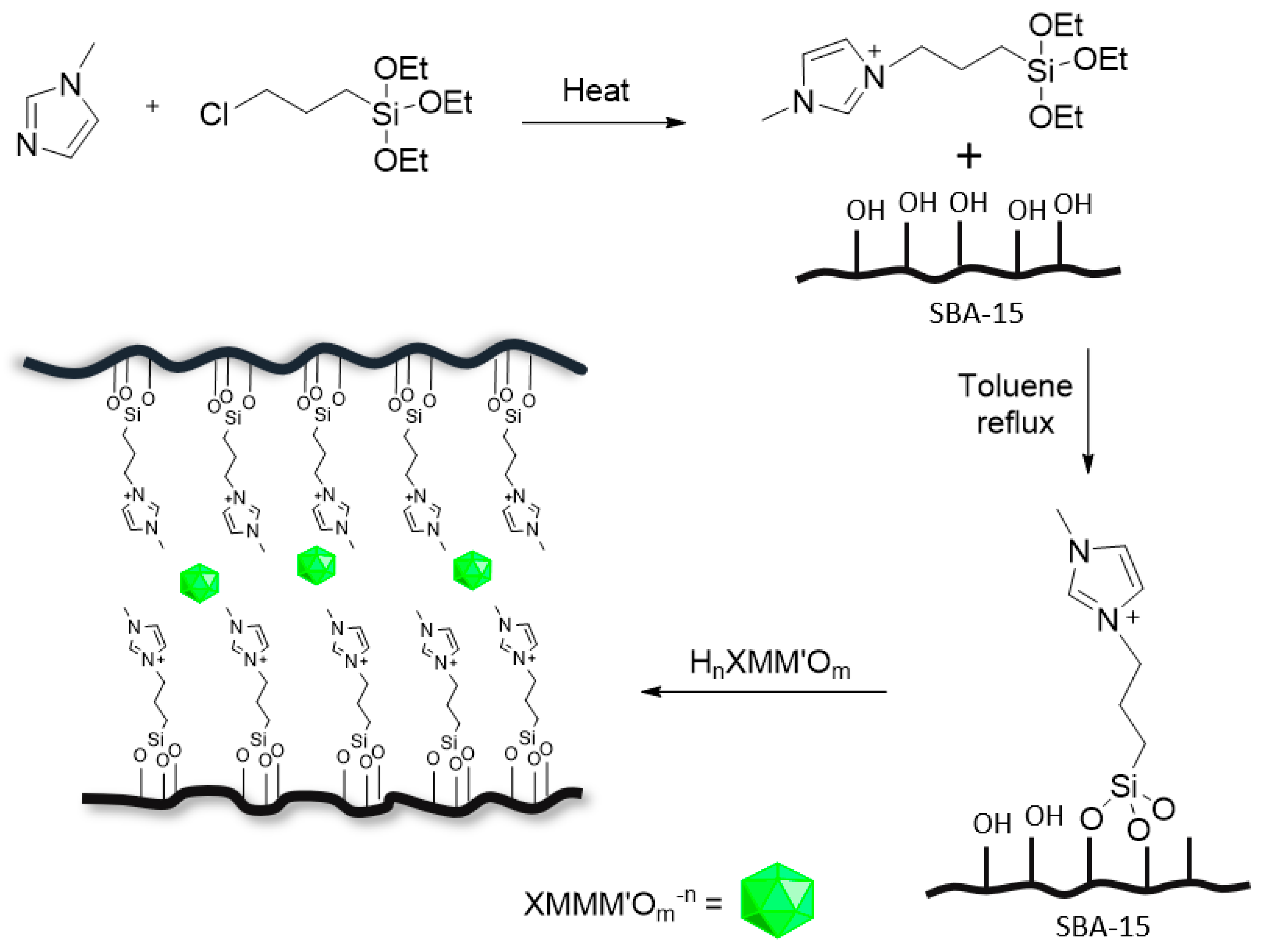
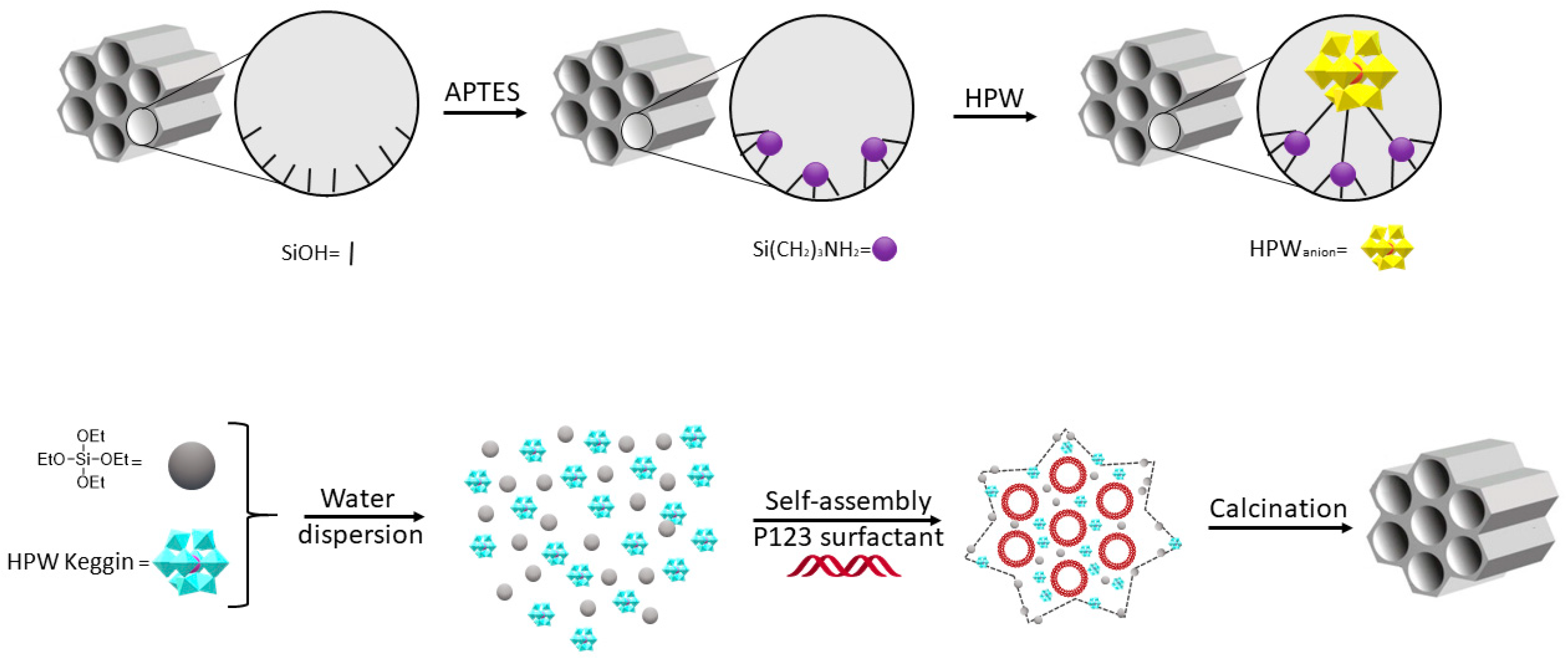
8. Polyoxometalates
8.1. Phosphomolybdic Containing POMs
8.2. Phosphotungstic Containing POMs
9. Miscellanea
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dos Santos, S.M.L.; Nogueira, K.A.B.; De Souza Gama, M.; Lima, J.D.F.; Da Silva Júnior, I.J.; De Azevedo, D.C.S. Synthesis and characterization of ordered mesoporous silica (SBA-15 and SBA-16) for adsorption of biomolecules. Microporous Mesoporous Mater. 2013, 180, 284–292. [Google Scholar] [CrossRef]
- Gaffar, I.; Rajamanickam, M. Mn-incorporated SBA-1 cubic mesoporous silicates: Synthesis and characterization. Mater. Chem. Phys. 2015, 161, 237–242. [Google Scholar]
- Chaudhary, V.; Sharma, S. An overview of ordered mesoporous material SBA-15: Synthesis, functionalization and application in oxidation reactions. J. Porous Mater. 2017, 24, 741–749. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Li, Q.; Stucky, G.D. Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mater. 2000, 12, 275–279. [Google Scholar] [CrossRef]
- Shen, S.; Chen, F.; Chow, P.S.; Phanapavudhikul, P.; Zhu, K.; Tan, R.B.H. Synthesis of SBA-15 mesoporous silica via dry-gel conversion route. Microporous Mesoporous Mater. 2006, 92, 300–308. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Yang, H.; Meng, Y.; Yu, C.; Tu, B.; Zhao, D. Understanding effect of wall structure on the hydrothermal stability of mesostructured silica SBA-15. J. Phys. Chem. B 2005, 109, 8723–8732. [Google Scholar] [CrossRef]
- Miyazawa, K.; Inagaki, S. Control of the microporosity within the pore walls of ordered mesoporous silica SBA-15. Chem. Commun. 2000, 21, 2121–2122. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Yuan, X.; Shen, J.; Kim, J. Direct synthesis, characterization and catalytic application of SBA-15 containing heteropolyacid H3PW12O40. J. Mol. Catal. A 2005, 229, 199–205. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Lashgari, N.; Badiei, A. Application of Organoamine-functionalized Mesoporous Silica (SBA-Pr-NH2) as a Nano Base Catalyst in Organic Reactions. Curr. Org. Chem. 2017, 21, 674–687. [Google Scholar] [CrossRef]
- Wu, S.H.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Han, Y.J.; Stucky, G.; Sotelo, J.L.; Ovejerob, G.; Melero, J.A. Synthesis and characterisation of iron-containing SBA-15 mesoporous silica. Stud. Surf. Sci. Catal. 2002, 142, 1109–1116. [Google Scholar]
- García-Martínez, J.C.; González Uribe, H.A.; González-Brambila, M.M.; Colín-Luna, J.A.; Escobedo-García, Y.E.; López-Gaona, A.; Alvarado-Perea, L. Selective adsorption of nitrogen compounds using silica-based mesoporous materials as a pretreatment for deep hydrodesulfurization. Catal. Today 2018, 305, 40–48. [Google Scholar] [CrossRef]
- Luan, Z. Alumination and ion exchange of mesoporous SBA-15 molecular sieves. Chem. Mater. 1999, 11, 1621–1627. [Google Scholar] [CrossRef]
- Venezia, A.M.; Di Carlo, G.; Liotta, L.F.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B 2011, 6, 529–539. [Google Scholar] [CrossRef]
- Mishra, G.S.; Machado, K.; Kumar, A. Highly selective n-alkanes oxidation to ketones with molecular oxygen catalyzed by SBA-15 supported rhenium catalysts. J. Ind. Eng. Chem. 2014, 20, 2228–2235. [Google Scholar] [CrossRef]
