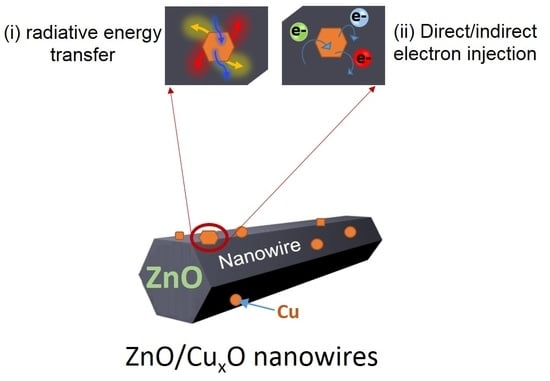
Duality in the Mechanism of Hexagonal ZnO/CuxO Nanowires Inducing Sulfamethazine Degradation under Solar or Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Description of the ZnO/CuxO (5%) Surface by Physical Methods
2.2. Redox Catalysis and Interfacial Potential during SMT Degradation
2.3. SMT-Degradation on ZnO/CuxO: Effect of Light Intensity
2.4. Determination of the ROS-Species during SMT-Degradation
2.5. IFCT Mechanisms Suggested under Solar and Visible Light: Involvement of the LSPR
3. Materials and Methods
3.1. Preparation of ZnO/CuxO Hexagonal Wurtzite Nanowires
3.2. Nanowires Properties Determined by Surface Physical Methods
3.3. X-ray Photoelectron Spectroscopy and Interfacial “In Situ” Potentials Determination
3.4. Irradiation Procedures and ROS Analysis.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.; Doerge, R.; Gaylor, W.; Miller, A.; Lorentzen, J.; Casciano, A.; Kadlubar, F.; Schwetz, A. An FDA Review of Sulfamethazine Toxicity. Regul. Toxicol. Pharmacol. 1999, 30, 217–222. [Google Scholar] [CrossRef] [PubMed]
- De Liguoro, M.; Fioretto, B.; Poltronieri, C.; Gallina, G. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere 2009, 75, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, M.; Pignatello, J.; Beltrán, L.; Granados, M.; Peccia, J. Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar). Environ. Sci. Technol. 2011, 45, 10020–10027. [Google Scholar] [CrossRef] [PubMed]
- Wegst-Uhrich, R.; Navarro, A.; Zimmerman, L.; Aga, S. Assessing antibiotic sorption in soil: A literature review and new case studies on sulfonamides and macrolides. Chem. Cent. J. 2014, 8, 5–20. [Google Scholar] [CrossRef]
- Ingerslev, F.; Halling-Sørensen, B. Biodegradability properties of sulfonamides in activated sludge. Environ. Toxicol. Chem. 2000, 19, 2467–2473. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, S.; Alder, C.; Giger, W.; Theiß, N.; Löffler, D.; Ternes, D.A. Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. J. Chromatogr. A 2005, 1085, 179–189. [Google Scholar] [CrossRef]
- Dolliver, H.; Kumar, K.; Gupta, S. Sulfamethazine uptake by plants from manure-amended soil. J. Eviron. Qual. 2007, 36, 1224–1230. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Ind. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Boxall, B.; Johnson, P.; Smith, J.; Sinclair, J.; Stutt, E.; Levy, S. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Xiaolei, B.; Weiwei, B. MCM-48 modified magnetic mesoporous nanocomposite as an attractive adsorbent for the removal of sulfamethazine from water. Water Res. 2013, 47, 4107–4114. [Google Scholar] [CrossRef] [PubMed]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, E.; Alberti, A.; Martucci, A.J. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. Hazard. Mater. 2010, 178, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sheng, P.; Ma, Y.; Wang, F.; Yu, Q. Roles of extracellular polymeric substances (EPS) in the migration and removal of sulfamethazine in activated sludge system. Water Res. 2013, 47, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Topp, E.; Chapman, R.; Devers-Lamrani, M.; Hartmann, A.; Marti, R.; Martin-Laurent, F.; Sabourin, L.; Scott, A.; Sumarah, M.J. Accelerated biodegradation of veterinary antibiotics in agricultural soil following long-term exposure, and isolation of a sulfamethazine-degrading Microbacterium sp. Environ. Qual. 2014, 42, 173–178. [Google Scholar] [CrossRef]
- Reis, J.; Reis, C.; Ricken, C.; Kolvenbach, B.; Manaia, M.; Corvini, F.; Nunes, C.J. Biodegradation of sulfamethoxazole and other sulfonamides by Achromobacter denitrificans PR1. Hazard. Mater. 2014, 280, 741–749. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, X.; Zhang, Y.; Li, J.; Lim, T. Synergistic catalytic degradation of antibiotic sulfamethazine in a heterogeneous sonophotolytic goethite/oxalate Fenton-like system. Appl. Catal. B Environ. 2013, 136, 294–301. [Google Scholar] [CrossRef]
- Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, M.; Mansilla, H. Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process. Water Res. 2010, 44, 2533–2540. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.; Oturan, A. Effect of the anode materials on the efficiency of the electro-Fenton process for the mineralization of the antibiotic sulfamethazine. Appl. Catal. B Environ. 2016, 199, 331–341. [Google Scholar] [CrossRef]
- Garoma, T.; Umamaheshwar, K.; Mumper, A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.J. Degradation of sulfamethazine by gamma irradiation in the presence of hydrogen peroxide. Hazard. Mater. 2013, 250, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xu, J.; Wang, S.; Zhang, Y.; He, Y.; Li, X. Photodegradation of sulfamethazine in an aqueous solution by a bismuth molybdate photocatalyst. Catal. Sci. Technol. 2013, 3, 1603–1611. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Xu, P.; Zeng, G.; Huang, D.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Liu, Y.; et al. In Situ Grown AgI/Bi12O17Cl2 Heterojunction Photocatalysts for Visible Light Degradation of Sulfamethazine: Efficiency, Pathway, and Mechanism. ACS Sustain. Chem. Eng. 2018, 6, 4174–4184. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.; Byrne, J.; Dionysiou, D.J. New insights into the mechanism of visible light photocatalysis. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Nozik, A. Photoelectrochemistry: Applications to solar energy conversion. Annu. Rev. Phys. Chem. 1978, 29, 189–222. [Google Scholar] [CrossRef]
- Mamba, G.; Kiwi, J.; Pulgarin, C.; Sanjines, R.; Giannakis, S.; Rtimi, S. Evidence for the degradation of an emerging pollutant by a mechanism involving iso-energetic charge transfer under visible light. Appl. Catal. B Environ. 2018, 233, 175–183. [Google Scholar] [CrossRef]
- Kung, Y.; Cai, L.; Pan, F.; Shen, W.; Su, H. Photonic Fano Resonance of Multishaped Cu2O Nanoparticles on ZnO Nanowires Modulating Efficiency of Hydrogen Generation in Water Splitting Cell. ACS Sustain. Chem. Eng. 2018, 6, 6590–6598. [Google Scholar] [CrossRef]
- Yu, J.; Kiwi, J.; Wang, T.; Pulgarin, C.; Rtimi, S. Evidence for a dual mechanism in the TiO2/CuxO photocatalyst during the degradation of sulfamethazine under solar or visible light: Critical issues. J. Photochem. Photobiol. A 2019, 375, 270–279. [Google Scholar] [CrossRef]
- Niedziolka-Jonsson, J.; Mackowski, S. Plasmonics with Metallic Nanowires. Materials 2019, 12, 1418–1432. [Google Scholar]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 12, 1612–1619. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. Acs Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef]
- Wang, H.; Tam, F.; Grady, N.K.; Halas, N.J. Cu nanoshells: Effects of interband transitions on the nanoparticle plasmon resonance. J. Phys. Chem. B 2005, 109, 18218–18222. [Google Scholar] [CrossRef]
- Singhal, S.; Kaur, J.; Namgyal, T.; Sharma, R. Cu-doped ZnO nanoparticles: Synthesis, structural and electrical properties. Phys. B Condens. Mater 2012, 407, 1223–1229. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Vuong, M.; Chinh, D.; Huy, T.; Lee, I. CuO-Decorated ZnO Hierarchical Nanostructures as Efficient and Established Sensing Materials for H2S Gas Sensors. Sci. Rep. 2016, 6, 26736. [Google Scholar]
- Ahmad, R.; Tripathy, N.; Ahn, S.; Bhat, S.; Mahmoudi, T.; Wang, Y.; Yoo, Y.; Kwon, W.; Yang, H.; Hahn, B.Y. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 5715–5718. [Google Scholar] [CrossRef]
- Skårman, B.; Grandjean, D.; Benfield, E.; Hinz, A.; Andersson, A.; Wallenberg, R.J. Carbon monoxide oxidation on nanostructured CuOx/CeO2 composite particles characterized by HREM, XPS, XAS, and high-energy diffraction. Catalysis 2002, 211, 119–133. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic synthesis of methane from acetic acid-new Kolbe reaction pathway. Am. Chem. Soc. 1978, 100, 2239–2240. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D. Essentials of Pharmaceutical Chemistry, 3rd ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2008; p. 69. [Google Scholar]
