Hydrogen Production from Aqueous Methanol Solutions Using Ti–Zr Mixed Oxides as Photocatalysts under UV Irradiation
Abstract
:1. Introduction
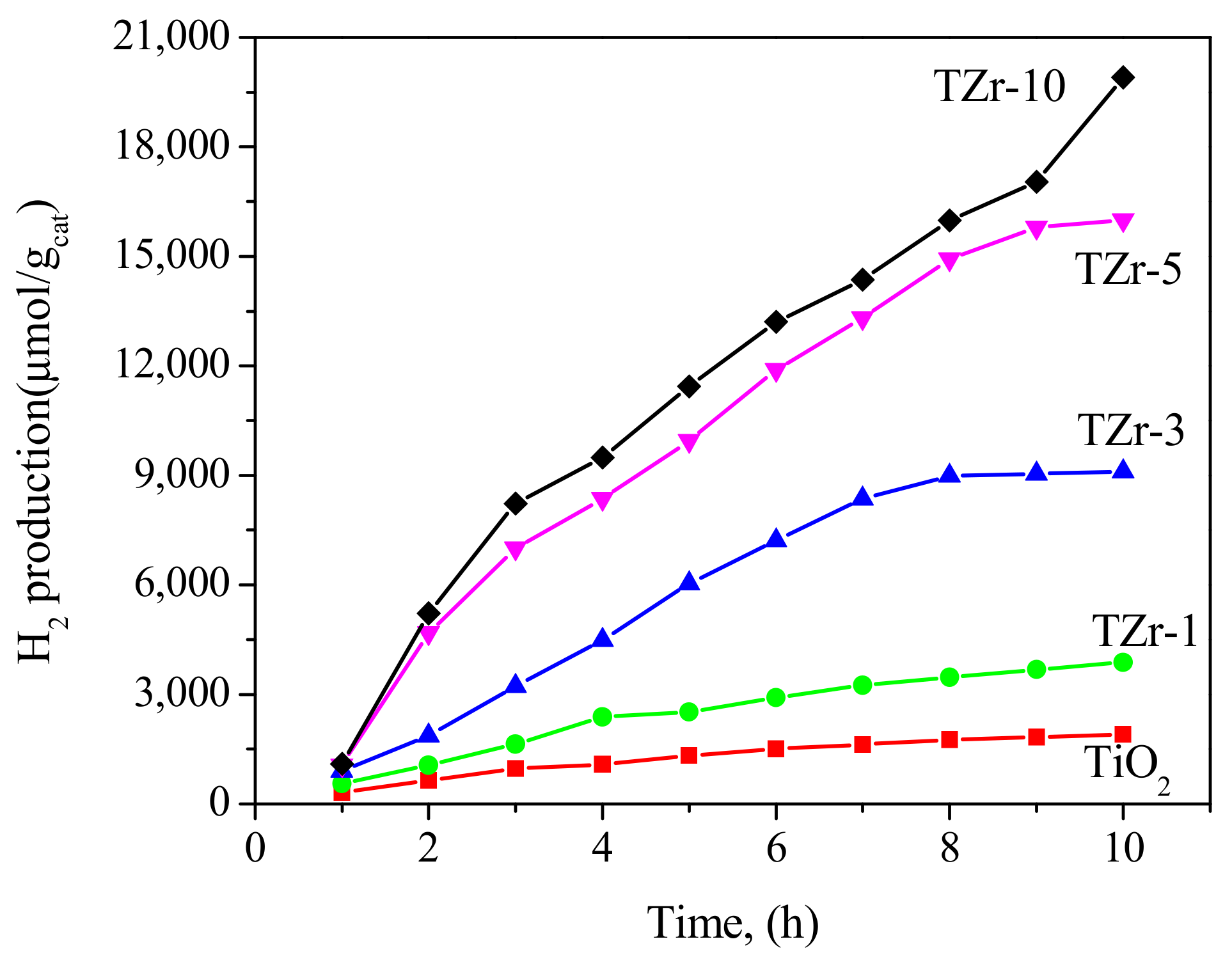
2. Results and Discussion
3. Photocatalytic Reactions
4. Experimental
4.1. Synthesis of Samples
4.2. Characterisation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Silva Veras, T.; Mozer, T.S.; Da Costa Rubim Messeder dos Santos, D.; Da Silva Cesar, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrog. Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Fox, M.A. Artificial photosynthesis: Solar splitting of water to Hydrogen and oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.S.; Mao, S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Moriya, Y.; Takatab, T.; Domen, K. Recent progress in the development of (oxy) nitride photocatalysts for water splitting under visible-light irradiation. Coord. Chem. Rev. 2013, 257, 1957–1969. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Kumaravela, V.; Mathewa, S.; Bartletta, J.; Pillaia, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Hernández-Gordillo, A.; Morales-Mendoza, G.; Lartundo-Rojas, L.; Mantilla, A.; Gómez, R. Enhancing the H2 evolution from water–methanol solution using Mn2+−Mn+3−Mn4+ redox species of Mn-doped TiO2 sol—gel photocatalysts. Catal. Today 2016, 266, 9–16. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Lopez, R.; Hernández-Gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO2–ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Guerrero-Araque, D.; Acevedo-Peña, P.; Ramírez-Ortega, D.; Calderon, H.A.; Gómez, R. Charge transfer processes involved in photocatalytic hydrogen production over CuO/ZrO2-TiO2 materials. Int. J. Hydrog. Energy 2017, 42, 9744–9753. [Google Scholar] [CrossRef]
- Yang, J.; Ferreira, J.M.F. On the Titania Phase Transition by Zirconia Additive in a Sol-Gel-Derived Powder. Mater. Res. Bull. 1998, 33, 389–394. [Google Scholar] [CrossRef]