- Hu, J.; Li, K.; Li, W.; Ma, F.; Guo, Y. Selective oxidation of styrene to benzaldehyde catalyzed by Schiff base-modified ordered mesoporous silica materials impregnated with the transition metal-monosubstituted Keggin-type polyoxometalates. Appl. Catal. A 2009, 364, 211–220. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Hao, S.; Guan, J.; Ding, H.; Shang, F.; Qiu, P.; Kan, Q. Heterogenization of functionalized Cu(II) and VO(IV) Schiff base complexes by direct immobilization onto amino-modified SBA-15: Styrene oxidation catalysts with enhanced reactivity. Appl. Catal. A 2010, 381, 274–281. [Google Scholar] [CrossRef]
- Jin, M.; Kim, J.H.; Kim, J.M.; Jeon, J.K.; Jurng, J.; Bae, G.N.; Park, Y.K. Benzene oxidation with ozone over MnOx/SBA-15 catalysts. Catal. Today 2013, 204, 108–113. [Google Scholar] [CrossRef]
- Neeli, C.K.P.; Narani, A.; Marella, R.K.; Rao, K.S.R.; Burri, D.R. Selective benzylic oxidation of alkylaromatics over Cu/SBA-15 catalysts under solvent-free conditions. Catal. Commun. 2013, 39, 5–9. [Google Scholar] [CrossRef]
- Cruz, P.; Perez, Y.; Hierro, I.D.; Fajardo, M. Copper, copper oxide nanoparticles and copper complexes supported on mesoporous SBA-15 as catalyst in the selective oxidation of benzyl alcohol in acqueous phase. Microporous Mesoporous Mater. 2016, 220, 136. [Google Scholar] [CrossRef]
- Liotta, L.F.; Pantaleo, G.; Puleo, F.; Venezia, A.M. Au/CeO2-SBA-15 catalysts for CO oxidation: Effect of ceria loading on physic-chemical properties and catalytic performances. Catal. Today 2012, 187, 10–19. [Google Scholar] [CrossRef]
- Zhang, L.X.; Shi, J.L.; Yu, J.; Hua, Z.L.; Zhao, X.G.; Ruan, M.L. A new in-situ reduction route for the synthesis of Pt nanoclusters in the channels of mesoporous silica SBA-15. Adv. Mater. 2002, 14, 1510–1513. [Google Scholar] [CrossRef]
- Xueguang, W.; Kyle, S.K.L.; Jerry, C.C.; Soofin, C. Direct Synthesis and Catalytic Applications of Ordered Large Pore Aminopropyl-Functionalized SBA-15 Mesoporous Materials. J. Phys. Chem. 2005, 109, 1763–1769. [Google Scholar]
- Haribandhu, C.; Subhajit, D.; Ashis, S. Synthesis and use of SBA-15 adsorbent for dye-loaded wastewater treatment. J. Environ. Chem. Eng. 2015, 3, 2866–2874. [Google Scholar]
- Tummino, M.L.; Testa, M.L.; Malandrino, M.; Gamberini, R.; Prevot, A.B.; Magnacca, G.; Laurenti, E. Green Waste-Derived Substances Immobilized on SBA-15 Silica: Surface Properties, Adsorbing and Photosensitizing Activities towards Organic and Inorganic Substrates. Nanomaterials 2019, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Łaniecki, M. Application of SBA-15 mesoporous material as the carrier for drug formulation systems. Papaverine hydrochloride adsorption and release study. Powder Technol. 2012, 230, 106–111. [Google Scholar] [CrossRef]
- Szewczyk, A.; Prokopowicz, M. Amino-modified mesoporous silica SBA-15 as bifunctional drug delivery system for cefazolin: Release profile and mineralization potential. Mater. Lett. 2018, 227, 136–140. [Google Scholar] [CrossRef]
- EU Regulations. Available online: http://www.dieselnet.com/standards/eu/fuel.php (accessed on 3 June 2013).
