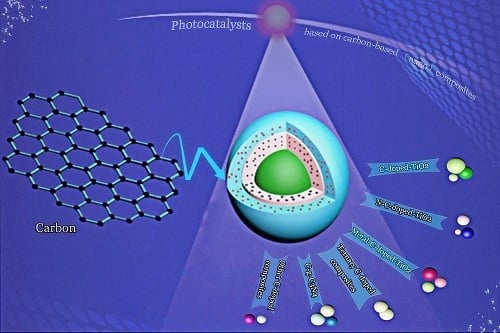
Recent Development of Photocatalysts Containing Carbon Species: A Review
Abstract
:1. Introduction
2. Photocatalysts of C-doped TiO2
2.1. Photocatalysts of TiO2/C Composites
2.2. Photocatalysts of N–C-Doped TiO2
2.3. Photocatalysts of Metal–C Doped TiO2 Composites
2.4. Photocatalysts of Other Co-Doped C/TiO2 Composites
3. Photocatalysts of C/g-C3N4 Composites
4. Photocatalysts of Ternary C-Doped Composites
5. Photocatalysts of Other C-doped Composites
6. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wu, Y.M.; Xing, M.Y.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Zhu, J.F.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Coll. Interface Sci. 2009, 14, 260–269. [Google Scholar] [CrossRef]
- Zhu, J.F.; Chakarov, D.; Zäch, M. Chapter 13: Nanostructured Materials for Photolytic Hydrogen Production. In Energy Efficiency and Renewable Energy through Nanotechnology; Zang, L., Ed.; Springer: London, UK, 2011; pp. 441–486. ISBN 978-0-85729-637-5. [Google Scholar]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C-Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.F. Photocatalytic hydrogen production. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 7881–7901. ISBN 978-0-387-89469-0. [Google Scholar]
- Zhu, J.F. Photocatalysts for hydrogen production. In Advanced Materials for Clean Energy; Xu, Q., Kobayashi, T., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 391–420. ISBN 978-1-4822-0578-7. [Google Scholar]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an environmental photocatalyst that generates oh radicals under visible light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Q.; Li, C.Y.; Yuan, S.; Ma, M.G.; Qiang, Y.H.; Zhu, J.F. ZnO based heterojunctions and their application in environmental photocatalysis. Nanotechnology 2016, 27, 402001. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.G.; Zhou, Y.J.; Fu, Y.J.; Guo, S.J.; Li, H.; Zhao, S.Q.; Huang, H.; Liu, Y.; Kang, Z.H. Carbon dots enhance the stability of CdS for visible-light-driven overall water splitting. Appl. Catal. B-Environ. 2017, 216, 114–121. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2 ultrathin nanosheets integrated on CdS nanorods to promote charge separation and migration and improve solar-driven photocatalytic hydrogen evolution. Chemsuschem 2017, 10, 1563–1570. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.L.; Cai, T.; Zhang, S.Q.; Liu, Y.T.; Song, Y.Z.; Dong, X.R.; Hu, L. Glucose-assisted synthesize 1D/2D nearly vertical CdS/MoS2 heterostructures for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2017, 321, 366–374. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Yan, S.C.; Lv, S.B.; Li, Z.S.; Zou, Z.G. Organic-inorganic composite photocatalyst of g-C3N4 and TaON with improved visible light photocatalytic activities. Dalton Trans. 2010, 39, 1488–1491. [Google Scholar] [CrossRef]
- Pan, C.S.; Xu, J.; Wang, Y.J.; Li, D.; Zhu, Y.F. Dramatic activity of C3N4/BiPO4 photocatalyst with core/shell structure formed by self-assembly. Adv. Funct. Mater. 2012, 22, 1518–1524. [Google Scholar] [CrossRef]
- Li, T.B.; Chen, G.; Zhou, C.; Shen, Z.Y.; Jin, R.C.; Sun, J.X. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances. Dalton Trans. 2011, 40, 6751–6758. [Google Scholar] [CrossRef]