- Chang, S.; Doong, R. Characterization of Zr-Doped TiO2 Nanocrystals Prepared by a Nonhydrolytic Sol-Gel Method at High Temperatures. J. Phys. Chem. B 2006, 110, 20808–20814. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedi, J.; Dionysiou, D.D.; Rodríguez, J.J.; Belver, C. Mixed Ti-Zr metal-organic-frameworks for the photodegradation of acetaminophen under solar irradiation. Appl. Catal. B Environ. 2019, 253, 253–262. [Google Scholar] [CrossRef]
- Daturi, M.; Cremona, A.; Milella, F.; Busca, G.; Vogna, E. Characterisation of Zirconia-Titania Powders Prepared by Coprecipitation. J. Eur. Ceram. Soc. 1998, 18, 1079–1087. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase. TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Kokporka, L.; Onsuratoom, S.; Puangpetch, T.; Chavadej, S. Sol-gel-synthesized mesoporous-assembled TiO2–ZrO2 mixed oxide nanocrystals and their photocatalytic sensitized H2 production activity under visible light irradiation. Mater. Sci. Semicond. Process. 2013, 16, 667–678. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Álvarez Galvan, M.C.; del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L. Water splitting on semiconductor catalysts under visible-light irradiation. CHEMSUSCHEM 2009, 2, 471–485. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrog. Energy 2002, 27, 991–1022. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yang, W.-D.; Huang, I.-L.; Wu, T.-S.; Chung, Z.-J. Hydrogen Production from Methanol/Water Photocatalytic Decomposition Using Pt/TiO2−xNx Catalyst. Energy Fuels 2009, 23, 2192–2196. [Google Scholar] [CrossRef]
- Jeon, M.K.; Park, J.W.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase and rutile forms of Cu-TiO2. J. Ind. Eng. Chem. 2007, 13, 84–91. [Google Scholar]
- Guzman, F.; Chuang, S.S.C.; Yang, C. Role of Methanol Sacrificing Reagent in the Photocatalytic Evolution of Hydrogen. Ind. Eng. Chem. Res. 2013, 52, 61–65. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grosse und der Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen. Nachr. Ges. Wiss. Göttingen 1918, 2, 98–100. [Google Scholar]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Puchkovska, G.; Naumov, V.; Khalyavka, T.; Kshnyakin, V.; Chernyak, V.; Baran, J. Titanium-Manganese Oxides. Optical and Photocatalytic Properties. Mater. Sci. Eng. B 2010, 175, 48–55. [Google