A Zn-MOF-Catalyzed Terpolymerization of Propylene Oxide, CO2, and β-butyrolactone
Abstract
:1. Introduction
2. Results and Discussion
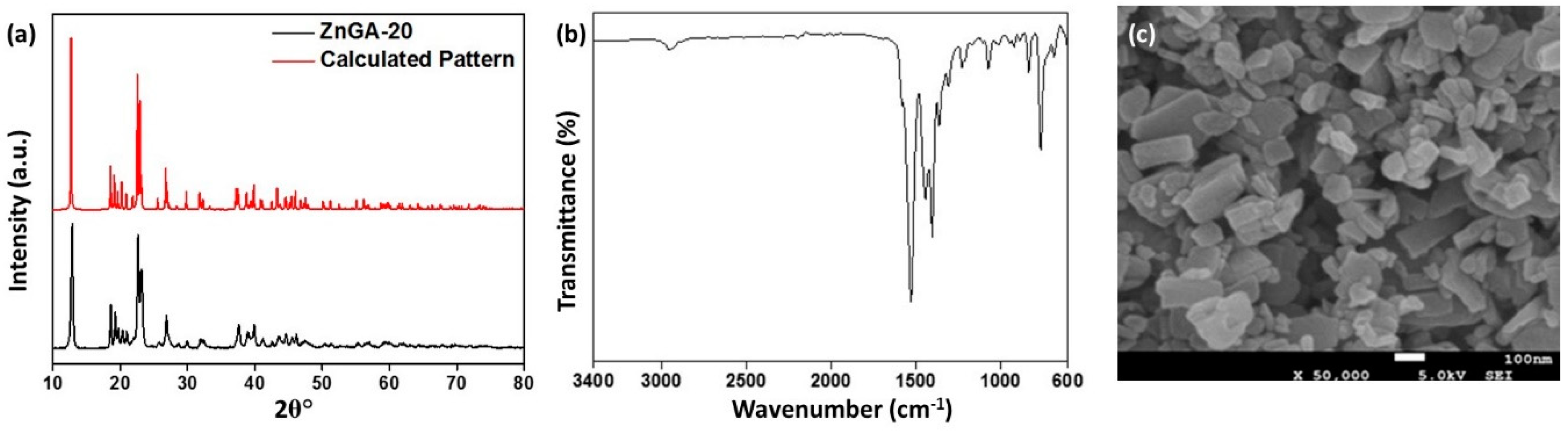
2.1. Synthesis and Characterization of Nano-Sized ZnGA
2.2. Terpolymerization of PO, BBL, and CO2 using ZnGA-20 as the Catalyst
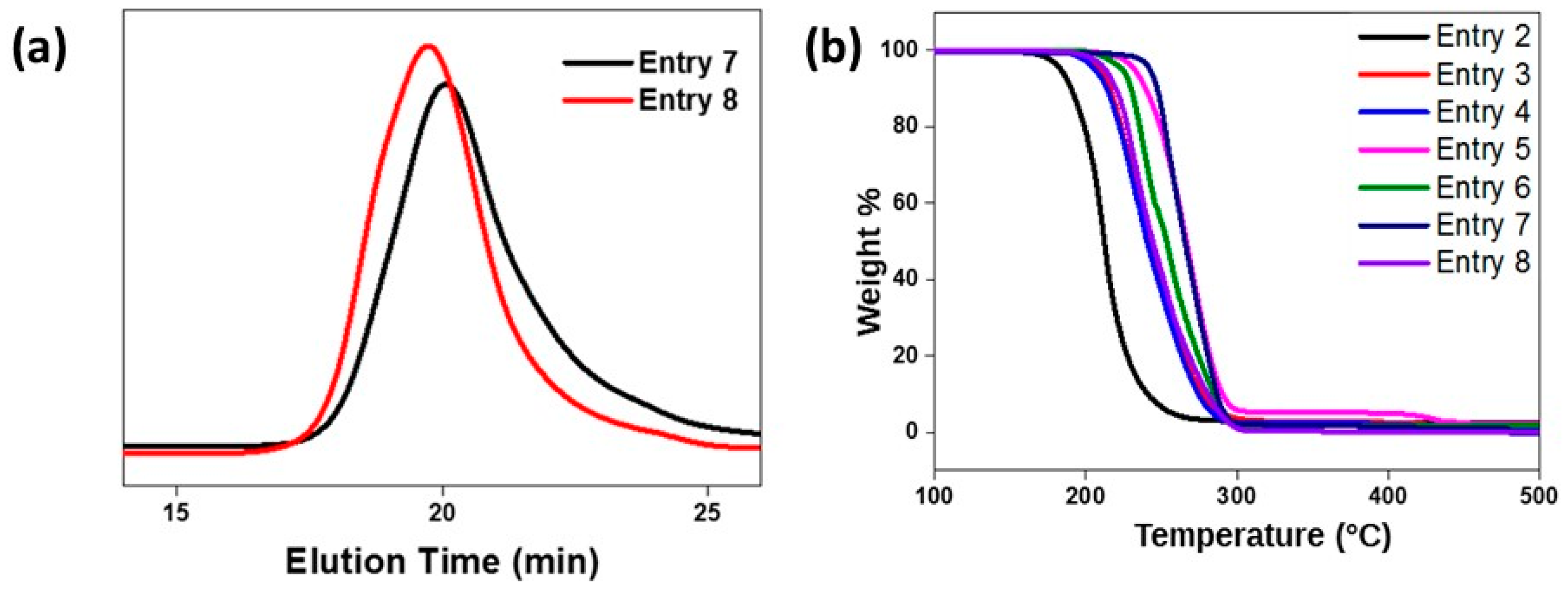
2.3. Properties of the Terpolymers
3. Materials and Methods
3.1. Synthesis of ZnGA-20
3.2. General Procedure for the Copolymerization of CO2 and PO
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Scharfenberg, M.; Hilf, J.; Frey, H. Functional polycarbonates from carbon dioxide and tailored epoxide monomers: Degradable materials and their application potential. Adv. Funct. Mater. 2018. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci. B Polym. Lett. 1969, 7, 287–292. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide with organometallic compounds. Makromolekul. Chem. 1969, 130, 210–220. [Google Scholar] [CrossRef]
- Soga, K.; Imai, E.; Hattori, I. Alternating copolymerization of CO2 and propylene-oxide with the catalysts prepared from Zn(OH)2 and various dicarboxylic-acids. Polym. J. 1981, 13, 407–410. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Stafford, N.W.; Katsurao, T. Supercritical carbon-dioxide as solvent for the copolymerization of carbon-dioxide and propylene-oxide using a heterogeneous zinc carboxylate catalyst. J. Mol. Catal. A Chem. 1995, 104, L1–L4. [Google Scholar] [CrossRef]
- Ree, M.; Bae, J.Y.; Jung, J.H.; Shin, T.J. A new copolymerization process leading to poly(propylene carbonate) with a highly enhanced yield from carbon dioxide and propylene oxide. J. Polym. Sci. A Polym. Chem. 1999, 37, 1863–1876. [Google Scholar] [CrossRef]
- Luinstra, G.A. Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: Catalysis and material properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Ang, R.R.; Sin, L.T.; Bee, S.T.; Tee, T.T.; Kadhum, A.A.H.; Rahmat, A.R.; Wasmi, B.A. Determination of zinc glutarate complexes synthesis factors affecting production of propylene carbonate from carbon dioxide and propylene oxide. Chem. Eng. J. 2017, 327, 120–127. [Google Scholar] [CrossRef]
- Scott, D.A.; Christopher, M.B.; Geoffrey, W.C. Carbon dioxide as a renewable C1 feedstock: Synthesis and characterization of polycarbonates from the alternating copolymerization of epoxides and CO2. In Feedstocks for the Future; Joseph, J.B., Martin, K.P., Eds.; American Chemical Society: Washington, DC, USA, 2006; Chapter 9; Volume 921, pp. 116–129. [Google Scholar]
- Van der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef] [Green Version]
- Sudakar, P.; Sivanesan, D.; Yoon, S. Copolymerization of epichlorohydrin and CO2 using zinc glutarate: An additional application of ZnGA in polycarbonate synthesis. Macromol. Rapid Commun. 2016, 37, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Luinstra, G.A.; Haas, G.R.; Molnar, F.; Bernhart, V.; Eberhardt, R.; Rieger, B. On the formation of aliphatic polycarbonates from epoxides with chromium (iii) and aluminum (iii) metal-salen complexes. Chem.-Eur. J. 2005, 11, 6298–6314. [Google Scholar] [CrossRef] [PubMed]