- Liu, R.H.; Huang, H.; Li, H.T.; Liu, Y.; Zhong, J.; Li, Y.Y.; Zhang, S.; Kang, Z.H. Metal nanoparticle/carbon quantum dot composite as a photocatalyst for high-efficiency cyclohexane oxidation. ACS Catal. 2014, 4, 328–336. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.Q.; Xu, Y.J. Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.J.; Li, Y.M.; Wang, Y.; Li, J.H. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A.; Ferraro, S.; Cespi, M.; Bonacucina, G.; Minicucci, M.; Di Cicco, A. Exfoliation of graphite into graphene in aqueous solution: an application as graphene/TiO2 nanocomposite to improve visible light photocatalytic activity. RSC Adv. 2016, 6, 93048–93055. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent advances in graphene based TiO2 nanocomposites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Rommozzi, E.; Zannotti, M.; Giovannetti, R.; D’Amato, C.A.; Ferraro, S.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Reduced graphene oxide/TiO2 nanocomposite: from synthesis to characterization for efficient visible light photocatalytic applications. Catalysts 2018, 8, 598. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.H.; Xu, Y.J. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Graphene nanomesh by ZnO nanorod photocatalysts. ACS Nano 2010, 4, 4174–4180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, M.Q.; Liu, S.Q.; Sun, Y.G.; Xu, Y.J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Gabashvili, A.; Jacob, D.S.; Yu, J.C.; Gedanken, A. Synthesis and characterization of TiO2@C core-shell composite nanoparticles and evaluation of their photocatalytic activities. Chem. Mater. 2006, 18, 2275–2282. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Neville, E.M.; Herron, R.; Thampi, K.R.; Donal MacElroy, J.M. Routes to visible light active C-doped TiO2 photocatalysts using carbon atoms from the Ti precursors. J. Photochem. Photobiol. A Chem. 2014, 289, 60–65. [Google Scholar] [CrossRef]
- Moghaddam, H.A.; Jafari, S.; Mohammadi, M.R. Enhanced efficiency of over 10% in dye-sensitized solar cells through C and N single- and co-doped TiO2 single-layer electrodes. New J. Chem. 2017, 41, 9453–9460. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Kazeminezhad, I.; Jaafarzadeh, N.; Ahmadi, M.; Ramezani, Z.; Martinez, S.S. The visible-light photodegradation of nonylphenol in the presence of carbon-doped TiO2 with rutile/anatase ratio coated on GAC: Effect of parameters and degradation mechanism. J. Hazard. Mater. 2018, 350, 108–120. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yin, S.; Dong, Q.; Sato, T. Blue/green/red colour emitting up-conversion phosphors coupled C-TiO2 composites with UV, visible and NIR responsive photocatalytic performance. Appl. Catal. B Environ. 2014, 156–157, 257–264. [Google Scholar] [CrossRef]
- Huang, Q.W.; Tian, S.Q.; Zeng, D.W.; Wang, X.X.; Song, W.L.; Li, Y.Y.; Xiao, W.; Xie, C.S. Enhanced photocatalytic activity of chemically bonded TiO2/graphene composites based on the effective interfacial charge transfer through C-Ti bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Bai, L.N.; Yan, J.M.; Jiang, Q.; Lian, J.S. Enhancing photocatalytic activity of disorder engineered C/TiO2 and TiO2 nanoparticles. J. Mater. Chem. A 2014, 2, 7439–7445. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Zhang, Y.Z.; Yang, G.; Deng, S.H.; Shen, F.; Peng, H.; Wang, L.L. Novel multi-layer cross-linked TiO2/C nanosheets and their photocatalytic properties. New J. Chem. 2014, 38, 1647–1654. [Google Scholar] [CrossRef]