- Sharipov, A.K.; Nigmatullin, V.R. Removal of Sulfur from Hydrotreated Diesel Fuel. Chem. Technol. Fuels Oils 2005, 41, 225–229. [Google Scholar] [CrossRef]
- González-García, O.; Cedeño-Caero, L. V-Mo based catalysts for oxidative desulfurization of diesel fuel. Catal. Today 2009, 148, 42–48. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Aghbolagh, Z.S.; Babaei, R. Oxidative desulfurization of gasoline catalyzed by IMID@PMA@CS nanocomposite as a high-performance amphiphilic nano catalyst. Environ. Prog. Sustain. Energy 2018, 37, 1891–1900. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Shaterian, M.; Akbarzadeh, F.; Khandan, S. Deep oxidative desulfurization of gasoline induced by PMoCu@MgCu2O4-PVA composite as a high-performance heterogeneous nanocatalyst. Chem. Eng. J. 2018, 333, 537–544. [Google Scholar] [CrossRef]
- Eßer, J.; Wasserscheid, P.; Jess, A. Deep desulfurization of oil refinery streams by extraction with ionic liquids. Green Chem. 2004, 6, 316–322. [Google Scholar] [CrossRef]
- Zeelani, G.G.; Lal Pal, S. A Review on Desulfurization Techniques of Liquid Fuels. Int. J. Sci. Res. 2016, 5, 2413–2419. [Google Scholar]
- Vsudevan, P.T.; Fierro, J.L.G. A Review of Deep Hydrodesulfurization Catalysis. Catal. Rev. Sci. Eng. 1996, 38, 2. [Google Scholar] [CrossRef]
- Tanimu, A.; Alhooshani, K. Advanced Hydrodesulfurization Catalysts: A Review of Design and Synthesis. Energy Fuels 2019, 33, 2810–2838. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Rufino, N.; Peza-Ledesma, C.L.; Javier, L.R.; Alonso-Núñe, G.; Pawelec, B.; Rivera-Muñoz, E.M. SBA-15 Mesoporous Silica as Catalytic Support for Hydrodesulfurization Catalysts-Review. Materials 2013, 6, 4139–4167. [Google Scholar] [CrossRef] [Green Version]
- Oyama, S.T.; Zhao, H.; Freund, H.J.; Asakura, K.; Włodarczyk, R.; Sierka, M. Unprecedented selectivity to the direct desulfurization (DDS) pathway in a highly active FeNi bimetallic phosphide catalyst. J. Catal. 2012, 285, 1–5. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Liu, Y.; Sun, X.P.; Tian, F.P.; Sun, F.X.; Liang, C.H.; You, W.S.; Han, C.R.; Li, C. Activated Carbons Chemically Modified by Concentrated H2SO4 for the Adsorption of the Pollutants from Wastewater and the Dibenzothiophene from Fuel Oils. Langmuir 2003, 19, 731–736. [Google Scholar] [CrossRef]
- Al-Shahrani, F.; Xiao, T.; Llewellyn, S.A.; Barri, S.; Jiang, Z.; Shi, H.; Martinie, G.; Green, M.L.H. Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst. Appl. Catal. B. 2007, 73, 311–316. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Schulz, H.; Böhringer, W.; Waller, P.; Ousmanov, F. Gas oil deep hydrodesulfurization: Refractory compounds and retarded kinetics. Catal. Today 1999, 49, 87–97. [Google Scholar] [CrossRef]
- Dou, S.-Y.; Wang, R. Recent Advances in New Catalysts for Fuel Oil Desulfurization. Curr. Org. Chem. 2017, 21, 1019–1036. [Google Scholar] [CrossRef]
- Zhu, W.; Li, H.; Jiang, X.; Yan, Y.; Lu, J.; Xia, J. Oxidative Desulfurization of Fuels Catalyzed by Peroxotungsten and Peroxomolybdenum Complexes in Ionic Liquids. Energy Fuels 2007, 21, 2514–2516. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Crucianelli, M. Advances in Nanostrostructured Transition Metal Catalysis Applied to Oxidative Desulfurization. In Applying Nanotechnology to the Desulfurization Process in Petroleum Engineering; Saleh, T.A., Ed.; IGI Global, 2016; pp. 1–555. [Google Scholar]
- Liu, Y.-Y.; Leus, K.; Sun, Z.; Li, X.; Depauw, H.; Wang, A.; Zhang, J.; Van Der Voort, P. Catalytic oxidative desulfurization of model and real diesel over a molybdenum anchored metal-organic framework. Microporous Mesoporous Mat. 2019, 277, 245–252. [Google Scholar] [CrossRef]
- Chan, N.Y.; Lin, T.-Y.; Yen, T.F. Superoxides: Alternative Oxidants for the Oxidative Desulfurization Process. Energy Fuels 2008, 22, 3326–3328. [Google Scholar] [CrossRef]
- Shaabani, A.; Behnam, M.; Rezayan, A.H. Tungstophosphoric Acid (H3PW12O40) Catalyzed Oxidation of Organic Compounds with NaBrO3. Catal. Commun. 2009, 10, 1074–1078. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Fierro, J.L.G. Highly efficient deep desulfurization of fuels by chemical oxidation. Green Chem. 2004, 6, 557–562. [Google Scholar] [CrossRef]
- Garcìa-Gutiérrez, J.L.; Fuentes, G.A.; Hernàndez-Teràn, M.E.; Murrieta, F.; Navarrete, J.; Jiménez-Cruz, F. Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3. Appl. Catal. 2006, 305, 15–20. [Google Scholar] [CrossRef]
- Ciclosi, M.; Dinoi, C.; Gonsalvi, L.; Peruzzini, M.; Manoury, E.; Poli, R. Oxidation of Thiophene Derivatives with H2O2 in Acetonitrile Catalyzed by [Cp*2M2O5] (M = Mo, W): A Kinetic Study. Organometallics 2008, 27, 2281–2286. [Google Scholar] [CrossRef]
- Liu, G.; Cao, Y.; Jiang, R.; Wang, L.; Zhang, X.; Mi, Z. Oxidative desulfurization of jet fuels and its impact on thermal-oxidative stability. Energy Fuel 2009, 23, 5978–5985. [Google Scholar] [CrossRef]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.; Lee, J.S.; Ayupov, A.; Chang, J.-S.; Kholdeeva, O. Cyclohexene Oxidation with H2O2 over Metal-Organic Framework MIL-125(Ti): The Effect of Protons on Reactivity. Catalysts 2019, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Di Giuseppe, A.; Crucianelli, M.; De Angelis, F.; Crestini, C.; Saladino, R. Efficient Oxidation of Thiophene Derivatives with Homogeneous and Heterogeneous MTO/H2O2 systems: A novel approach for Oxidative Desulfurization (ODS) of Diesel Fuel. Appl. Catal. B 2009, 89, 239–245. [Google Scholar] [CrossRef]
- Saladino, R.; Crucianelli, M.; De Angelis, F. Methyltrioxorhenium catalysis in nonconventional solvents: A great catalyst in safe reaction medium. ChemSusChem 2010, 3, 524–540. [Google Scholar]
- Meman, N.M.; Pourkhalil, M.; Rashidi, A.; Zarenezhad, B. Synthesis, characterization and operation of a functionalized multi-walled CNT supported MnOx nanocatalyst for deep oxidative desulfurization of four petroleum fractions. J. Ind. Eng. Chem. 2014, 20, 4054–4058. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A.; Venture, M. Photochemical conversion of solar energy. ChemSusChem 2008, 1, 26–58. [Google Scholar] [CrossRef]
- Ramirez-Verduzco, L.F.; De los Reyes, J.A.; Torres-Garcia, E. Solvent Effect in Homogeneous and Heterogeneous Reactions to Remove Dibenzothiophene by an Oxidation-Extraction Scheme. Ind. Eng. Chem. Res. 2008, 47, 5353–5361. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, B.; Rodríguez, H.; Rodil, E.; Arce, A.; Soto, A. Extractive and oxidative-extractive desulfurization of fuels with ionic liquids. Fuel 2014, 117, 882–889. [Google Scholar] [CrossRef]
- Taguchi, A.; Schuth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.J.; Cui, Y.C.; Luo, G.S. Direct synthesis of mesoporous TiO2 and its catalytic performance in DBT oxidative desulfurization. Microporous Mesoporous Mater. 2008, 116, 378–385. [Google Scholar] [CrossRef]
- Tanev, P.T.; Chibwe, M.; Pinnavaia, T.J. Titanium-containing mesoporous molecular sieves for catalytic oxidation of aromatic compounds. Nature 1994, 368, 321–323. [Google Scholar] [CrossRef]
- Chica, A.; Corma, A.; Dómine, M.E. Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixed-bed reactor. J. Catal. 2006, 242, 299–308. [Google Scholar] [CrossRef]