- Luinstra, G.A.; Borchardt, E. Material properties of poly(propylene carbonates). Adv. Polym. Sci. 2012, 245, 29–48. [Google Scholar]
- Kobayashi, M.; Inoue, S.; Tsuruta, T. Diethylzinc-dihydric phenol system as catalyst for copolymerization of carbon dioxide with propylene oxide. Macromolecules 1971, 4, 658–659. [Google Scholar] [CrossRef]
- Inoue, S.; Tsuruta, T.; Kobayashi, M.; Koinuma, H. Reactivities of some organozinc initiators for copolymerization of carbon-dioxide and propylene oxide. Makromolekul. Chem. 1972, 155. [Google Scholar] [CrossRef]
- Kobayashi, M.; Inoue, S.; Tsuruta, T. Copolymerization of carbon-dioxide and epoxide by dialkylzinc-carboxylic acid system. J. Polym. Sci. A Polym. Chem. 1973, 11, 2383–2385. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tang, Y.L.; Tsuruta, T.; Inoue, S. Copolymerization of carbon-dioxide and epoxide using dialkylzinc-dihydric phenol system as catalyst. Makromol. Chem. 1973, 169, 69–81. [Google Scholar] [CrossRef]
- Kuran, W.; Pasynkiewicz, S.; Skupinska, J. Investigations on catalytic systems diethylzinc-dihydroxybenzenes and trihydroxybenzenes in copolymerization of carbon-dioxide with propylene-oxide. Makromol. Chem. 1976, 177, 1283–1292. [Google Scholar] [CrossRef]
- Kuran, W.; Pasynkiewicz, S.; Skupinska, J.; Rokicki, A. Alternating copolymerization of carbon-dioxide and propylene-oxide in presence of organometallic catalysts. Makromol. Chem. 1976, 177, 11–20. [Google Scholar] [CrossRef]
- Soga, K.; Uenishi, K.; Hosoda, S.; Ikeda, S. Copolymerization of carbon-dioxide and propylene-oxide with new catalysts. Makromol. Chem. 1977, 178, 893–897. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making plastics from carbon dioxide: Salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Fitch, S.B. (tetramethyltetraazaannulene) chromium chloride: A highly active catalyst for the alternating copolymerization of epoxides and carbon dioxide. Inorg. Chem. 2007, 46, 5474–5476. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Frantz, E.B. Manganese(III) schiff base complexes: Chemistry relevant to the copolymerization of epoxides and carbon dioxide. Inorg. Chem. 2007, 46, 5967–5978. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.R.; Sin, L.T.; Bee, S.T.; Tee, T.T.; Kadhum, A.A.H.; Rahmat, A.R.; Wasmi, B.A. A review of copolymerization of green house gas carbon dioxide and oxiranes to produce polycarbonate. J. Clean. Prod. 2015, 102, 1–17. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A 2016. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Romain, C.; Shaw, J.; Williams, C.K. Sequence selective polymerization catalysis: A new route to aba block copoly(ester-b-carbonate-b-ester). Macromolecules 2015, 48, 6047–6056. [Google Scholar] [CrossRef]