- Lin, Y.T.; Weng, C.H.; Chen, F.Y. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Hassan, M.E.; Cong, L.; Liu, G.L.; Zhu, D.W.; Cai, J.B. Synthesis and characterization of C-doped TiO2 thin films for visible-light-induced photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2014, 294, 89–94. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Huang, J.F.; Fei, J.; Cao, L.Y.; Li, C.Y. In situ synthesis of mesoporous C-doped TiO2 single crystal with oxygen vacancy and its enhanced sunlight photocatalytic properties. Dyes Pigments 2017, 144, 203–211. [Google Scholar] [CrossRef]
- Dong, F.; Wang, H.Q.; Wu, Z.B. One-step “green” synthetic approach for mesoporous c-doped titanium dioxide with efficient visible light photocatalytic activity. J. Phys. Chem. C 2009, 113, 16717–16723. [Google Scholar] [CrossRef]
- Dong, F.; Guo, S.; Wang, H.Q.; Li, X.F.; Wu, Z.B. Enhancement of the visible light photocatalytic activity of C-doped TiO2 nanomaterials prepared by a green synthetic approach. J. Phys. Chem. C 2011, 115, 13285–13292. [Google Scholar] [CrossRef]
- Zhou, G.B.; Liu, X.W.; Nan, C.Y.; Liu, Y.X.; Wang, D.S.; Chen, X.Q. C/N-sensitized self-assembly of mesostructured TiO2 nanospheres with significantly enhanced photocatalytic activity. New J. Chem. 2013, 37, 2582–2588. [Google Scholar] [CrossRef]
- Shi, J.W.; Chen, J.W.; Cui, H.J.; Fu, M.L.; Luo, H.Y.; Xu, B.; Ye, Z.L. One template approach to synthesize C-doped titania hollow spheres with high visible-light photocatalytic activity. Chem. Eng. J. 2012, 195–196, 226–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.Y.; Chen, J.R.; Cheng, L.; Chang, J.; Sheng, W.C.; Hu, C.Y.; Cao, S.S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.H.; Li, B.B.; Wang, H.K.; Shi, J.W.; Li, G.D.; Niu, C.M. C-doped mesoporous anatase TiO2 comprising 10 nm crystallites. J. Coll. Interface Sci. 2016, 476, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Purbia, R.; Borah, R.; Paria, S. Carbon-doped mesoporous anatase TiO2 multi-tubes nanostructures for highly improved visible light photocatalytic activity. Inorg. Chem. 2017, 56, 10107–10116. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.J.; Ma, M.G. Synthesis, structure, and applications of lignin-based carbon materials: A review. Sci. Adv. Mater. 2019, 11, 18–32. [Google Scholar] [CrossRef]
- Liu, S.; Ma, C.; Ma, M.G.; Li, J.F. Recent advances in carbon nanomaterials derived from biomass. Sci. Adv. Mater. 2019, 11, 5–17. [Google Scholar] [CrossRef]
- Liu, S.; Fu, L.H.; Liu, Y.J.; Meng, L.Y.; Dong, Y.Y.; Li, Y.Y.; Ma, M.G. Cu/C or Cu2O/C composites: Selective synthesis, characterization, and applications in water treatment. Sci. Adv. Mater. 2016, 8, 2045–2053. [Google Scholar] [CrossRef]
- Wang, B.; Liu, B.; Ji, X.X.; Ma, M.G. Synthesis, characterization, and photocatalytic properties of bamboo charcoal/TiO2 composites using four sizes powder. Materials 2018, 11, 670. [Google Scholar] [CrossRef]
- Liu, B.; Liu, S.; Meng, L.Y.; Li, Y.Y.; Wang, B.; Ma, M.G. Microwave-hydrothermal synthesis and photocatalytic properties of biomass charcoal/TiO2 nanocomposites. J. Saudi Chem. Soc. 2018, 22, 509–518. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.L.; Yang, J.; Wang, B.; Ma, M.G.; Xu, F.; Sun, R.C.; Zhang, X.M. Enhanced photocatalytic activity of CdS-decorated TiO2/carbon core-shell microspheres derived from microcrystalline cellulose. Materials 2016, 9, 245. [Google Scholar] [CrossRef]
- Wang, X.P.; Lim, T.T. Solvothermal synthesis of C–N codoped TiO2 and photocatalytic evaluation for bisphenol A degradation using a visible-light irradiated LED photoreactor. Appl. Catal. B Environ. 2010, 100, 355–364. [Google Scholar] [CrossRef]