Scholar] [CrossRef]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of Nanocrystal Sizes: A Comparison of TEM, SAXS, and XRD Studies of Highly Monodisperse CoPt3 Particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Perspectives on the Physical Chemistry of Semiconductor Nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Roy, M.; Pompella, A.; Kubacki, J.; Piosik, A.; Psiuk, B.; Klimontko, J.; Szade, J.; Roy, R.A.; Hedzelek, W. Photofunctionalization of dental zirconia oxide: Surface modification to improve bio-integration preserving crystal stability. Colloids Surf. B Biointerfaces 2017, 156, 194–202. [Google Scholar] [CrossRef]
- Avilés-García, O.; Espino-Valencia, J.; Romero-Romero, R.; Rico-Cerda, J.L.; Arroyo-Albiter, M.; Solís-Casados, D.A.; Natividad-Rangel, R. Enhanced Photocatalytic Activity of Titania by Co-Doping with Mo and W. Catalysts 2018, 8, 631. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Li, J.; Liu, J.; Wang, L.; Zhang, H.; Zhang, Z.; Zhao, B. Visible-light-driven photocatalyst of La-N-codoped TiO2 nano-photocatalyst: Fabrication and its enhanced photocatalytic performance and mechanism. J. Ind. Eng. Chem. 2015, 25, 16–21. [Google Scholar] [CrossRef]
- Lin, G. Hierarchically macro–mesoporous ZrO2–TiO2 composites with enhanced photocatalytic activity. Ceram. Int. 2015, 41, 575–5749. [Google Scholar]
- Linnik, O.; Shestopal, N.; Smirnova, N.; Eremenko, A.; Korduban, O.; Kandyba, V.; Kryshchuk, T.; Socol, G.; Stefan, N.; Popescu-Pelin, G.; et al. Correlation between electronic structure and photocatalytic properties of non-metal doped TiO2/ZrO2 thin films obtained by pulsed laser deposition method. Vacuum 2015, 114, 166–171. [Google Scholar] [CrossRef]
- Foster, A.S.; Sulimov, V.B.; Gejo, F.L.; Shluger, A.L.; Nieminen, R.M. Structure and electrical levels of point defects in monoclinic zirconia. Phys. Rev. B Condens. Matter 2001, 64. [Google Scholar] [CrossRef]
