Chromium Oxide Supported on Silicalite-1 Zeolite as a Novel Efficient Catalyst for Dehydrogenation of Isobutane Assisted by CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
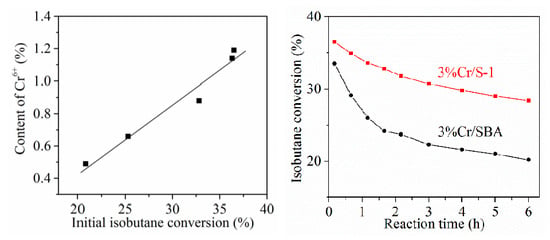
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Zhu, K.; Gao, F.; Wang, C.; Liu, J.; Peden, C.H.F.; Wang, Y. Direct conversion of bio-ethanol to isobutene on nanosized ZnxZryOz mixed oxides with balanced acid–base sites. J. Am. Chem. Soc. 2011, 133, 11096–11099. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, W.E. Isobutylene. J. Chem. Health Saf. 2013, 20, 35–37. [Google Scholar] [CrossRef]
- Ding, J.F.; Qin, Z.F.; Li, X.K.; Wang, G.F.; Wang, J.G. Catalytic dehydrogenation of isobutane in the presence of carbon dioxide over nickel supported on active carbon. J. Mol. Catal. A 2010, 315, 221–225. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.L.; Liu, Y.M.; Cao, Y.; Guo, L.; He, H.Y.; Fan, K.N. Study in support effect of In2O3/MOx (M = Al, Si, Zr) catalysts for dehydrogenation of propane in the presence of CO2. Appl. Catal. A 2011, 407, 20–28. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Zenczak, K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2—A comparative study. J. Mol. Catal. A 2011, 349, 1–12. [Google Scholar] [CrossRef]
- Baek, J.; Yun, H.J.; Yun, D.; Choi, Y.; Yi, J. Preparation of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2: Insight into the nature of catalytically active chromium sites. ACS Catal. 2012, 2, 1893–1903. [Google Scholar] [CrossRef]
- Wu, J.L.; Chen, M.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Sucrose-templated mesoporous β-Ga2O3 as a novel efficient catalyst for dehydrogenation of propane in the presence of CO2. Catal. Commun. 2013, 30, 61–65. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E. Oxidative dehydrogenation of ethane with CO2 over flame-made Ga-loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Amini, M. The beneficial utilization of natural zeolite in preparation of Cr/clinoptilolite nanocatalyst used in CO2-oxidative dehydrogenation of ethane to ethylene. J. Ind. Eng. Chem. 2015, 31, 142–155. [Google Scholar] [CrossRef]
- Wei, C.L.; Xue, F.Q.; Miao, C.X.; Yue, Y.H.; Yang, W.M.; Hua, W.M.; Gao, Z. Dehydrogenation of isobutane with carbon dioxide over SBA-15-supported vanadium oxide catalysts. Catalysts 2016, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.L.; Xue, F.Q.; Miao, C.X.; Yue, Y.H.; Yang, W.M.; Hua, W.M.; Gao, Z. Dehydrogenation of isobutane to isobutene with carbon dioxide over SBA-15-supported chromia-ceria catalysts. Chin. J. Chem. 2017, 35, 1619–1626. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lei, T.Q.; Miao, C.X.; Hua, W.M.; Yue, Y.H.; Gao, Z. Ga2O3/NaZSM-5 for C2H6 dehydrogenation in the presence of CO2: Conjugated effect of silanol. Micropor. Mesopor. Mater. 2018, 268, 235–242. [Google Scholar] [CrossRef]
- Lei, T.Q.; Guo, H.Y.; Miao, C.X.; Hua, W.M.; Yue, Y.H.; Gao, Z. Mn-doped CeO2 nanorod supported Au catalysts for dehydrogenation of ethane with CO2. Catalysts 2019, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.B.; Zhu, Z.H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenations—A review. Energy Fuels 2004, 18, 1126–1139. [Google Scholar] [CrossRef]
- Mukherjee, D.; Parkb, S.E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An ecobenign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Ding, J.F.; Qin, Z.F.; Li, X.K.; Wang, G.F.; Wang, J.G. Coupling dehydrogenation of isobutane in the presence of carbon dioxide over chromium oxide supported on active carbon. Chin. Chem. Lett. 2008, 19, 1059–1062. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, E. Dehydrogenation of isobutane in the presence of carbon dioxide over supported vanadium oxide catalysts. React. Kinet. Catal. Lett. 2006, 88, 293–300. [Google Scholar] [CrossRef]