- Yan, X.M.; Mei, P.; Lei, J.; Mi, Y.; Xiong, L.; Guo, L. Synthesis and characterization of mesoporous phosphotungstic acid/TiO2 nanocompositive as a novel oxidative desulfurization catalyst. J. Mol. Chem. Catal. A Chem. 2009, 304, 52–57. [Google Scholar] [CrossRef]
- Seung-Tae, Y.; Kwang-Eun, J.; Soon-Yong, J.; Wha-Seung, A. Synthesis of mesoporous TS-1 using a hybrid SiO2-TiO2 xerogel for catalytic oxidative desulfurization. Mater. Res. Bull. 2012, 47, 4398–4402. [Google Scholar]
- Sengupta, A.; Kamble, P.D.; Basu, J.K.; Sengupta, S. Kinetic study and optimization of oxidative desulfurization of benzothiophene using mesoporous titanium silicate-1 catalyst. Ind. Eng. Chem. Res. 2012, 51, 147–157. [Google Scholar] [CrossRef]
- Tangestaninejad, S.; Mirkhani, V.; Moghadam, M.; Mohammadpoor-Baltork, I.; Shams, E.; Salavati, H. Hydrocarbon oxidation catalyzed by vanadium polyoxometalate supported on mesoporous MCM-41 under ultrasonic irradiation. Ultrason. Sonochem. 2008, 15, 438–447. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Fang, Y.; Zhu, X.; Zhen, W.; Wang, R.; Ma, J. Phosphotungstic acid containing ionic liquid immobilized on magnetic mesoporous silica rod catalyst for the oxidation of dibenzothiophene with H2O2. Catal. Commun. 2013, 38, 54–58. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wu, L.; Ye, F.; Xiao, J.; Li, Z. One-pot photocatalysis-assisted adsorptive desulfurization of diesel over doped-TiO2 under ambient conditions. RSC Adv. 2014, 4, 56567–56570. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.J.; Xu, J.H.; Luo, G.S. Synthesis of TS-1 on porous glass beads for catalytic oxidative desulfurization. Chem Eng. J. 2015, 259, 552–561. [Google Scholar] [CrossRef]
- Sentorun-Shalaby, C.; Saha, S.K.; Ma, X.L.; Song, C.S. Mesoporous-molecular-sievesupported nickel sorbents for adsorptive desulfurization of commercial ultralow-sulfur diesel fuel. Appl. Catal. B. 2011, 101, 718–726. [Google Scholar] [CrossRef]
- Ren, X.; Miao, G.; Xiao, Z.; Ye, F.; Li, Z.; Wang, H.; Xiao, J. Catalytic adsorptive desulfurization of model diesel fuel using TiO2/SBA-15 under mild conditions. Fuel 2016, 174, 118–125. [Google Scholar] [CrossRef]
- Hao, X.; Quach, L.; Korah, J.; Spieker, W.A.; Regalbuto, J.R. The control of platinum impregnation by PZC alteration of oxides and carbon. J. Mol. Catal. A Chem. 2004, 219, 97–107. [Google Scholar] [CrossRef]
- Lee, K.X.; Valla, J.A. Adsorptive desulfurization of liquid hydrocarbons using zeolite-based sorbents: A comprehensive review. React. Chem. Eng. 2019, 4, 1357–1386. [Google Scholar] [CrossRef]
- Miao, G.; Ye, F.; Wu, L.; Ren, X.; Xiao, J.; Li, Z.; Wang, H. Selective adsorption of thiophenic compounds from fuel over TiO2/SiO2 under UV-irradiation. J. Hazard. Mater. 2015, 300, 426–432. [Google Scholar] [CrossRef]
- Rourke, C.O.; Bowler, D.R. Adsorption of thiophene-conjugated sensitizers on TiO2 anatase (101). J. Phys. Chem. 2010, 114, 20240–20248. [Google Scholar]
- Zhuravlev, L.T. Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir 1987, 3, 316–318. [Google Scholar] [CrossRef]
- Collins, F.M.; Lucy, A.R.; Sharp, C. Oxidative desulphurization of oils via hydrogen peroxide and heteropolyanion catalysis. J. Mol. Catal. A Chem. 1997, 117, 397–403. [Google Scholar] [CrossRef]
- Gao, G.; Cheng, S.; An, Y.; Si, X.; Fu, X.; Liu, Y.; Zhang, H.; Wu, P.; He, M.Y. Oxidative Desulfurization of Aromatic Sulfur Compounds over Titanosilicates. ChemCatChem. 2010, 2, 459–466. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Removal of refractory organosulfur compounds via oxidation with hydrogen peroxide on amorphous Ti/SiO2 catalysts. Energy Environ. Sci. 2010, 3, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Bérubé, F.; Khadhraoui, A.; Janicke, M.T.; Kleitz, F.; Kaliaguine, S. Optimizing Silica Synthesis for the Preparation of Mesoporous Ti-SBA-15 Epoxidation Catalysts. Ind. Eng. Chem. Res. 2010, 49, 6977–6985. [Google Scholar] [CrossRef]
- Berube’, F.; Nohair, B.; Kleitz, F.; Kaliaguine, S. Controlled Postgrafting of Titanium Chelates for Improved Synthesis of Ti-SBA-15 Epoxidation Catalysts. Chem. Mater. 2010, 22, 1988–2000. [Google Scholar] [CrossRef]
- Choi, M.; Heo, W.; Kleitz, F.; Ryoo, R. Facile synthesis of high quality mesoporous SBA-15 with enhanced control of the porous network connectivity and wall thickness. Chem. Commun. 2003, 1340–1341. [Google Scholar] [CrossRef] [PubMed]
- Kleitz, F.; Bérubé, F.; Guillet-Nicolas, R.; Yang, C.M.; Thommes, M. Probing Adsorption, Pore Condensation, and Hysteresis Behavior of Pure Fluids in Three-Dimensional Cubic Mesoporous KIT-6 Silica. J. Phys. Chem. C 2010, 114, 9344–9355. [Google Scholar] [CrossRef]
- Cedeňo-Caero, L.; Ramos-Luna, M.; Méndez-Cruz, M.; Ramírez-Solís, J. Oxidative desulfurization of dibenzothiophene compounds with titania based catalysts. Catal. Today 2011, 172, 189–194. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Lu, J.; Han, B.; Li, M.; Xiu, J.; Ying, P.; Can, L. Direct Synthesis and Characterization of Titanium-Substituted Mesoporous Molecular Sieve SBA-15. Chem. Mater. 2002, 14, 3413–3421. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kim, M.-J.; Kleitz, F.; Nair, M.M.; Guillet Nicolas, R.; Jeong, K.-E.; Chae, H.-J.; Kim, C.-U.; Jeong, S.-Y. Tailor-Made Mesoporous Ti-SBA-15 Catalysts for Oxidative Desulfurization of Refractory Aromatic Sulfur Compounds in Transport Fuel. ChemCatChem 2012, 4, 687–697. [Google Scholar] [CrossRef]
- Bianconi, A.; Fritsch, E.; Calas, G.; Petiau, J. X-ray-absorption near-edge structure of 3d transition elements in tetrahedral coordination: The effect of bond-length variation. Phys. Rev. B Condens. Matter. 1985, 32, 4292–4295. [Google Scholar] [CrossRef]
- Laredo, G.C.; Leyva, S.; Alvarez, R.; Mares, M.T.; Castillo, J.; Cano, J.L. Nitrogen compounds characterization in atmospheric gas oil and light cycle oil from a blend of Mexican crudes. Fuel 2002, 81, 1341–1350. [Google Scholar] [CrossRef]
- Cho, K.-S.; Le, Y.-K. Effects of nitrogen compounds, aromatics, and aprotic solvents on the oxidative desulfurization (ODS) of light cycle oil over Ti-SBA-15 catalyst. Appl. Catal. B 2014, 147, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, A.; Wang, D.; Dumeignil, F.; Amano, H.; Qian, E.W.; Kabe, T. Oxidative desulfurization and denitrogenation of a light gas oil using an oxidation/adsorption continuous flow process. Appl. Catal. A 2005, 279, 279–287. [Google Scholar] [CrossRef]
- Caero, L.C.; Navarro, J.F.; Gutierrez-Alejandre, A. Oxidative desulfurization of synthetic diesel using supported catalysts: Part II. Effect of oxidant and nitrogen-compounds on extraction–oxidation process. Catal. Today 2006, 116, 562–568. [Google Scholar] [CrossRef]
- Jia, Y.; Li, G.; Ning, G.; Jin, C. The effect of N-containing compounds on oxidative desulphurization of liquid fuel. Catal. Today 2009, 140, 192–196. [Google Scholar] [CrossRef]
- Mirante, F.; Ribeiro, S.O.; De Castro, B.; Granadeiro, C.M.; Balula, S.S. Sustainable Desulfurization Processes Catalyzed by TitaniumPolyoxometalate@TM-SBA-15. Top. Catal. 2017, 60, 1140–1150. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Kovaleva, L.A.; Maksimovskaya, R.I.; Maksimov, G.M. Kinetics and mechanism of thioether oxidation with H2O2 in the presence of Ti (IV)-substituted heteropolytungstates. J. Mol. Catal. A Chem. 2000, 158, 223–229. [Google Scholar] [CrossRef]