- Youssef, M.; Yildiz, B. Intrinsic point-defect equilibria in tetragonal ZrO2: Density functional theory analysis with finite-temperature effects. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Ma, W.; Herbert, F.W.; Senanayake, S.D.; Yildiz, B. Non-equilibrium oxidation states of zirconium during early stages of metal oxidation. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Rodríguez-Castellón, E.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. Measuring and interpreting quantum efficiency for hydrogen photo-production using Pt-titania catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]






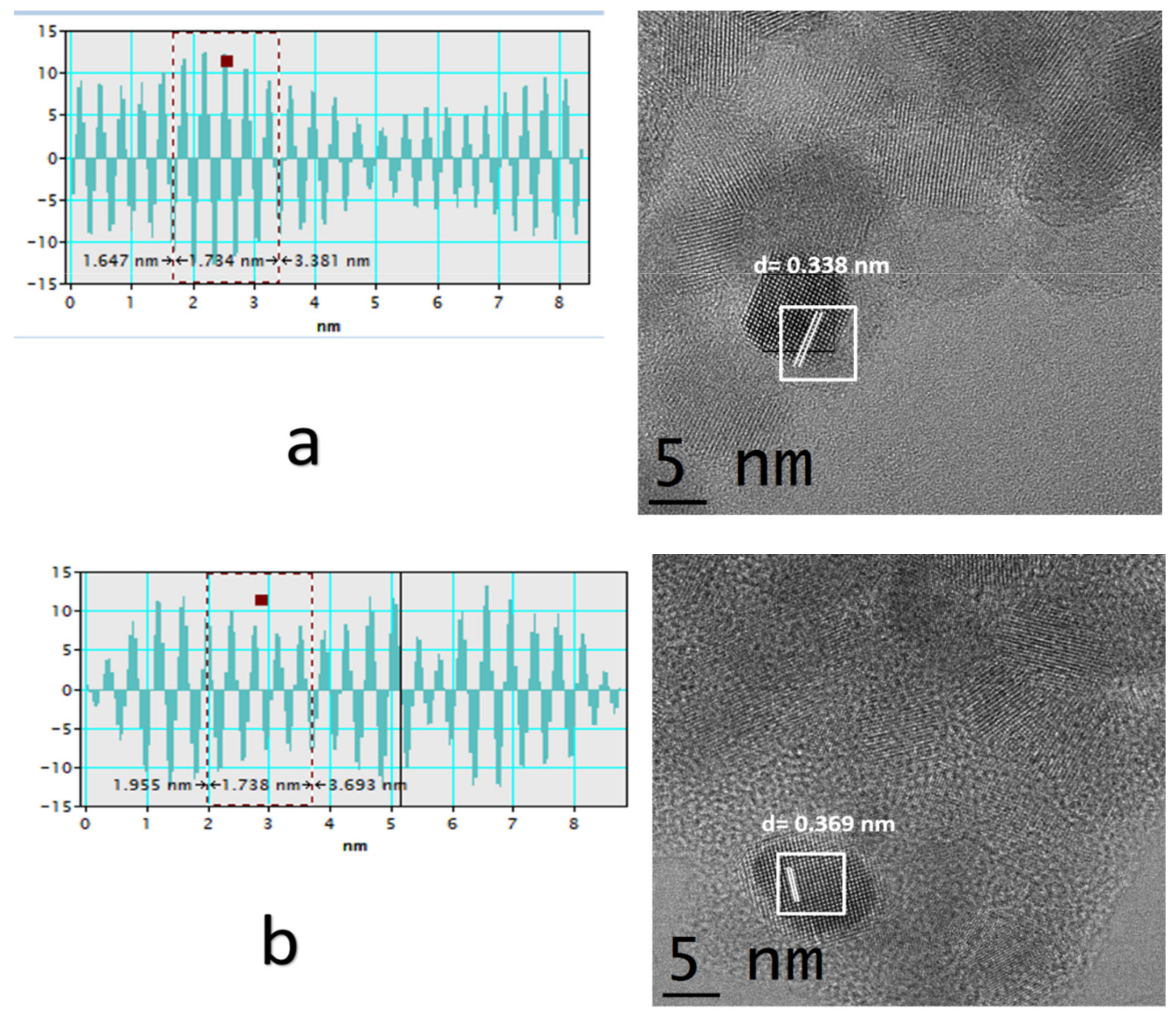

| Zr | S(BET) | Mean Pore Size | Eg | Cell Parameter a | Crystallite Size | H2 Production |
|---|---|---|---|---|---|---|
| (wt.%) | (m2/g) | (nm) | (eV) | (nm) | (nm) | (μmoL/h) |
| 1.0 | 91 | 5.6 | 3.05 | 0.560 | 1.76 | 387 |
| 3.0 | 147 | 7.7 | 3.14 | 0.580 | 0.98 | 900 |
| 5.0 | 157 | 7.8 | 3.20 | 0.660 | 0.96 | 1600 |
| 10.0 | 138 | 9.5 | 3.15 | 0.780 | 1.36 | 2100 |
| TiO2 | 64 | 6.5 | 3.20 | 0.377 | 5.02 | 190 |
| Sample | Composition | ||||||
|---|---|---|---|---|---|---|---|
| Ti 2p, at.% | Zr 3d, at.% | O 1s, at.% | Ti, wt.% | Zr, wt.% | O, wt.% | O 1s/(Ti 2p + Zr 3d) | |
| TZr-1 | 37.8 | 1.0 | 61.3 | 54.6 | 1.0 | 44.40 | 1.58 |
| TZr-3 | 36.9 | 1.2 | 61.9 | 53.55 | 2.5 | 43.95 | 1.62 |
| TZr-5 | 36.2 | 1.9 | 62.0 | 52.15 | 5.0 | 42.85 | 1.63 |
| TZr-10 | 35.8 | 2.9 | 61.4 | 47.95 | 9.0 | 43.05 | 1.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Larios, A.; Rico, J.L.; Anaya-Esparza, L.M.; Vargas, O.A.G.; González-Silva, N.; Gómez, R. Hydrogen Production from Aqueous Methanol Solutions Using Ti–Zr Mixed Oxides as Photocatalysts under UV Irradiation. Catalysts 2019, 9, 938. https://doi.org/10.3390/catal9110938
Pérez-Larios A, Rico JL, Anaya-Esparza LM, Vargas OAG, González-Silva N, Gómez R. Hydrogen Production from Aqueous Methanol Solutions Using Ti–Zr Mixed Oxides as Photocatalysts under UV Irradiation. Catalysts. 2019; 9(11):938. https://doi.org/10.3390/catal9110938
Chicago/Turabian StylePérez-Larios, Alejandro, Jose L. Rico, Luis M. Anaya-Esparza, O.A. González Vargas, Napoleón González-Silva, and Ricardo Gómez. 2019. "Hydrogen Production from Aqueous Methanol Solutions Using Ti–Zr Mixed Oxides as Photocatalysts under UV Irradiation" Catalysts 9, no. 11: 938. https://doi.org/10.3390/catal9110938