- Romain, C.; Zhu, Y.; Dingwall, P.; Paul, S.; Rzepa, H.S.; Buchard, A.; Williams, C.K. Chemoselective polymerizations from mixtures of epoxide, lactone, anhydride, and carbon dioxide. J. Am. Chem. Soc. 2016, 138, 4120–4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romain, C.; Williams, C.K. Chemoselective polymerization control: From mixed-monomer feedstock to copolymers. Angew. Chem. Int. Ed. 2014, 53, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.T.; Jung, J.W.; Ree, M.; Kim, H. Terpolymerization of CO2 with propylene oxide and epsilon-caprolactone using zinc glutarate catalyst. Macromolecules 2003, 36, 8210–8212. [Google Scholar] [CrossRef]
- Ree, M.; Hwang, Y.; Kim, J.S.; Kim, H.; Kim, G.; Kim, H. New findings in the catalytic activity of zinc glutarate and its application in the chemical fixation of CO2 into polycarbonates and their derivatives. Catal. Today 2006, 115, 134–145. [Google Scholar] [CrossRef]
- Zou, Y.N.; Xiao, M.; Li, X.H.; Wang, S.J.; Meng, Y.Z. Biodegradability enhanced terpolymer of propylene oxide and ethylene oxide with carbon dioxide using zinc glutarate as catalyst. Polym. Polym. Compos. 2007, 15, 53–58. [Google Scholar] [CrossRef]
- Song, P.F.; Xiao, M.; Du, F.G.; Wang, S.J.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Synthesis and properties of aliphatic polycarbonates derived from carbon dioxide, propylene oxide and maleic anhydride. J. Appl. Polym. Sci. 2008, 109, 4121–4129. [Google Scholar] [CrossRef]
- Song, P.F.; Wang, S.J.; Xiao, M.; Du, F.G.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Cross-linkable and thermally stable aliphatic polycarbonates derived from CO2, propylene oxide and maleic anhydride. J. Polym. Res. 2009, 16, 91–97. [Google Scholar] [CrossRef]
- Wu, J.S.; Xiao, M.; He, H.; Wang, S.J.; Han, D.M.; Meng, Y.Z. Copolymerization of propylene oxide and carbon dioxide in the presence of diphenylmethane diisoyanate. J. Polym. Res. 2011, 18, 1479–1486. [Google Scholar] [CrossRef]
- Liu, Y.L.; Xiao, M.; Wang, S.J.; Xia, L.; Hang, D.M.; Cui, G.F.; Meng, Y.Z. Mechanism studies of terpolymerization of phthalic anhydride, propylene epoxide, and carbon dioxide catalyzed by ZnGA. RSC Adv. 2014, 4, 9503–9508. [Google Scholar] [CrossRef]
- Nornberg, B.; Luinstra, G.A. Influence of norbornene dicarboxylic anhydride on the copolymerization of carbon dioxide and propylene oxide. Eur. Polym. J. 2015, 73, 297–307. [Google Scholar] [CrossRef]
- Tang, L.; Luo, W.H.; Xiao, M.; Wang, S.J.; Meng, Y.Z. One-pot synthesis of terpolymers with long l-lactide rich sequence derived from propylene oxide, CO2, and l-lactide catalyzed by zinc adipate. J. Polym. Sci. Pol. A Chem. 2015, 53, 1734–1741. [Google Scholar] [CrossRef]
- Luo, W.H.; Xiao, M.; Wang, S.J.; Han, D.M.; Meng, Y.Z. Gradient terpolymers with long epsilon-caprolactone rich sequence derived from propylene oxide, CO2, and ε-caprolactone catalyzed by zinc glutarate. Eur. Polym. J. 2016, 84, 245–255. [Google Scholar] [CrossRef]