- Maksimov, G.M.; Maksimovskaya, R.I.; Kholdeeva, O.A.; Fedotov, M.A.; Zaikovskii, V.I.; Vasil’ev, V.G.; Arzumanov, S.S. Structure and properties of H8(PW11TiO39)2O heteropoly acid. J. Struct. Chem. 2009, 50, 618–627. [Google Scholar] [CrossRef]
- Balula, S.S.; Cunha-Silva, L.; Santos, I.C.M.S.; Estrada, A.C.; Fernandes, A.C.; Cavaleiro, J.A.S.; Pires, J.; Freire, C.; Cavaleiro, A.M.V. Mono-substituted silicotungstates as active catalysts for sustainable oxidations: Homo- and heterogeneous performance. New J. Chem. 2013, 37, 2341–2350. [Google Scholar] [CrossRef]
- Balula, S.S.; Santos, I.C.M.S.; Cunha-Silva, L.; Carvalho, A.P.; Pires, J.; Freire, C.; Cavaleiro, J.A.S.; de Castro, B.; Cavaleiro, A.M.V. Phosphotungstates as catalysts for monoterpenes oxidation: Homo- and heterogeneous performance. Catal. Today 2013, 203, 95–102. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Maksimovskaya, R.I. Titanium- and zirconium-monosubstituted polyoxometalates as molecular models for studying mechanisms of oxidation catalysis. J. Mol. Catal. A Chem. 2007, 262, 7–24. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Chen, W.; Wang, M.; Song, Y.F. Highly efficient extraction and oxidative desulfurization system using Na7H2LaW10O36·32 H2O in [bmim]BF4 at room temperature. Chem. A Eur. J. 2012, 18, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huang, W.; Li, H.; Zhang, M.; Jiang, W.; Chen, G.; Han, C. Polyoxometalate-based ionic liquids as catalysts for deep desulfurization of fuels. Fuel Process. Technol. 2011, 92, 1842–1848. [Google Scholar] [CrossRef]
- Caero, L.C.; Hernández, E.; Pedraza, F.; Murrieta, F. Oxidative desulfurization of synthetic diesel using supported catalysts: Part, I. Study of the operation conditions with a vanadium oxide based catalyst. Catal. Today 2005, 107–108, 564–569. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Naito, T.; Hirai, T. Vanadosilicate Molecular Sieve as a Catalyst for Oxidative Desulfurization of Light Oil. Ind. Eng. Chem. Res. 2003, 42, 6034–6039. [Google Scholar] [CrossRef]
- Anisimov, A.V.; Fedorova, E.V.; Lesnugin, A.Z.; Senyavin, V.M.; Aslanov, L.A.; Rybakov, V.B.; Tarakanova, A.V. Vanadium peroxocomplexes as oxidation catalysts of sulfur organic compounds by hydrogen peroxide in bi-phase systems. Catal. Today 2003, 78, 319–325. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Pinault, M.; Wang, C.; Fang, F.; Pfefferle, L.; Haller, G.L. Synthesis, characterization, and catalytic performance of highly dispersed vanadium grafted SBA-15 catalyst. J. Catal. 2008, 253, 74–90. [Google Scholar] [CrossRef]
- Cedeño-Caero, L.; Gomez-Bernal, H.; Fraustro-Cuevas, A.; Guerra-Gomez, H.D.; Cuevas-Garcia, R. Oxidative desulfurization of synthetic diesel using supported catalysts. Part III. Support effect on vanadium-based catalysts. Catal. Today 2008, 133–135, 244–254. [Google Scholar] [CrossRef]
- Rivoira, L.; Martínez, M.L.; Anunziata, O.; Beltramone, A. Vanadium oxide supported on mesoporous SBA-15 modified with Al and Ga as a highly active catalyst in the ODS of DBT. Microporous Mesoporous Mater. 2017, 254, 96–113. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Chmelka, B.F.; Stucky, G.D. Triblock-copolymer-directed syntheses of large-pore mesoporous silica fibers. Chem. Mater. 1998, 10, 2033–2036. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cao, Y.; Yi, W.; Feng, W.L.; Dai, W.L.; Yan, S.R.; He, H.Y.; Fan, K.N. Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane. J. Catal. 2004, 224, 417–428. [Google Scholar] [CrossRef]
- Kumar, R.; Sithambaram, S.; Suib, S.L. Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves-Effect of acidity of the catalyst. J. Catal. 2009, 262, 304–313. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Cussa, J.; Martínez, M.L.; Beltramone, A.R. Experimental design optimization of the ODS of DBT using vanadium oxide supported on mesoporous Ga-SBA-15. Catal. Today 2018. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Yao, X.; Jiang, W.; Zhang, M.; Li, H.; Liu, H.; Zhu, W.; Li, H. One-pot extraction and aerobic oxidative desulfurization with highly dispersed V2O5/SBA-15 catalyst in ionic liquids. RSC Adv. 2017, 7, 39383. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Xu, J. The oxygen activated by the active vanadium species for the selective oxidation of benzene to phenol. Catal. Lett. 2006, 111, 203. [Google Scholar] [CrossRef]
- Jia, Y.; Li, G.; Ning, G. Efficient oxidative desulfurization (ODS) of model fuel with H2O2 catalyzed by MoO3/γ-Al2O3 under mild and solvent free conditions. Fuel Process. Technol. 2011, 92, 106–111. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Huang, X.; Li, J.; Duan, Y.; Yan, L.; Yao, Y.; Liao, X. Molybdenum anchored on NH2-modified spherical SiO2: A highly efficient and stable catalyst for oxidative desulfurization of fuel oil. Appl. Organomet. Chem. 2018, 32, e4521. [Google Scholar] [CrossRef]
- Han, X.; Wang, A.; Wang, X.; Li, X.; Wang, Y.; Hu, Y. Catalytic performance of P-modified MoO3/SiO2 in oxidative desulfurization by cumene hydroperoxide. Catal. Commun. 2013, 42, 6–9. [Google Scholar] [CrossRef]
- Chang, J.; Wang, A.; Liu, J.; Li, X.; Hu, Y. Oxidation of dibenzothiophene with cumene hydroperoxide on MoO3/SiO2 modified with alkaline earth metals. Catal. Today 2010, 149, 122–126. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, X.; Yu, W.; Wu, Y.; Qin, Y.; Sun, Z.; Song, L. Effects of surface acidities of MCM-41 modified with MoO3 on adsorptive desulfurization of gasoline. Appl. Surf. Sci. 2012, 263, 1–7. [Google Scholar] [CrossRef]
- Ramos, J.M.; Wang, J.A.; Chen, L.F.; Arellano, U.; Ramírez, S.P.; Sotelo, R.; Schachat, P. Synthesis and catalytic evaluation of CoMo/SBA-15 catalysts for oxidative removal of dibenzothiophene from a model diesel. Catal. Commum. 2015, 72, 57–62. [Google Scholar] [CrossRef]
- La Parola, V.; Degnello, G.; Tewell, C.R.; Venrzial, A.M. Structural characterization of silica supported CoMo catalysts by UV, Raman spectroscopy, XPS and X-ray diffraction techniques. Appl. Catal. A Gen. 2002, 235, 171–180. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. (Eds.) Practical Surface analysis, Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; Wiley: New York, NY, USA, 1990; p. 607. [Google Scholar]
- González, J.; Wang, J.A.; Chen, L.; Manríquez, M.; Salmones, J.; Limas, R.; Arellano, U. Quantitative determination of oxygen defects, surface lewis acidity, and catalytic properties of mesoporous MoO3/SBA-15 catalysts. J. Solid State Chem. 2018, 263, 100–111. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Narasimharao, K.; Bagabas, A.A.; Mokhtar, M. Effect of iron oxide loading on the phase transformation and physicochemical properties of nanosized mesoporous ZrO2. Mater. Res. Bull. 2012, 47, 3463–3472. [Google Scholar] [CrossRef]
- Epifani, M.; Imperatori, P.; Mirenghi, L.; Schioppa, M.; Siciliano, P. Synthesis and Characterization of MoO3 Thin Films and Powders from a Molybdenum Chloromethoxide. Chem. Mater. 2004, 16, 5495–5501. [Google Scholar] [CrossRef]
- He, T.; Yao, J. Photochromism of molybdenum oxide. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 125–143. [Google Scholar] [CrossRef]