- Yuan, R.X.; Li, Y.; Yan, H.B.; Wang, H.; Song, J.; Zhang, Z.S.; Fan, W.B.; Chen, J.G.; Liu, Z.W.; Liu, Z.T.; et al. Insights into the vanadia catalyzed oxidative dehydrogenation of isobutane with CO2. Chin. J. Catal. 2014, 35, 1329–1336. [Google Scholar] [CrossRef]
- Shimada, H.; Akazawa, T.; Ikenaga, N.; Suzuki, T. Dehydrogenation of isobutane to isobutene with iron-loaded activated carbon catalyst. Appl. Catal. A 1998, 168, 243–250. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, E. Catalytic dehydrogenation of isobutane in the presence of carbon dioxide. React. Kinet. Catal. Lett. 2005, 86, 195–201. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, E. Activity of vanadium magnesium oxide supported catalysts in the dehydrogenation of isobutane. Catal. Lett. 2006, 111, 79–85. [Google Scholar] [CrossRef]
- Shi, X.J.; Ji, S.F.; Wang, K. Oxidative Dehydrogenation of ethane to ethylene with carbon dioxide over Cr–Ce/SBA-15 catalysts. Catal. Lett. 2008, 125, 331–339. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Zhou, L.B.; Xu, J.X.; Miao, C.X.; Hua, W.M.; Yue, Y.H.; Gao, Z. Chromium-based catalysts for ethane dehydrogenation: Effect of SBA-15 support. Micropor. Mesopor. Mater. 2016, 234, 370–376. [Google Scholar] [CrossRef]
- Ohishi, Y.; Kawabata, T.; Shishido, T.; Takaki, K.; Zhang, Q.H.; Wang, Y.; Takehira, K. Dehydrogenation of ethylbenzene with CO2 over Cr-MCM-41 catalyst. J. Mol. Catal. A 2005, 230, 49–58. [Google Scholar] [CrossRef]
- Li, J.X.; Shi, C.H.; Zhang, H.F.; Zhang, X.F.; Wei, Y.Y.; Jiang, K.; Zhang, B.G. Silicalite-1 zeolite membrane: Synthesis by seed method and application in organics removal. Chemosphere 2019, 218, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Tang, C.Y.; Zhong, S.L.; Wang, B.; Zhou, J.J.; Zhou, R.F. Synthesis optimization of (h0h)-oriented silicalite-1 membranes for butane isomer separation. Sep. Purif. Technol. 2019, 214, 51–60. [Google Scholar] [CrossRef]
- Heitmann, G.P.; Dahlhoff, G.; Hölderich, W.F. Catalytically active sites for the Beckmann rearrangement of cyclohexanone oxime to ε-Caprolactam. J. Catal. 1999, 186, 12–19. [Google Scholar] [CrossRef]
- Lanzafame, P.; Barbera, K.; Perathoner, S.; Centi, G.; Aloise, A.; Migliori, M.; Macario, A.; Nagy, J.B.; Giordano, G. The role of acid sites induced by defects in the etherification of HMF on Silicalite-1 catalysts. J. Catal. 2015, 330, 558–568. [Google Scholar] [CrossRef]
- Shi, L.H.; Liu, G.D.; Guo, H.C. Efficient Pt/Silicalite-1 catalyst for isomerization of n-heptane. Catal. Commun. 2017, 101, 111–115. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.F.; Lu, C.Y.; Zou, X.L.; Cheng, H.W.; Ning, J.Y.; Lu, X.G.; Zhou, Z.F. Hydrogen production from coke oven gas by CO2 reforming over a novel Ni-doped Silicalite-1. Catal. Lett. 2018, 148, 1424–1434. [Google Scholar] [CrossRef] [Green Version]
- Niu, R.Y.; Liu, P.C.; Li, W.; Wang, S.; Li, J.P. High performance for oxidation of low-concentration methane using ultra-low Pd in silicalite-1 zeolite. Micropor. Mesopor. Mater. 2019, 284, 235–240. [Google Scholar] [CrossRef]
- Sang, S.; Chang, F.; Liu, Z.; He, C.; He, Y.; Xu, L. Difference of ZSM-5 zeolites synthesized with various templates. Catal. Today 2004, 93–95, 729–734. [Google Scholar] [CrossRef]
- Zaki, M.I.; Fouad, N.E.; Leyrev, J.; Knözinger, H. Physicochemical investigation of calcined chromia-coated silica and alumina catalysts—Characterization of chromium-oxygen species. Appl. Catal. 1986, 21, 359–377. [Google Scholar] [CrossRef]