- Fernandez, J.; Nadtochenko, V.; Kiwi, J. Photobleaching of Orange II within seconds using the oxone/Co 2+ reagent through Fenton-like chemistry. Chem. Commum. 2003, 18, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Parks, G. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Tacic, A.; Nicolic, V.; Nicolic, L.; Savic, I. Antimicrobial sulfonamide drugs. Adv. Technol. 2017, 6, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Liu, P.; Chen, L. Comparison of surface plasmon resonance responses to dry/wet air for Ag, Cu, and Au/SiO2. Appl. Opt. 2012, 51, 1357–1360. [Google Scholar] [CrossRef]
- Khanehzaeri, H.; Ahmad, M.; Shameli, K.; Ajdari, Z. Synthesis and characterization of Cu@ Cu2O core shell nanoparticles prepared in seaweed Kappaphycus alvarezii Media. J. Electrochem. Sci. 2014, 9, 8189–8198. [Google Scholar]
- Liu, X.; Swihart, M. Heavily-doped colloidal semiconductor and metal oxide nanocrystals: An emerging new class of plasmonic nanomaterials. Chem. Soc. Rev. 2014, 43, 3908–3920. [Google Scholar] [CrossRef]
- Mendelsberg, J.; McBride, M.; Duong, T.; Bailey, M.; Llordes, A.; Milliron, J.; Helms, A. Dispersible Plasmonic Doped Metal Oxide Nanocrystal Sensors that Optically Track Redox Reactions in Aqueous Media with Single-Electron Sensitivity. Adv. Opt. Mater. 2015, 3, 1293–1300. [Google Scholar] [CrossRef]
- Sulzberger, B.; Canonica, S.; Egli, T.; Giger, W.; Klausen, J.; von Gunten, U. Oxidative transformations of contaminants in natural and in technical systems. Chimia 1997, 51, 900–907. [Google Scholar]
- Wardman, P.J. Reduction potentials of one-electron couples involving free radicals in aqueous solution. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Jing, L.; Zhou, W.; Tian, G.; Fu, H. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef]
- Yu, J.; Kiwi, J.; Zivkovic, I.; Rønnow, H.M.; Wang, T.; Rtimi, S. Quantification of the local magnetized nanotube domains accelerating the photocatalytic removal of the emerging pollutant tetracycline. Appl. Cat. B Environm. 2019, 248, 450–458. [Google Scholar] [CrossRef]
- Tamaekong, N.; Phanichphant, S.; Wisitsoraat, A.; Liewhiran, C. Core/Shell of p-CuxO/n-ZnO Nanowire Arrays for H2S Gas Sensor. Solid. State Phenom. 2018, 283, 7–15. [Google Scholar] [CrossRef]
- Elfadill, N.; hashim, M.; Saron, K.; Charour, K.; Qaeed, M.; Bououdina, M. Ultraviolet–visible photo-response of p-Cu2O/n-ZnO heterojunction prepared on flexible (PET) substrate. Mater. Chem. Phys. 2015, 156, 54–60. [Google Scholar] [CrossRef]
- Maccato, C.; Berreca, D.; Carraro, G.; Gasparotto, A.; Gombac, V.; Fornasiero, P. CuOx-TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Solutions. Surf. Coat. Technol. 2013, 230, 219–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Stefanakos, M.R.E.; Goswami, D. Synthesis, characterization, and applications of ZnO nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
















| C1s | N1s | O1s | Na1s | Cu2p | Zn2p | |
|---|---|---|---|---|---|---|
| Before SMT degradation | 13.78 | 0.00 | 42.58 | 0.00 | 7.32 | 36.32 |
| After SMT degradation | 14.02 | 0.00 | 41.99 | 0.00 | 6.92 | 37.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Kiwi, J.; Wang, T.; Pulgarin, C.; Rtimi, S. Duality in the Mechanism of Hexagonal ZnO/CuxO Nanowires Inducing Sulfamethazine Degradation under Solar or Visible Light. Catalysts 2019, 9, 916. https://doi.org/10.3390/catal9110916
Yu J, Kiwi J, Wang T, Pulgarin C, Rtimi S. Duality in the Mechanism of Hexagonal ZnO/CuxO Nanowires Inducing Sulfamethazine Degradation under Solar or Visible Light. Catalysts. 2019; 9(11):916. https://doi.org/10.3390/catal9110916
Chicago/Turabian StyleYu, Jiajie, John Kiwi, Tianhe Wang, Cesar Pulgarin, and Sami Rtimi. 2019. "Duality in the Mechanism of Hexagonal ZnO/CuxO Nanowires Inducing Sulfamethazine Degradation under Solar or Visible Light" Catalysts 9, no. 11: 916. https://doi.org/10.3390/catal9110916