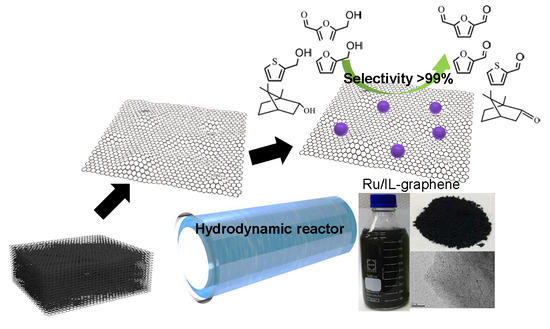
High-Throughput Production of Heterogeneous RuO2/Graphene Catalyst in a Hydrodynamic Reactor for Selective Alcohol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of IL–Graphene and RuO2/IL–Graphene
3.3. Material Characterization
3.4. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable n-heterocyclic carbene complex catalysts and beyond: A perspective on recycling strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.C. A review of biomass energy—Shortcomings and concerns. J. Chem. Technol. Biotechnol. 2016, 91, 1933–1945. [Google Scholar] [CrossRef]
- Lange, J.P.; Heide, E.; Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Ma, J.; Du, Z.; Xu, J.; Chu, Q.; Pang, Y. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran, and synthesis of a fluorescent material. ChemSusChem 2011, 4, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Shun, N.; Atsuki, S.; Kohki, E. Direct hydroxymethylation of furaldehydes with aqueous formaldehyde over a reusable sulfuric functionalized resin catalyst. ACS Omega 2018, 3, 5988–5993. [Google Scholar] [CrossRef]
- Das, V.K.; Shifrina, Z.B.; Bronstein, L.M. Graphene and graphene-like materials in biomass conversion: Paving the way to the future. J. Mater. Chem. A 2017, 5, 25131–25143. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal. 2018, 8, 6301–6333. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective benzyl alcohol oxidation over Pd catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Mali, M.; Mastrorilli, P.; Monopoli, A. Oxidation of benzyl alcohols to aldehydes and ketones under air in water using a polymer supported palladium catalyst. J. Mol. Catal. A Chem. 2014, 386, 114–119. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Fan, W. Graphene-based catalysis for biomass conversion. Catal. Sci. Technol. 2015, 5, 3845–3858. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Shi, G. Graphene based catalysts. Energy Environ. Sci. 2012, 5, 8848–8868. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Wang, D.; Niu, W.; Tan, M.; Wu, M.; Zheng, X.; Li, Y.; Tsubaki, N. Pt nanocatalysts supported on reduced graphene oxide for selective conversion of cellulose or cellobiose to sorbitol. ChemSusChem 2014, 7, 1398–1406. [Google Scholar] [CrossRef]
- Jin, X.; Dang, L.; Lohrman, J.; Subramaniam, B.; Ren, S.; Chaudhari, R.V. Lattice-matched bimetallic CuPd-graphene nanocatalysts for facile conversion of biomass-derived polyols to chemicals. ACS Nano 2013, 7, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.M.; Kang, H.G.; Kim, H.J.; Hong, S.B.; Jeon, H.; Hwang, S.Y.; Seo, D.; Kwak, B.E.; Han, Y.K.; Choi, B.G.; et al. Hydraulic power manufacturing for highly scalable and stable 2D nanosheet dispersions and their film electrode application. Adv. Funct. Mater. 2018, 28, 1802952–1802963. [Google Scholar] [CrossRef]
- Jeon, H.; Jeong, J.M.; Kang, H.G.; Kim, H.J.; Park, J.; Kim, D.H.; Jung, Y.M.; Hwang, S.Y.; Han, Y.K.; Choi, B.G. Scalable water-based production of highly conductive 2D nanosheets with ultrahigh volumetric capacitance and rate capability. Adv. Energy Mater. 2018, 8, 1800227–1800237. [Google Scholar] [CrossRef]
- Hong, S.B.; Jeong, J.M.; Kang, H.G.; Seo, D.; Cha, Y.; Jeon, H.; Lee, G.Y.; Irshad, M.; Kim, D.H.; Hwang, S.Y.; et al. Fast and scalable hydrodynamic synthesis of MnO2/defect-free graphene nanocomposites with high rate capability and long cycle life. ACS Appl. Mater. Interfaces 2018, 10, 35250–35259. [Google Scholar] [CrossRef]