- De Filippis, P.; Scarsella, M.; Verdone, N. Oxidative Desulfurization I: Peroxyformic Acid Oxidation of Benzothiophene and Dibenzothiophene. Ind. Eng. Chem. Res. 2009, 48, 1372–1375. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Sheldon, R.A. Activities and stabilities of heterogeneous catalysts in selectiveliquid phase oxidations: Recent developments. Appl. Catal. A 2001, 212, 175–187. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lei, L.C.; Zhu, J.K.; Yang, B.; Li, Z.J. Deep desulfurization of fuels by extraction with 4-dimethylaminopyridinium-based ionic liquids. Energy Fuels 2013, 27, 4617–4623. [Google Scholar] [CrossRef]
- Chen, X.C.; Song, D.D.; Asumana, C.; Yu, G.R. Deep oxidative desulfurization of diesel fuels by Lewis acidic ionic liquids based on 1-n-butyl-3-methylimidazolium metal chloride. J. Mol. Catal. A 2012, 359, 8–13. [Google Scholar] [CrossRef]
- Nie, Y.; Dong, Y.X.; Bai, L.; Dong, H.F.; Zhang, X.P. Fast oxidative desulfurization of fuel oil using dialkylpyridinium tetrachloroferrates ionic liquids. Fuel 2013, 103, 997–1002. [Google Scholar] [CrossRef]
- Zhu, W.S.; Zhang, J.T.; Li, H.M.; Chao, Y.H.; Jiang, W.; Yin, S.; Liu, H. Fenton-like ionic liquids/H2O2 system: One-pot extraction combined with oxidation desulfurization of fuel. RSC Adv. 2012, 2, 658–664. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhu, W.S.; Li, H.M.; Jiang, W.; Jiang, Y.Q.; Huang, W.L.; Yan, Y.S. Deep oxidative desulfurization of fuels by Fenton-like reagent in ionic liquids. Green Chem. 2009, 11, 1801–1807. [Google Scholar] [CrossRef]
- Ding, W.; Zhu, W.; Xiong, J.; Yang, L.; Wei, A.; Zhang, M.; Li, H. Novel heterogeneous iron-based redox ionic liquid supported on SBA-15 for deep oxidative desulfurization of fuels. Chem. Eng. J. 2015, 266, 213–221. [Google Scholar] [CrossRef]
- Bordoloi, A.; Sahoo, S.; Lefebvre, F.; Halligudi, S. Heteropoly acid-based supported ionic liquid-phase catalyst for the selective oxidation of alcohols. J. Catal. 2008, 259, 232–239. [Google Scholar] [CrossRef]
- Rivoira, L.; Juárez, J.; Martínez, M.L.; Beltramone, A. Iron-modified mesoporous materials as catalysts for ODS of sulfur compounds. Catal. Today 2018. [Google Scholar] [CrossRef]
- Sanjini, N.S.; Velmathi, S. Iron impregnated SBA-15, a mild and efficient catalyst for the catalytic hydride transfer reduction of aromatic nitro compounds. RSC Adv. 2014, 4, 15381–15388. [Google Scholar] [CrossRef]
- Chaliha, S.; Bhattacharyya, K.G. Fe(III)-, Co(II)- and Ni(II)-impregnated MCM41 for wet oxidative destruction of 2,4-dichlorophenol in water. Catal. Today 2009, 141, 225–233. [Google Scholar] [CrossRef]
- Barpanda, P.; Recham, N.; Chotard, J.N.; Djellab, K.; Walker, W.; Armand, M.; Tarascon, J.M. Structure and electrochemical properties of novel mixed Li(Fe1−xMx)SO4F (M = Co, Ni, Mn) phases fabricated by low temperature ionothermal synthesis. J. Mater. Chem. 2010, 20, 1659–1668. [Google Scholar] [CrossRef]
- Otsuki, T.; Nonaka, N.; Takashima, W.; Qian, A.; Ishihara, T.; Imai, T.; Kabe, T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Ramos, J.M.; Wang, J.A.; Flores, S.O.; Chen, L.F.; Nava, N.; Navarrete, J.; Domínguez, J.M.; Szpunar, J.A. Ultrasound-assisted synthesis and catalytic activity of mesostructured FeOx/ SBA-15 and FeOx/Zr-SBA-15 catalysts for the oxidative desulfurization of model diesel. Catal. Today 2018. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tharamani, C.N.; Bordoloi, A.; Schuhmann, W.; Muhler, M. Mesoporous nitrogen-rich carbon materials as catalysts for the oxygen reduction reaction. ChemSusChem 2012, 5, 637–641. [Google Scholar]
- Su, F.C.; Mathew, S.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Abdalghani, I.; Botta, G.; Crucianelli, M.; Passacantando, M.; Di Vacri, M.L.; Saladino, R. Preparation of wrapped carbon nanotubes poly(4-vinylpyridine)/MTO based heterogeneous catalysts for the oxidative desulfurization (ODS) of model and synthetic diesel fuel. Appl. Catal. B Environ. 2017, 200, 392–401. [Google Scholar] [CrossRef]
- Jin, X.; Balasubramanian, V.V.; Selvan, S.T.; Sawant, D.P.; Chari, M.A.; Lu, G.Q.; Vinu, A. Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content: A metal-free basic catalyst. Angew. Chem. Int. Ed. 2009, 48, 7884–7887. [Google Scholar] [CrossRef]
- Goyal, R.; Sarkar, B.; Lucus, N.; Bordoloi, A. Acid–Base Cooperative Catalysis over Mesoporous Nitrogen-Rich Carbon. ChemCatChem 2014, 6, 3091–3095. [Google Scholar] [CrossRef]
- Li, X.; Huang, S.; Xu, Q.; Yang, Y. Preparation of WO3-SBA-15 mesoporous molecular sieve and its performance as an oxidative desulfurization catalyst. Transit. Met. Chem. 2009, 34, 943–947. [Google Scholar] [CrossRef]
- Wang, H.; Gong, Y.; Wang, Y. Cellulose-based hydrophobic carbon aerogels as versatile and superior adsorbents for sewage treatment. RSC Adv. 2014, 4, 45753. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Li, H.; Antonietti, M. Metal-free oxidation of sulfides by carbon nitride with visible light illumination at room temperature. Green Chem. 2012, 14, 1904–1908. [Google Scholar] [CrossRef]
- Goyal, R.; Dumbre, D.; Sivakumar Konathala, L.N.; Pandey, M.; Bordoloi, A. Oxidative coupling of aniline and desulfurization over nitrogen rich mesoporous carbon. Catal. Sci. Technol. 2015, 5, 3632–3638. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Zhao, M.; Lv, Y.; Li, G.; Hu, C.J. Partial Oxidation of Toluene in CH3COOH by H2O2 in the Presence of VO (acac)2 Catalyst. J. Phys. Chem. C 2009, 113, 14270–14278. [Google Scholar] [CrossRef]
- Nair, S.; Tatarchuk, B.J. Supported silver adsorbents for selective removal of sulfur species from hydrocarbon fuels. Fuel 2010, 89, 3218–3225. [Google Scholar] [CrossRef]
- Samokhvalov, A.; Duin, E.C.; Nair, S. Study ofthe surface chemical reactions of thiophene with Ag/titania by the complementary temperature-programmed electron spin resonance, temperature-programmed desorption, and X-ray photoelectron spectroscopy: Adsorption, desorption, and sorbent regeneration mechanisms. J. Phys. Chem. C 2010, 114, 4075–4085. [Google Scholar]
- Mckinley, S.G.; Angelici, R.J. Deep desulfurization by selective adsorption of dibenzothiophenes on Ag+/SBA-15 and Ag+/SiO2. Chem. Commun. 2003, 2620–2621. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Z.; Liu, B. Adsorption of benzothiophene and dibenzothiophene on ion-impregnated activated carbons and ion-exchanged Y zeolites. Energy Fuels 2008, 22, 3858–3863. [Google Scholar] [CrossRef]
- Xiao, J.; Bian, G.A.; Zhang, W.; Li, Z. Adsorption of dibenzothiophene on Ag/Cu/ Fe-supported activated carbons prepared by ultrasonic-assisted impregnation. J. Chem. Eng. 2010, 55, 5818–5823. [Google Scholar] [CrossRef]
- Ghosh, S.; Acharyya, S.S.; Tiwari, R.; Sarkar, B.; Singha, R.K.; Pendem, C.; Sasaki, T.; Bal, R. Selective Oxidation of Propylene to Propylene Oxide over Silver-Supported Tungsten Oxide Nanostructure with Molecular Oxygen. ACS Catal. 2014, 4, 2169–2174. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini-Monfared, H.; Abbasi, V.; Khoshroo, M.R. Selective oxidation of hydrocarbons under air using recoverable silver ferrite–graphene (AgFeO2–G) nanocomposite: A good catalyst for green chemistry. Inorg. Chem. Commun. 2016, 67, 72–79. [Google Scholar] [CrossRef]