- Dolat, D.; Quici, N.; Kusiak-Nejman, E.; Morawski, A.W.; Puma, G.L. One-step, hydrothermal synthesis of nitrogen, carbon co-doped titanium dioxide (N,C TiO2) photocatalysts. Effect of alcohol degree and chain length as carbon dopant precursors on photocatalytic activity and catalyst deactivation. Appl. Catal. B Environ. 2012, 115–116, 81–89. [Google Scholar] [CrossRef]
- Wang, D.H.; Jia, L.; Wu, X.L.; Lu, L.Q.; Xu, A.W. One-step hydrothermal synthesis of N-doped TiO2/C nanocomposites with high visible light photocatalytic activity. Nanoscale 2012, 4, 576–584. [Google Scholar] [CrossRef]
- Wu, D.Y.; Wang, L.Z. Low-temperature synthesis of anatase C-N-TiO2 photocatalyst with enhanced visible-light-induced photocatalytic activity. Appl. Surf. Sci. 2013, 271, 357–361. [Google Scholar] [CrossRef]
- Ming, H.; Huang, H.; Pan, K.M.; Li, H.T.; Liu, Y.; Kang, Z.H. C/TiO2 nanohybrids co-doped by N and their enhanced photocatalytic ability. J. Solid State Chem. 2012, 192, 305–311. [Google Scholar] [CrossRef]
- Wang, M.G.; Han, J.; Hu, Y.M.; Guo, R. Mesoporous C, N-codoped TiO2 hybrid shells with enhanced visible light photocatalytic performance. RSC Adv. 2017, 7, 15513–15520. [Google Scholar] [CrossRef]
- Peng, Y.P.; Chen, H.L.; Huang, C.P. The synergistic effect of photoelectrochemical (PEC) reactions exemplified by concurrent perfluorooctanoic acid (PFOA) degradation and hydrogen generation over carbon and nitrogen codoped TiO2 nanotube arrays (C-N-TNTAs) photoelectrode. Appl. Catal. B-Environ. 2017, 209, 437–446. [Google Scholar] [CrossRef]
- Venkatkarthick, R.; Davidson, D.J.; Vasudevan, S.; Sozhan, G.; Ravichandran, S. An investigation of interfacial and photoelectrochemical performance of thermally prepared C,N-codoped TiO2 photoanodes for water splitting. Chemistryselect 2017, 2, 288–294. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Y.; Zhou, L.G.; Li, X.K.; Guo, X.Y. In situ C,N-codoped mesoporous TiO2 nanocrystallites with high surface areas and worm-like structure for efficient photocatalysis. J. Porous Mater. 2018, 25, 571–579. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, Z.Y.; Wang, Y.B.; Chen, D.M.; Yang, D. Photocatalytic properties of porous C-doped TiO2 and Ag/C-doped TiO2 nanomaterials by eggshell membrane templating. J. Nanopart. Res. 2009, 11, 375–384. [Google Scholar] [CrossRef]
- Zhang, L.; Han, M.D.; Tan, O.K.; Tse, M.S.; Wang, Y.X.; Sze, C.C. Facile fabrication of Ag/C-TiO2 nanoparticles with enhanced visible light photocatalytic activity for disinfection of Escherichia coli and Enterococcus faecalis. J. Mater. Chem. B 2013, 1, 564–570. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, C.X.; Fang, P.F.; Wei, J.H.; Xiong, R. Mo + C co-doped TiO2 using thermal oxidation for enhancing photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Yin, S.; Dong, Q.; Guo, C.S.; Kimura, T.; Matsushita, J.; Sato, T. Photocatalytic properties of Nd and C codoped TiO2 with the whole range of visible light absorption. J. Phys. Chem. C 2013, 117, 8345–8352. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.B.; Tong, Z.W.; Sun, Y.Y.; Jiang, Z.Y. One-pot fabrication of C-Fe-co-doped TiO2 sheets with dominant {001} facets for enhanced visible-light photocatalytic activity. Ind. Engineer. Chem. Res. 2014, 53, 19249–19256. [Google Scholar]