- Amiri, A.; Naraghi, M.; Ahmadi, G.; Soleymaniha, M.; Shanbedi, M. A review on liquid-phase exfoliation for scalable production of pure graphene, wrinkled, crumpled and functionalized graphene and challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Rao, K.S.; Senthilnathan, J.; Liu, Y.-F.; Yoshimura, M. Role of peroxide ions in formation of graphene nanosheets by electrochemical exfoliation of graphite. Sci. Rep. 2014, 4, 4237–4242. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, S.; Cho, D.H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.P.; Jung, G.Y.; Shin, E.; Kim, W.G.; et al. Direct exfoliation and dispersion of two-dimensional materials in pure water via temperature control. Nat. Commun. 2015, 6, 8294–8302. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 2009, 19, 3680–3695. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, J.; Li, C.; Li, B.; Li, D.; Liu, Z.; Cai, Q.; Zhang, J.; Liu, Y. Fabrication of novel ternary three-dimensional RuO2/graphitic-C3N4@reduced graphene oxide aerogel composites for supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 4982–4991. [Google Scholar] [CrossRef]
- Nie, J.; Liu, H. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on manganese oxide catalysts. J. Catal. 2014, 316, 57–66. [Google Scholar] [CrossRef]
- Antonyraj, C.A.; Kim, B.; Kim, Y.; Shin, S.; Lee, K.-Y.; Kim, I.; Cho, J.K. Heterogeneous selective oxidation of 5-hydroxymethyl-2-furfural (HMF) into 2,5-diformylfuran catalyzed by vanadium supported activated carbon in MIBK, extracting solvent for HMF. Cat. Commun. 2014, 57, 64–68. [Google Scholar] [CrossRef]
- Antonyraj, C.A.; Jeong, J.; Kim, B.; Shin, S.; Kim, S.; Lee, K.-Y.; Cho, J.K. Selective oxidation of HMF to DFF using Ru/γ-alumina catalyst in moderate boiling solvents toward industrial production. J. Ind. Eng. Chem. 2013, 19, 1056–1059. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Z.; Liu, B.; Chen, S.; Zhang, Z. Catalytic oxidation of biomass derived 5-hydroxymethylfurfural (HMF) over Ru(III) incorporated zirconium phosphate catalyst. J. Ind. Eng. Chem. 2016, 38, 181–185. [Google Scholar] [CrossRef]
- Fang, R.; Lauque, R.; Li, Y. Selective aerobic oxidation of biomass-derived HMF to 2,5-diformylfuran using a MOF-derived magnetic hollow Fe–Co nanocatalyst. Green Chem. 2016, 18, 3152–3157. [Google Scholar] [CrossRef]





| Entry | Catalyst | Conv. [b] (%) | Select. (%) |
|---|---|---|---|
| 1 | RuO2/IL-graphene [a] | >99 | >99 |
| 2 | IL-graphene [b] | No reaction | No reaction |
| 3 | RuCl3·nH2O [c] | 5 | <5 |
| 4 | Ru(OH)x/Bulk C [d] | 44 | >99 |
| 5 | Ru(OH)x/SNP | 4 | >99 |
| 6 | Ru(OH)x/MoS2 | >99 | >99 |
| 7 | Ru(OH)x/Al2O3 | 52 | >99 |
| 8 | Ru(OH)x/MnO2 | 82 | >99 |
| 9 | Ru(OH)x/CeO2 | 45 | >99 |
| 10 | Ru(OH)x/ZrO2 | 23 | >99 |
| 11 | No catalyst | No reaction | No reaction |
| Entry | Substrate | Time (h) | Conv. (%) | Yield (%) | Select. (%) | Product |
|---|---|---|---|---|---|---|
| 1 |  | 12 | 60 | 60 | >99 |  |
| 2 |  | 12 | 70 | 70 | >99 |  |
| 3 |  | 2 | >99 | >99 | >99 |  |
| 4 |  | 1 | >99 | >99 | >99 |  |
| 5 |  | 0.16 (10 min) | >99 | >99 | >99 |  |
| 6 |  | 18 | >95 | >95 | >99 |  |
| 7 |  | 6 | >93 | >93 | >99 |  |
| 8 |  | 8 | >99 | >99 | >99 |  |
| 9 |  | 6 | >92 | >92 | >99 |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-M.; Jin, S.B.; Yoon, J.H.; Yeo, J.G.; Lee, G.Y.; Irshad, M.; Lee, S.; Seo, D.; Kwak, B.E.; Choi, B.G.; et al. High-Throughput Production of Heterogeneous RuO2/Graphene Catalyst in a Hydrodynamic Reactor for Selective Alcohol Oxidation. Catalysts 2019, 9, 25. https://doi.org/10.3390/catal9010025
Jeong J-M, Jin SB, Yoon JH, Yeo JG, Lee GY, Irshad M, Lee S, Seo D, Kwak BE, Choi BG, et al. High-Throughput Production of Heterogeneous RuO2/Graphene Catalyst in a Hydrodynamic Reactor for Selective Alcohol Oxidation. Catalysts. 2019; 9(1):25. https://doi.org/10.3390/catal9010025
Chicago/Turabian StyleJeong, Jae-Min, Se Bin Jin, Jo Hee Yoon, Jae Goo Yeo, Geun Young Lee, Mobina Irshad, Seongwoo Lee, Donghyuk Seo, Byeong Eun Kwak, Bong Gill Choi, and et al. 2019. "High-Throughput Production of Heterogeneous RuO2/Graphene Catalyst in a Hydrodynamic Reactor for Selective Alcohol Oxidation" Catalysts 9, no. 1: 25. https://doi.org/10.3390/catal9010025