- Fellah, M.F.; Santen, R.A.; Onal, I. Epoxidation of ethylene by silver oxide (Ag2O) cluster: A density functional theory study. Catal. Lett. 2011, 141, 762–771. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, Y. Effect of support on the activity of Ag-based catalysts for formaldehyde oxidation. Sci. Rep. 2015, 5, 12950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, X.; He, H.; Yu, Y.; Yuan, T.; Tian, Z.; Wang, J.; Li, Y. Effect of the pressure on the catalytic oxidation of volatile organic compounds over Ag/Al2O3 catalyst. Appl. Catal. B Environ. 2009, 89, 659–664. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Z.; Li, X.; Wen, M. Studies of silver species for low-temperature CO oxidation on Ag/SiO2 catalysts. Sep. Purif. Technol. 2010, 72, 395–400. [Google Scholar] [CrossRef]
- Ye, F.; Miao, G.; Wu, L.; Wu, Y.; Li, Z.; Song, C.; Xiao, J. [O]-induced reactive adsorptive desulfurization of liquid fuel over Ag X O@SBA-15 under ambient conditions. Chem. Eng. Sci. 2017, 168, 225–234. [Google Scholar] [CrossRef]
- Dellasega, D.; Facibeni, A.; Fonzo, F.D. Nanostructured Ag4O4 films with enhanced antibacterial activity. Nanotechnology 2008, 19, 475602. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, G. Influence of catalyst morphology on the performance of electrolytic silver catalysts for the partial oxidation of methanol to formaldehyde. Appl. Catal. A Gen. 2004, 266, 257–273. [Google Scholar] [CrossRef]
- Derouin, J.; Farber, R.G.; Heslop, S.L.; Killelea, D.R. Formation of surface oxides and Ag2O thin films with atomic oxygen on Ag(111). Surf. Sci. 2015, 641, L1–L4. [Google Scholar] [CrossRef]
- Car, P.-E.; Patzke, G.R. The Fascination of Polyoxometalate Chemistry. Inorganics 2015, 3, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-S.; Yang, G.-Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef]
- Ammam, M. Polyoxometalates: Formation, structures, principal properties, main deposition methods and application in sensing. J. Mater. Chem. A 2013, 1, 6291–6312. [Google Scholar] [CrossRef]
- Omwoma, S.; Gore, C.T.; Ji, Y.; Hu, C.; Song, Y.-F. Environmentally benign polyoxometalate materials. Coord. Chem. Rev. 2015, 286, 17–29. [Google Scholar] [CrossRef]
- Narasimharao, K.; Brown, D.R.; Lee, A.F.; Newman, A.D.; Siril, P.F.; Tavener, S.J.; Wilson, K. Structure–activity relations in Cs-doped heteropoly acid catalysts for biodiesel production. J. Catal. 2007, 248, 226–234. [Google Scholar] [CrossRef]
- Contreras Coronel, N.; da Silva, M.J. Lacunar Keggin Heteropolyacid Salts: Soluble, Solid and Solid-Supported Catalysts. J. Clust. Sci. 2018, 29, 195–205. [Google Scholar] [CrossRef]
- Lotfian, N.; Heravi, M.M.; Mirzaei, M.; Heidari, B. Applications of inorganic-organic hybrid architectures based on polyoxometalates in catalyzed and photocatalyzed chemical transformations. Appl. Organomet. Chem. 2019, 33, 4808. [Google Scholar] [CrossRef]
- Ye, J.-J.; Wu, C.-D. Immobilization of polyoxometalates in crystalline solids for highly efficient heterogeneous catalysis. Dalton Trans. 2016, 45, 10101–10112. [Google Scholar] [CrossRef]
- Dufaud, V.; Lefebvre, F. Inorganic Hybrid Materials with Encapsulated Polyoxometalates. Materials 2010, 3, 682–703. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.N.; Park, H.C.; Choi, H.S. A Comprehensive Review on Catalytic Oxidative Desulfurization of Liquid Fuel Oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yan, J.; Mei, P.; Lei, J. Synthesis, characterization and catalytic properties of mesoporous HPMo/SiO2 composite. J. Wuhan Univ. Technol. 2008, 23, 834–838. [Google Scholar] [CrossRef]
- Yan, X.; Lei, J.; Liu, D.; Guo, L.; Wu, Y. Oxidation reactivities of organic sulfur compounds in fuel oil using immobilized heteropoly acid as catalyst. J. Wuhan Univ. Technol. 2007, 22, 320–324. [Google Scholar] [CrossRef]
- Kaleta, W.; Nowinska, K. Immobilisation of Heteropoly Anions in Si-MCM-41 Channels by Means of Chemical Bonding to Aminosilane Groups. Chem. Commun. 2001, 535–536. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Zhou, L.; Su, W.; Sun, Y.; Zhou, Y. Deep Desulfurization of Transportation Fuels by Characteristic Reaction Resided in Adsorbents. AIChE J. 2009, 55, 1872–1881. [Google Scholar] [CrossRef]
- Li, J.; Hu, B.; Tan, J.; Zhuang, J. Deep oxidative desulfurization of fuels catalyzed by molybdovanadophosphoric acid on amino-functionalized SBA-15 using hydrogen peroxide as oxidant. Transit. Met. Chem. 2013, 38, 495–501. [Google Scholar] [CrossRef]
- Chamack, M.; Mahjoub, A.R.; Aghayan, H. Catalytic performance of vanadium-substituted molybdophosphoric acid supported on zirconium modified mesoporous silica in oxidative desulfurization. Chem. Eng. Res. Des. 2015, 94, 565–572. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wang, Y.; Liu, H.; Qiu, J.; Wang, J.; Han, W.; Yeung, K.L. Investigating the role of zeolite nanocrystal seeds in the synthesis of mesoporous catalysts with zeolite wall structure. Chem. Mater. 2011, 23, 4469–4479. [Google Scholar] [CrossRef]
- Chamack, M.; Mahjoub, A.R.; Aghayan, H. Cesium salts of tungsten-substituted molybdophosphoric acid immobilized onto platelet mesoporous silica: Efficient catalysts for oxidative desulfurization of dibenzothiophene. Chem. Eng. J. 2014, 255, 686–694. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Jatupisarnpong, J. Acidic and cesium salts of polyoxometalates with and without vanadium supported on MCM-41 as catalysts for oxidation of cyclohexane with H2O2. Appl. Petrochem. Res. 2013, 3, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Torres-Garcia, E.; Galano, A.; Rodriguez-Gattorno, G. Oxidative desulfurization (ODS) of organosulfur compounds catalyzed by peroxo-metallate complexes of WOx–ZrO2: Thermochemical, structural, and reactivity indexes analyses. J. Catal. 2011, 282, 201–208. [Google Scholar] [CrossRef]
- Zhuang, J.; Jin, X.; Shen, X.; Tan, J.; Nie, L.; Xiong, J.; Hu, B. Preparation of Ionic Liquid-modified SBA-15 Doped with Molybdovanadophosphoric Acid for Oxidative Desulfurization. Bull. Korean Chem. Soc. 2015, 36, 1784–1790. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.; Ding, W.; Yang, L.; Zhang, M.; Jiang, W.; Zhao, Z.; Li, H. Hydrophobic mesoporous silica-supported heteropolyacid induced by ionic liquid as a high efficiency catalyst for the oxidative desulfurization of fuel. RSC Adv. 2015, 5, 16847–16855. [Google Scholar] [CrossRef]
- Cai, T.-F.; Li, H.-P.; Zhao, H. A Study on the Oxidation-extraction Desulfurization of FCC Gasoline Over Supported Phosphotungstic Acid/H2O2/Dimethyl Sulfoxide (DMSO). Pet. Sci. Technol. 2014, 32, 1713–1719. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Yuan, X.; Shen, J.; Qi, Y. One step non-hydrodesulfurization of fuel oil: Catalyzed oxidation adsorption desulfurization over HPWA-SBA-15. J. Mol. Catal. A 2007, 262, 114–118. [Google Scholar] [CrossRef]
- Estephane, G.; Lancelot, C.; Blanchard, P.; Toufaily, J.; Hamiye, T.; Lamonier, C. Sulfur compounds reactivity in the ODS of model and real feeds on W–SBA based catalysts. RSC Adv. 2018, 8, 13714–13721. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Zhou, Y.; Wang, S.; Long, L.; Zhou, L. Catalytic Oxidation of Thiophenes with Air and PW/SBA-15. Ads. Sci. Technol. 2007, 25, 385–394. [Google Scholar] [CrossRef]
- Lu, C.; Fu, H.; Li, H.; Zhao, H.; Cai, T. Oxidation-extraction desulfurization of model oil over Zr-ZSM-5/SBA-15 and kinetic study. Front. Chem. Sci. Eng. 2014, 8, 203–211. [Google Scholar] [CrossRef]
- Qi, H.-X.; Zhai, S.-R.; Zhang, W.; Zhai, B.; An, Q.-D. Recyclable HPW/PEHA/ZrSBA-15 toward efficient oxidative desulfurization of DBT with hydrogen peroxide. Catal. Commun. 2015, 72, 53–56. [Google Scholar] [CrossRef]