- Guo, M.L. Synergistic effect of C, Ag-codoped TiO2 photocatalyst within the GGA plus U framework. RSC Adv. 2015, 5, 434–439. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, Y.; Zheng, S.K. First principles study on the electronic structure and optical property of Nd-C codoped anatase TiO2. Mater.-Rio De Janeiro 2016, 21, 301–306. [Google Scholar]
- Pham, T.D.; Lee, B.K. Novel capture and photocatalytic conversion of CO2 into solar fuels by metals co-doped TiO2 deposited on PU under visible light. Appl. Catal. A-Gen. 2017, 529, 40–48. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Tichagwa, L.; Mamphweli, S.; Petrik, L. Silver/carbon codoped titanium dioxide photocatalyst for improved dye degradation under visible light. Int. J. Photoenergy 2017, 2017, 3079276. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.Z. Controllable one-pot synthesis and enhanced visible light photocatalytic activity of Tunable C-Cl-Co-doped TiO2 nanocrystals with high surface area. J. Phys. Chem. C 2010, 114, 940–946. [Google Scholar] [CrossRef]
- Bai, H.W.; Kwan, K.S.Y.; Liu, Z.Y.; Song, X.X.; Lee, S.S.; Sun, D.D. Facile synthesis of hierarchically meso/nanoporous S- and C-co-doped TiO2 and its high photocatalytic efficiency in H2 generation. Appl. Catal. B Environ. 2013, 129, 294–300. [Google Scholar] [CrossRef]
- Xu, P.; Xu, T.; Lu, J.; Gao, S.M.; Hosmane, N.S.; Huang, B.B.; Dai, Y.; Wang, Y.B. Visible-light-driven photocatalytic S- and C- codoped meso/nanoporous TiO2. Energy Environ. Sci. 2010, 3, 1128–1134. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H.; Chen, C.; Li, X.; Niu, M.C.; Gao, X.Y.; Yang, Y.T. Effect of calcination temperature on the structure and visible-light photocatalytic activities of (N, S and C) co-doped TiO2 nano-materials. Appl. Surf. Sci. 2015, 332, 172–180. [Google Scholar] [CrossRef]
- Li, Y.H.; Sun, Y.J.; Dong, F.; Ho, W.K. Enhancing the photocatalytic activity of bulk g-C3N4 by introducing mesoporous structure and hybridizing with grapheme. J. Coll. Interface Sci. 2014, 436, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.X.; Sun, J.M. Remarkably enhanced photocatalytic activity of ordered mesoporous carbon/g-C3N4 composite photocatalysts under visible light. Dalton Trans. 2014, 43, 7236–7244. [Google Scholar] [CrossRef]
- Zou, Y.J.; Shi, J.W.; Ma, D.D.; Fan, Z.Y.; Lu, L.; Niu, C.M. In situ synthesis of C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution. Chem. Eng. J. 2017, 322, 435–444. [Google Scholar] [CrossRef]
- Li, Y.P.; Wu, S.L.; Huang, L.Y.; Wang, J.L.; Xu, H.; Li, H.M. Synthesis of carbon-doped g-C3N4 composites with enhanced visible-light photocatalytic activity. Mater. Lett. 2014, 137, 281–284. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, L.X.; Huang, W.M.; Kong, Q.L.; Fan, X.Q.; Wang, M.; Shi, J.L. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light. Carbon 2016, 99, 111–117. [Google Scholar] [CrossRef]
- Wang, F.L.; Chen, P.; Feng, Y.P.; Xie, Z.J.; Liu, Y.; Su, Y.H.; Zhang, Q.X.; Wang, Y.F.; Yao, K.; Lv, W.Y. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B-Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Wen, J.Q.; Xie, J.; Yang, Z.H.; Shen, R.C.; Li, H.Y.; Luo, X.Y.; Chen, X.B.; Li, X. Fabricating the Robust g-C3N4 Nanosheets/carbons/NiS multiple heterojunctions for enhanced photocatalytic H2 generation: An insight into the trifunctional roles of nanocarbons. ACS Sustain. Chem. Eng. 2017, 5, 2224–2236. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Zhang, H.; Yang, B.; Xiao, K.; Guo, T.; Zhang, J.J.; Shao, H.X.; Wang, Y.B.; Yu, G. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation. Appl. Catal. B-Environ. 2018, 233, 35–45. [Google Scholar] [CrossRef]