- Grzybowska, B.; Sloczynski, J.; Grabowski, R.; Wcislo, K.; Kozlowska, A.; Stoch, J.; Zielinski, J. Chromium oxide alumina catalysts in oxidative dehydrogenation of isobutane. J. Catal. 1998, 178, 687–700. [Google Scholar] [CrossRef]
- Gao, B.; Luo, Y.J.; Miao, C.X.; Yue, Y.H.; Yang, W.M.; Hua, W.M.; Gao, Z. Oxidative dehydrogenation of 1-butene to 1,3-butadiene using CO2 over Cr-SiO2 catalysts prepared by sol-gel method. Chem. Res. Chin. Univ. 2018, 34, 609–615. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Wachs, I.E.; Schoonheydt, R.A. Surface chemistry and spectroscopy of chromium in inorganic oxides. Chem. Rev. 1996, 96, 3327–3349. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Bare, S.R.; Weckhuysen, B.; Wachs, I.E. In situ spectroscopic investigation of molecular structures of highly dispersed vanadium oxide on silica under various conditions. J. Phys. Chem. B 1998, 102, 10842–10852. [Google Scholar] [CrossRef] [Green Version]
- Takehira, K.; Ohishi, Y.; Shishido, T.; Kawabata, T.; Takaki, K.; Zhang, Q.H.; Wang, Y. Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J. Catal. 2004, 224, 404–416. [Google Scholar] [CrossRef]
- Cherian, M.; Rao, M.S.; Yang, W.T.; Jehng, J.M.; Hirt, A.M.; Deo, G. Oxidative dehydrogenation of propane over Cr2O3/Al2O3 and Cr2O3 catalysts: Effects of loading, precursor and surface area. Appl. Catal. A 2002, 233, 21–33. [Google Scholar] [CrossRef]
- Yim, S.D.; Nam, I.S. Characteristics of chromium oxides supported on TiO2 and Al2O3 for the decomposition of perchloroethylene. J. Catal. 2004, 221, 601–611. [Google Scholar] [CrossRef]
- Zhu, Q.J.; Takiguchi, M.; Setoyama, T.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Oxidative dehydrogenation of propane with CO2 over Cr/H[B]MFI catalysts. Catal. Lett. 2011, 141, 670–677. [Google Scholar] [CrossRef]
- Ye, X.N.; Hua, W.M.; Yue, Y.H.; Dai, W.L.; Miao, C.X.; Xie, Z.K.; Gao, Z. Ethylbenzene dehydrogenation to styrene in the presence of carbon dioxide over chromia-based catalysts. New J. Chem. 2014, 28, 373–378. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.W.; Beato, P. Structure-deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196–205. [Google Scholar] [CrossRef]
- Liu, G.D.; Liu, J.X.; He, N.; Miao, C.L.; Wang, J.L.; Xin, Q.; Guo, H.C. Silicalite-1 zeolite acidification by zinc modification and its catalytic properties for isobutane conversion. RSC Adv. 2018, 8, 18663–18671. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.H.; Song, H.L.; Chou, L.J.; Zhao, J.; Yang, J.; Yan, L. Insight into the structure and molybdenum species in mesoporous molybdena–alumina catalysts for isobutane dehydrogenation. Catal. Sci. Technol. 2017, 7, 3258–3267. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite characterization. 1. Structure, adsorptive Capacity, and IR spectroscopy of the framework and hydroxyl modes. J. Phys. Chem. 1992, 96, 4985–4990. [Google Scholar] [CrossRef]
- Benamor, T.; Michelin, L.; Lebeau, B.; Marichal, C. Flash induction calcination: A powerful tool for total template removal and fine tuning of the hydrophobic/hydrophilic balance in SBA-15 type silica mesoporous materials. Micropor. Mesopor. Mater. 2012, 147, 370–376. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Ikenaga, N.; Nishitani-Gamo, M.; Ando, T.; Suzuki, T. Dehydrogenation of light alkanes over oxidized diamond-supported catalysts in the presence of carbon dioxide. Catal. Today 2003, 84, 149–157. [Google Scholar] [CrossRef]