- Lei, J.; Chen, L.; Yang, P.; Du, X.; Yan, X. Oxidative desulfurization of diesel fuel by mesoporous phosphotungstic acid/SiO2: The effect of preparation methods on catalytic performance. J. Porous Mater. 2013, 20, 1379–1385. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Granadeiro, C.M.; Almeida, P.L.; Pires, J.; Capel-Sanchez, M.C.; Campos-Martin, J.M.; Gago, S.; de Castro, B.; Balula, S.S. Oxidative desulfurization strategies using Keggin-type polyoxometalate catalysts: Biphasic versus solvent-free systems. Catal. Today 2019, 333, 226–236. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Nogueira, L.S.; Gago, S.; Almeida, P.L.; Corvo, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Desulfurization process conciliating heterogeneous oxidation and liquid extraction: Organic solvent or centrifugation/water? Appl. Catal. A 2017, 542, 359–367. [Google Scholar] [CrossRef]
- Pham, X.N.; Tran, D.L.; Pham, T.D.; Nguyen, Q.M.; van Thi, T.T.; Van, H.D. One-step synthesis, characterization and oxidative desulfurization of 12-tungstophosphoric heteropolyanions immobilized on amino functionalized SBA-15. Adv. Powder Technol. 2018, 29, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Pham, X.N.; van Doan, H. Activity and stability of amino-functionalized SBA-15 immobilized 12-tungstophosphoric acid in the oxidative desulfurization of a diesel fuel model with H2O2. Chem. Eng. Commun. 2019, 206, 1139–1151. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Dong, X.; Wu, P.; Hu, H.; Xue, G. Copper(II)-Substituted Polyoxotungstates Immobilized on Amine-Functionalized SBA-15: Efficient Heterogeneous Catalysts for Liquid Phase Oxidative Reaction. Catal. Lett. 2016, 146, 2468–2477. [Google Scholar] [CrossRef]
- Julião, D.; Mirante, F.O.; Ribeiro, S.O.; Gomes, A.C.; Valença, R.; Ribeiro, J.C.; Pillinger, M.; de Castro, B.; Gonçalves, I.S.; Balula, S.S. Deep oxidative desulfurization of diesel fuels using homogeneous and SBA-15-supported peroxophosphotungstate catalysts. Fuel 2019, 241, 616–624. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Duarte, B.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Improving the Catalytic Performance of Keggin [PW12O40]3− for Oxidative Desulfurization: Ionic Liquids versus SBA-15 Composite. Materials 2018, 11, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Xiong, J.; Wang, J.; Ding, W.; Yang, L.; Zhang, M.; Zhu, W.; Li, H. Structure and catalytic oxidative desulfurization properties of SBA-15 supported silicotungstic acid ionic liquid. J. Porous Mater. 2016, 23, 823–831. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.; Ding, W.; Yang, L.; Chao, Y.; Li, H.; Zhu, F.; Li, H. Phosphotungstic Acid Immobilized on Ionic Liquid-Modified SBA-15: Efficient Hydrophobic Heterogeneous Catalyst for Oxidative Desulfurization in Fuel. Ind. Eng. Chem. Res. 2014, 53, 19895–19904. [Google Scholar] [CrossRef]
- Wang, L.; Fan, J.; Tian, B.; Yang, H.; Yu, C.; Tu, B.; Zhao, D. Synthesis and characterization of small pore thick-walled SBA-16 templated by oligomeric surfactant with ultra-long hydrophilic chains. Microporous Mesoporous Mater. 2004, 67, 135–141. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Bisio, C.; Marchese, L.; Pastore, H.O. Surface acidity of novel mesostructured silicas with framework aluminum obtained by SBA-16 related synthesis. Microporous Mesoporous Mater. 2008, 111, 632–635. [Google Scholar] [CrossRef]
- Shah, A.T.; Li, B.; Ali Abdalla, Z.E. Direct synthesis of Ti-containing SBA-16-type mesoporous material by the evaporation-induced self-assembly method and its catalytic performance for oxidative desulfurization. J. Colloid Interface Sci. 2009, 336, 707–711. [Google Scholar] [CrossRef]
- Boccuti, M.R.; Rao, K.M.; Zecchina, A.; Leofanti, G.; Petrini, G. Spectroscopic Characterization of Silicalite and Titanium-Silicalite. Stud. Surf. Sci. Catal. 1989, 48, 133–144. [Google Scholar]
- Araújo, R.S.; Azevedo, D.C.S.; Rodríguez-Castellón, E.; Jiménez-López, A.; Cavalcante, C.L. Al and Ti-containing mesoporous molecular sieves: Synthesis, characterization and redox activity in the anthracene oxidation. J. Mol. Catal. A Chem. 2008, 281, 154–163. [Google Scholar] [CrossRef]
- Eimer, G.A.; Chanquia, C.M.; Sapag, K.; Herrero, E.R. The role of different parameters of synthesis in the final structure of Ti-containing mesoporous materials. Microporous Mesoporous Mater. 2008, 116, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Zepeda, T.A. Comparison and performance of different sulphided Ti-loaded mesostructured silica-supported CoMo catalysts in deep HDS. Appl. Catal. A 2008, 347, 148–161. [Google Scholar] [CrossRef]






| Catalyst Modification | Operating Conditions a | Sulfur Removal (%) b | Catalyst Recycle (n° of Runs) c | Reference |
|---|---|---|---|---|
| TiO2/SiO2 sol-gel method | BT, DBT, MDBT, and DMDBT, UV-irrad., air, toluene/dodecane, 25 °C, 2 h. | n.c. | 5 | [78] |
| TiO2/SBA-15 grafting insertion | BT, DBT, 4-MDBT, and 4,6-DMDBT, CHP, n-heptane/toluene, 80 °C. | >99 | n.a. | [91] |
| V2O5/SBA-15 wetness impregnation | Selective extraction phase DBT, 4-MDTB, 4,6-DMDTB, [Bmim]BF4, O2, 120 °C, 6 h. | 99 | 6 | [116] |
| CoMo/SBA-15 wetness impregnation | DBT, hexadecane, H2O2, 60 °C, 1 h. | 90 | n.a. | [125] |
| MoO3/SBA-15 wetness impregnation | Formic acid as co-oxidant species, 4,6-DMDBT, H2O2, hexadecane, 70 °C, 1 h. | 99 | n.a. | [130] |
| [pmim]FeCl4/SBA-15 grafting insertion | BT and DBT, H2O2, octane, 30 °C, 1 h. | 94.3 | n.a. | [137] |
| Fe/SBA-15 wetness impregnation | BT, DBT and 4,6-DMDBT, H2O2, n-dodecane and CH3CN, 60 °C, 15 min. | 90 | 4 | [139] |
| WOx/SBA-15 grafting insertion | DBT, H2O2, n-dodecane, 100 °C, 2 h. | >99 | n.a. | [155] |
| MoV/Zr/SBA-15 wetness impregnation | DBT, TBHP, n-hexane, 60 °C, 75 min. | 98.5 | 4 | [187] |
| Cs2Mo8W4/SBA-15 wetness impregnation | DBT, TBHP, n-hexane, 60 °C, 80 min. | >99 | 4 | [189] |
| HPMo/IL-SBA-15 wetness impregnation | DBT, H2O2, n-octane, 60 °C, 90 min. | >90 | 5 | [193] |
| HPW/PEHA/Zr/SBA-15 layer by layer strategy | DBT, H2O2, n-octane, 60 °C, 60 min. | 95 | 6 | [199] |
| HPW/SiO2-EISA self-assembly | BT, DBT and 4,6-DMDBT, H2O2, petroleum ether/CH3CN, 60 °C, 2 h | >99 | 7 | [200] |
| PW11/H2N-SBA-15 wetness impregnation | BT, DBT, 4-MDBT and 4,6-DMDBT, H2O2, n-octane and CH3CN, 70 °C, 60 min. | >99 | 8 | [201] |
| PW4/tma-SBA-15 wetness impregnation | BT, DBT and 4,6-DMDBT, H2O2, n-octane and [Bmim]PF6 or MeCN, 70 °C, 3 h | >99 | 10 | [206] |
| HSiW/IL-SBA-15 wetness impregnation | BT, DBT and 4,6-DMDBT, H2O2, n-octane, 60 °C, 2 h | 96 | 8 | [208] |
| TiO2/SBA-16 self-assembly | DBT, n-octane, 60 °C, 3 h | >99 | 5 | [213] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crucianelli, M.; Bizzarri, B.M.; Saladino, R. SBA-15 Anchored Metal Containing Catalysts in the Oxidative Desulfurization Process. Catalysts 2019, 9, 984. https://doi.org/10.3390/catal9120984
Crucianelli M, Bizzarri BM, Saladino R. SBA-15 Anchored Metal Containing Catalysts in the Oxidative Desulfurization Process. Catalysts. 2019; 9(12):984. https://doi.org/10.3390/catal9120984
Chicago/Turabian StyleCrucianelli, Marcello, Bruno Mattia Bizzarri, and Raffaele Saladino. 2019. "SBA-15 Anchored Metal Containing Catalysts in the Oxidative Desulfurization Process" Catalysts 9, no. 12: 984. https://doi.org/10.3390/catal9120984