- Song, P.F.; Xu, H.D.; Mao, X.D.; Liu, X.J.; Wang, L. A one-step strategy for aliphatic poly(carbonate-ester)s with high performance derived from CO2, propylene oxide and l-lactide. Polym. Adv. Technol. 2017, 28, 736–741. [Google Scholar] [CrossRef]
- Hwang, Y.; Kim, H.; Ree, M. Zinc glutarate catalyzed synthesis and biodegradability of poly(carbonate-co-ester)s from CO2, propylene oxide, and ε-caprolactone. Macromol. Symp. 2005, 224, 227–237. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Mecking, S. Nature or petrochemistry? Biologically degradable materials. Angew. Chem. Int. Ed. 2004, 43, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.B.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Carpentier, J.-F. Discrete metal catalysts for stereoselective ring-opening polymerization of chiral racemic β-lactones. Macromol. Rapid Commun. 2010, 31, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.A. Applications of phb—A microbially produced biodegradable thermoplastic. Phys. Technol. 1985, 16, 32–36. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Rieth, L.R.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. Single-site beta-diiminate zinc catalysts for the ring-opening polymerization of beta-butyrolactone and beta-valerolactone to poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 2002, 124, 15239–15248. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.W.; Coates, G.W. Fluorinated beta-lactones and poly(beta-hydroxyalkanoate)s: Synthesis via epoxide carbonylation and ring-opening polymerization. Tetrahedron 2008, 64, 6973–6978. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.W.; Treitler, D.S.; Dunn, E.W.; Castro, P.M.; Roisnel, T.; Thomas, C.M.; Coates, G.W. Polymerization of enantiopure monomers using syndiospecific catalysts: A new approach to sequence control in polymer synthesis. J. Am. Chem. Soc. 2009, 131, 16042–16044. [Google Scholar] [CrossRef] [PubMed]
- Brule, E.; Gaillard, S.; Rager, M.N.; Roisnel, T.; Guerineau, V.; Nolan, S.P.; Thomas, C.M. Polymerization of racemic beta-butyrolactone using gold catalysts: A simple access to biodegradable polymers. Organometallics 2011, 30, 2650–2653. [Google Scholar] [CrossRef]
- Ebrahimi, T.; Aluthge, D.C.; Hatzildriakos, S.G.; Mehrkhodavandi, P. Highly active chiral zinc catalysts for immortal polymerization of beta-butyrolactone form melt processable syndio-rich poly(hydroxybutyrate). Macromolecules 2016, 49, 8812–8824. [Google Scholar] [CrossRef]
- Rajendiran, S.; Natarajan, P.; Yoon, S. A covalent triazine framework-based heterogenized al-co bimetallic catalyst for the ring-expansion carbonylation of epoxide to beta-lactone. RSC Adv. 2017, 7, 4635–4638. [Google Scholar] [CrossRef]
- Rajendiran, S.; Park, G.; Yoon, S. Direct conversion of propylene oxide to 3-hydroxy butyric acid using a cobalt carbonyl ionic liquid catalyst. Catalysts 2017, 7, 228. [Google Scholar] [CrossRef]