- Mimura, N.; Okamoto, M.; Yamashita, H.; Oyama, S.T.; Murata, K. Oxidative dehydrogenation of ethane over Cr/ZSM-5 catalysts using CO2 as an oxidant. J. Phys. Chem. B 2006, 110, 21764–21770. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, R.X.; Yue, Y.H.; Yang, W.M.; Gu, S.Y.; Miao, C.X.; Hua, W.M.; Gao, Z. Chromium oxide supported on ZSM-5 as a novel efficient catalyst for dehydrogenation of propane with CO2. Micropor. Mesopor. Mater. 2011, 145, 194–199. [Google Scholar] [CrossRef]
- Butt, T.; Tosheva, L. Synthesis of colloidal silicalite-1 at high temperatures. Micropor. Mesopor. Mater. 2014, 187, 71–76. [Google Scholar] [CrossRef]
- Smith, M.A.; Zoelle, A.; Yang, Y.; Rioux, R.M.; Hamilton, N.G.; Amakawa, K.; Nielsen, P.K.; Trunschke, A. Surface roughness effects in the catalytic behavior of vanadia supported on SBA-15. J. Catal. 2014, 312, 170–178. [Google Scholar] [CrossRef]











| Sample | SBET | Vmicroa | Vmeso | Vtotalb | TM | H2 Uptake | Cr6+ |
|---|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | (°C) | (mmol/g) | (%) c | |
| Silicalite-1 | 379 | 0.18 | 0.12 | 0.30 | - | - | - |
| 0.5%Cr/S-1 | 378 | 0.17 | 0.05 | 0.22 | 424 | 0.141 | 0.49 |
| 1%Cr/S-1 | 368 | 0.16 | 0.06 | 0.22 | 377 | 0.189 | 0.66 |
| 2%Cr/S-1 | 358 | 0.16 | 0.05 | 0.21 | 369 | 0.253 | 0.88 |
| 3%Cr/S-1 | 350 | 0.16 | 0.04 | 0.20 | 364 | 0.342 | 1.19 |
| 7%Cr/S-1 | 345 | 0.13 | 0.05 | 0.18 | 372 (213) d | 0.330 | 1.14 |
| SBA-15 | 655 | 0.06 | 1.02 | 1.08 | - | - | - |
| 3%Cr/SBA | 469 | 0.02 | 0.69 | 0.71 | 373 | 0.304 | 1.05 |
| Sample | Sample Description | Eb (eV) a | Cr6+/Cr3+ b | |
|---|---|---|---|---|
| Cr3+ | Cr6+ | |||
| A | Fresh 3%Cr/S-1 | 576.9 | 579.2 | 2.82 |
| B | Sample A reacted for 6 h in the presence of CO2 | 577.1 | 579.5 | 1.19 |
| C | Sample A reacted for 6 h in the absence of CO2 | 576.7 | 579.6 | 0.91 |
| D | Sample C subsequently treated with CO2 at 570 °C for 0.5 h | 577.0 | 579.4 | 1.97 |
| E | Fresh 3%Cr/SBA | 576.7 | 579.2 | 2.42 |
| F | Sample E reacted for 6 h in the presence of CO2 | 576.8 | 579.3 | 0.72 |
| Catalyst | Conversion (%) | Selectivity (%) | H2/CO c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| i-C4H10 | CO2 | i-C4H8 | CH4 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 b | ||
| 0.5%Cr/S-1 | 20.8 (14.3) | 3.5 (1.7) | 79.7 (82.5) | 3.9 (3.2) | 0 (0) | 0 (0) | 10.7 (9.4) | 0.9 (0.7) | 4.8 (4.2) | 4.2 (5.6) |
| 1%Cr/S-1 | 25.3 (17.6) | 4.8 (4.4) | 76.1 (79.0) | 4.8 (4.4) | 0.6 (0.3) | 0.4 (0.1) | 10.9 (11.4) | 1.3 (0.7) | 5.9 (4.1) | 2.6 (3.1) |
| 2%Cr/S-1 | 32.8 (25.0) | 10.6 (5.6) | 72.6 (77.7) | 5.3 (4.4) | 0.7 (0.3) | 0.6 (0.3) | 11.0 (10.1) | 1.8 (1.2) | 8.0 (6.0) | 1.9 (2.1) |
| 3%Cr/S-1 | 36.5 (28.4) | 13.3 (6.9) | 71.2 (75.4) | 5.6 (4.9) | 0.8 (0.5) | 0.9 (0.6) | 11.4 (10.6) | 2.0 (1.5) | 8.1 (6.5) | 1.8 (2.0) |
| 7%Cr/S-1 | 36.3 (27.9) | 11.7 (6.2) | 69.9 (74.6) | 5.8 (5.2) | 1.0 (0.7) | 1.0 (0.7) | 11.6 (10.5) | 2.2 (1.7) | 8.5 (6.6) | 2.0 (2.4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Miao, C.; Yue, Y.; Yang, W.; Hua, W.; Gao, Z. Chromium Oxide Supported on Silicalite-1 Zeolite as a Novel Efficient Catalyst for Dehydrogenation of Isobutane Assisted by CO2. Catalysts 2019, 9, 1040. https://doi.org/10.3390/catal9121040
Luo Y, Miao C, Yue Y, Yang W, Hua W, Gao Z. Chromium Oxide Supported on Silicalite-1 Zeolite as a Novel Efficient Catalyst for Dehydrogenation of Isobutane Assisted by CO2. Catalysts. 2019; 9(12):1040. https://doi.org/10.3390/catal9121040
Chicago/Turabian StyleLuo, Yajun, Changxi Miao, Yinghong Yue, Weimin Yang, Weiming Hua, and Zi Gao. 2019. "Chromium Oxide Supported on Silicalite-1 Zeolite as a Novel Efficient Catalyst for Dehydrogenation of Isobutane Assisted by CO2" Catalysts 9, no. 12: 1040. https://doi.org/10.3390/catal9121040