- Li, S.K.; Huang, F.Z.; Wang, Y.; Shen, Y.H.; Qiu, L.G.; Xie, A.J.; Xu, S.J. Magnetic Fe3O4@C@Cu2O composites with bean-like core/shell nanostructures: Synthesis, properties and application in recyclable photocatalytic degradation of dye pollutants. J. Mater. Chem. 2011, 21, 7459–7466. [Google Scholar] [CrossRef]
- Zhou, M.J.; Hu, Y.; Liu, Y.; Yang, W.L.; Qian, H.S. Microwave-assisted route to fabricate coaxial ZnO/C/CdS nanocables with enhanced visible light-driven photocatalytic activity. CrystEngComm 2012, 14, 7686–7693. [Google Scholar] [CrossRef]
- Cai, J.B.; Wu, X.Q.; Li, S.X.; Zheng, F.Y.; Zhu, L.C.; Lai, Z.H. Synergistic effect of double-shelled and sandwiched TiO2@Au@C hollow spheres with enhanced visible-light-driven photocatalytic activity. ACS Appl. Mater. Interfaces 2015, 7, 3764–3772. [Google Scholar] [CrossRef]
- Yang, S.J.; Im, J.H.; Kim, T.; Lee, K.; Park, C.R. MOF-derived ZnO and ZnO@C composites with high photocatalytic activity and adsorption capacity. J. Hazard. Mater. 2011, 186, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, B.B.; Zhao, Z.B.; Yu, C.; Hu, C.; Wu, S.J.; Qiu, J.S. Furfural-induced hydrothermal synthesis of ZnO@C gemel hexagonal microrods with enhanced photocatalytic activity and stability. ACS Appl. Mater. Interfaces 2014, 6, 8560–8566. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.S.; Xue, J.J.; Zhou, Y.M.; Zhang, Z.W.; Wu, X. A facile route for the preparation of ZnO/C composites with high photocatalytic activity and adsorption capacity. CrystEngComm 2014, 16, 4478. [Google Scholar] [CrossRef]
- Mu, J.B.; Guo, Z.C.; Che, H.W.; Zhang, X.L.; Bai, Y.M.; Hou, J.X. Electrospinning of C-doped ZnO nanofibers with high visible-light photocatalytic activity. J. Sol-Gel Sci. Technol. 2016, 78, 99–109. [Google Scholar] [CrossRef]
- Wang, S.B.; Zhang, X.W.; Li, S.; Fang, Y.; Pan, L.; Zou, J.J. C-doped ZnO ball-in-ball hollow microspheres for efficient photocatalytic and photoelectrochemical applications. J. Hazard. Mater. 2017, 331, 235–245. [Google Scholar] [CrossRef]
- Chen, Y.L.; Cao, X.X.; Kuang, J.D.; Chen, Z.; Chen, J.L.; Lin, B.Z. The gas-phase photocatalytic mineralization of benzene over visible-light-driven Bi2WO6@C microspheres. Catal. Commun. 2010, 12, 247–250. [Google Scholar] [CrossRef]
- Sun, H.G.; Zhao, X.; Zhang, L.; Fan, W.L. Origin of the enhanced visible photocatalytic activity in (N, C)-codoped ZnS studied from density functional theory. J. Phys. Chem. C 2011, 115, 2218–2227. [Google Scholar] [CrossRef]
- Lu, H.X.; Lei, J.; Li, X.X.; Shao, G.; Hou, T.C.; Fan, B.B.; Chen, D.L.; Zhang, L.W.; Wang, H.L.; Xu, H.L. Synthesis and characterization of carbon-doped ZnSn(OH)6 with enhanced photoactivity by hydrothermal method. Cryst. Res. Technol. 2016, 51, 11–15. [Google Scholar] [CrossRef]












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.-J.; Ma, M.-G.; Zhu, J.-F. Recent Development of Photocatalysts Containing Carbon Species: A Review. Catalysts 2019, 9, 20. https://doi.org/10.3390/catal9010020
Shi Z-J, Ma M-G, Zhu J-F. Recent Development of Photocatalysts Containing Carbon Species: A Review. Catalysts. 2019; 9(1):20. https://doi.org/10.3390/catal9010020
Chicago/Turabian StyleShi, Zheng-Jun, Ming-Guo Ma, and Jie-Fang Zhu. 2019. "Recent Development of Photocatalysts Containing Carbon Species: A Review" Catalysts 9, no. 1: 20. https://doi.org/10.3390/catal9010020