- Kernbichl, S.; Reiter, M.; Adams, F.; Vagin, S.; Rieger, B. CO2-controlled one-pot synthesis of AB, ABA block, and statistical terpolymers from β-butyrolactone, epoxides, and CO2. J. Am. Chem. Soc. 2017, 139, 6787–6790. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Q.; Lin, J.L.; Zhang, H.L. Crystal structure of zinc glutarate, Zn(C5H6H4). Z. Krist.-New Cryst. Struct. 2000, 215, 535–536. [Google Scholar]
- Jiang, Z.-R.; Wang, H.; Hu, Y.; Lu, J.; Jiang, H.-L. Polar group and defect engineering in a metal–organic framework: Synergistic promotion of carbon dioxide sorption and conversion. ChemSusChem 2015, 8, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Toyao, T.; Fujiwaki, M.; Miyahara, K.; Kim, T.-H.; Horiuchi, Y.; Matsuoka, M. Design of zeolitic imidazolate framework derived nitrogen-doped nanoporous carbons containing metal species for carbon dioxide fixation reactions. ChemSusChem 2015, 8, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Chen, S.; Liu, X.-Q.; Sun, L.-B.; Lu, J.; Jiang, H.-L. Metal–organic framework-templated catalyst: Synergy in multiple sites for catalytic CO2 fixation. ChemSusChem 2017, 10, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.-S.; Shi, Y.; Xu, H.; Zhao, B. A multifunctional mof as a recyclable catalyst for the fixation of CO2 with aziridines or epoxides and as a luminescent probe of cr(vi). Dalton Trans. 2018, 47, 4545–4553. [Google Scholar] [CrossRef] [PubMed]

| Entry | Zn:PO:BBL | CO2 (MPa) | TON b | Conversion (%) c | PPC (%) | PHB (%) | PE (%) d | Mn (kg/mol) e | PDI e | Tg (°C) f | T5% (°C) g | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PO | BBL | |||||||||||
| 1 h | 1:15:13 | 1.5 | 7.3 | 58.0 | 46.5 | 62.0 | 36.0 | 2.5 | -- | -- | -- | -- |
| 2 | 1:90:10 | 1.5 | 24.0 | 47.4 | 21.6 | 93.0 | 4.0 | 3.0 | 69.8 | 3.7 | 37 | 183.4 |
| 3 | 1:75:25 | 1.5 | 24.6 | 54.6 | 22.4 | 87.0 | 10.0 | 3.0 | 45.1 | 3.5 | 34 | 209.9 |
| 4 | 1:50:50 | 1.5 | 18.5 | 58.1 | 12.6 | 82.0 | 15.0 | 2.0 | 41.4 | 4.9 | 30 | 206.1 |
| 5 | 1:25:75 | 1.5 | 9.5 | 48.6 | 8.3 | 67.0 | 29.0 | 3.0 | 25.9 | 5.6 | 20 | 231.9 |
| 6 | 1:202.5:67.5 | 1.5 | 60.8 | 49.4 | 22.4 | 86.0 | 11.0 | 4.0 | 127.5 | 2.5 | 34 | 224.2 |
| 7 | 1:135:135 | 1.5 | 67.5 | 78.5 | 19.3 | 82.0 | 17.0 | 2.0 | 83.5 | 2.6 | 35 | 242.2 |
| 8 | 1:202.5:67.5 | 3.0 | 74.4 | 62.5 | 25.0 | 88.0 | 10.0 | 2.0 | 123.0 | 2.6 | 41 | 212.8 |
| 9 i | 1:202.5:67.5 | 15 | na | 0.0 | 0.0 | nd | nd | nd | na | na | na | na |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padmanaban, S.; Dharmalingam, S.; Yoon, S. A Zn-MOF-Catalyzed Terpolymerization of Propylene Oxide, CO2, and β-butyrolactone. Catalysts 2018, 8, 393. https://doi.org/10.3390/catal8090393
Padmanaban S, Dharmalingam S, Yoon S. A Zn-MOF-Catalyzed Terpolymerization of Propylene Oxide, CO2, and β-butyrolactone. Catalysts. 2018; 8(9):393. https://doi.org/10.3390/catal8090393
Chicago/Turabian StylePadmanaban, Sudakar, Sivanesan Dharmalingam, and Sungho Yoon. 2018. "A Zn-MOF-Catalyzed Terpolymerization of Propylene Oxide, CO2, and β-butyrolactone" Catalysts 8, no. 9: 393. https://doi.org/10.3390/catal8090393





