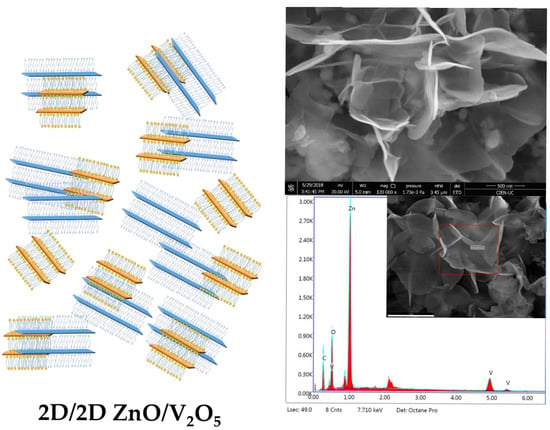
Enhancement Photocatalytic Activity of the Heterojunction of Two-Dimensional Hybrid Semiconductors ZnO/V2O5
Abstract
:1. Introduction
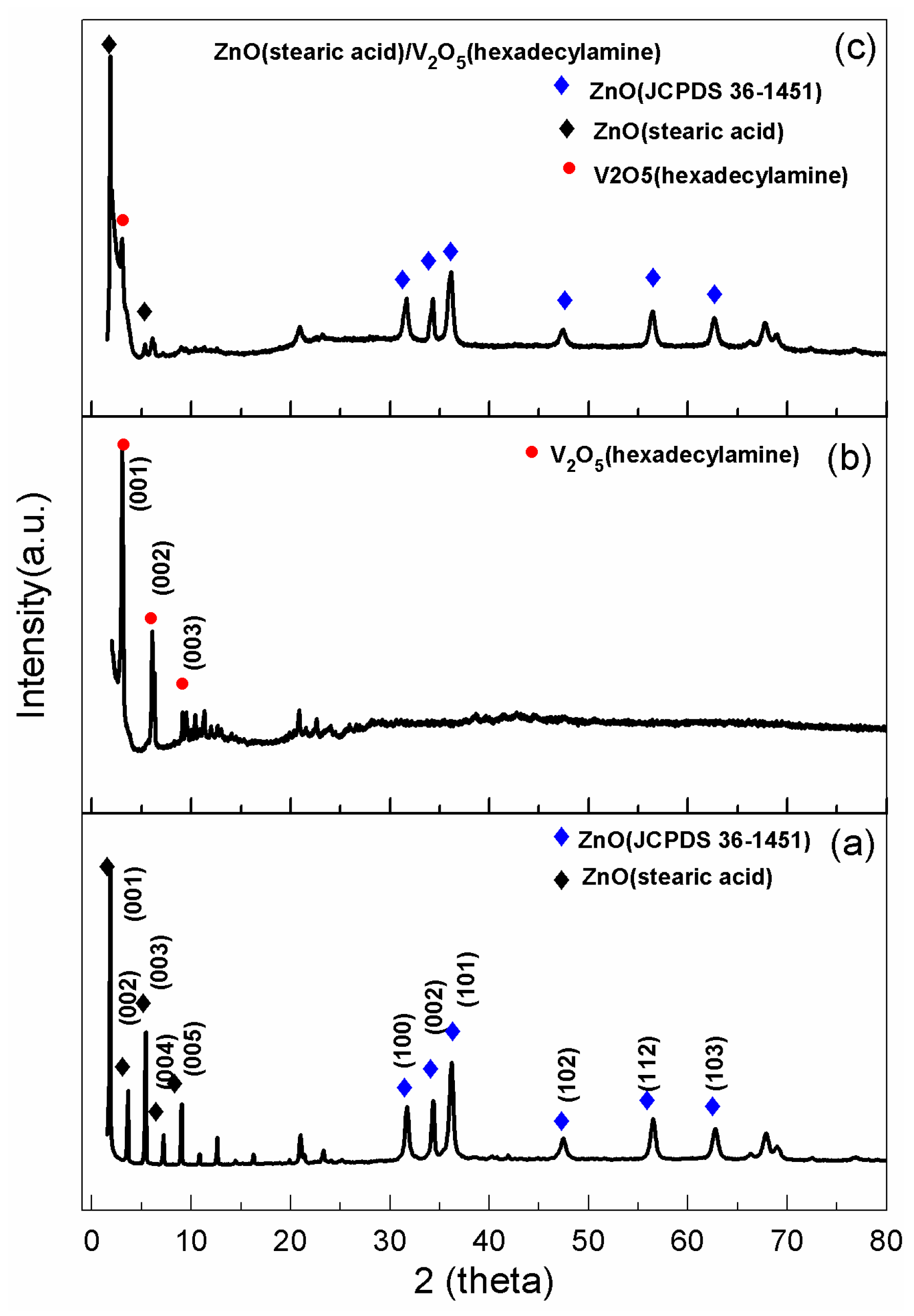
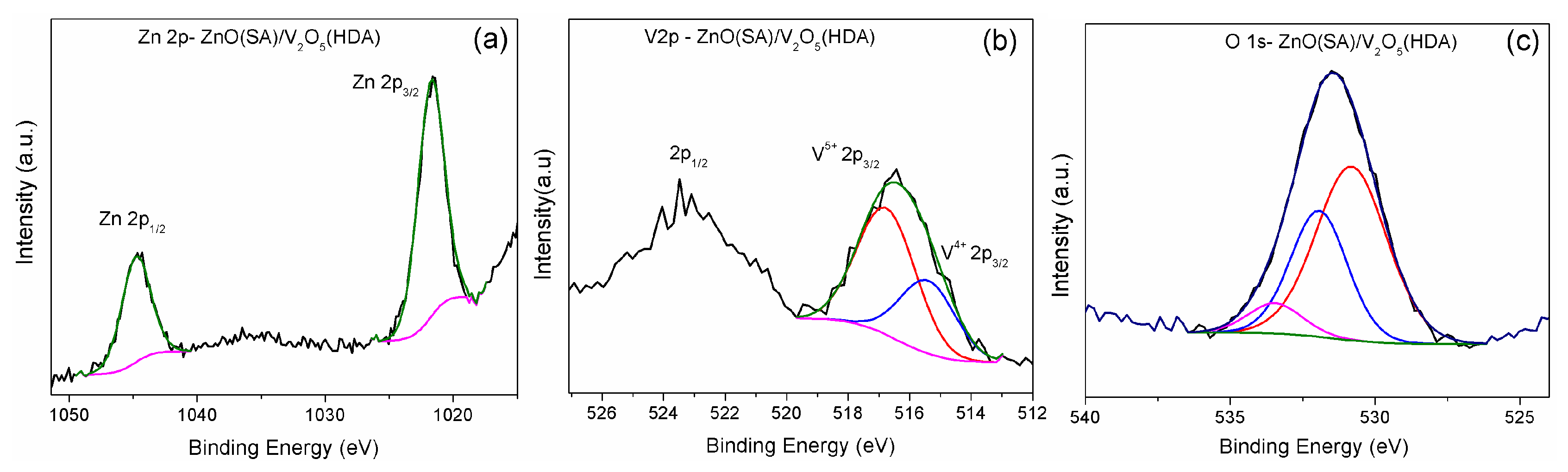
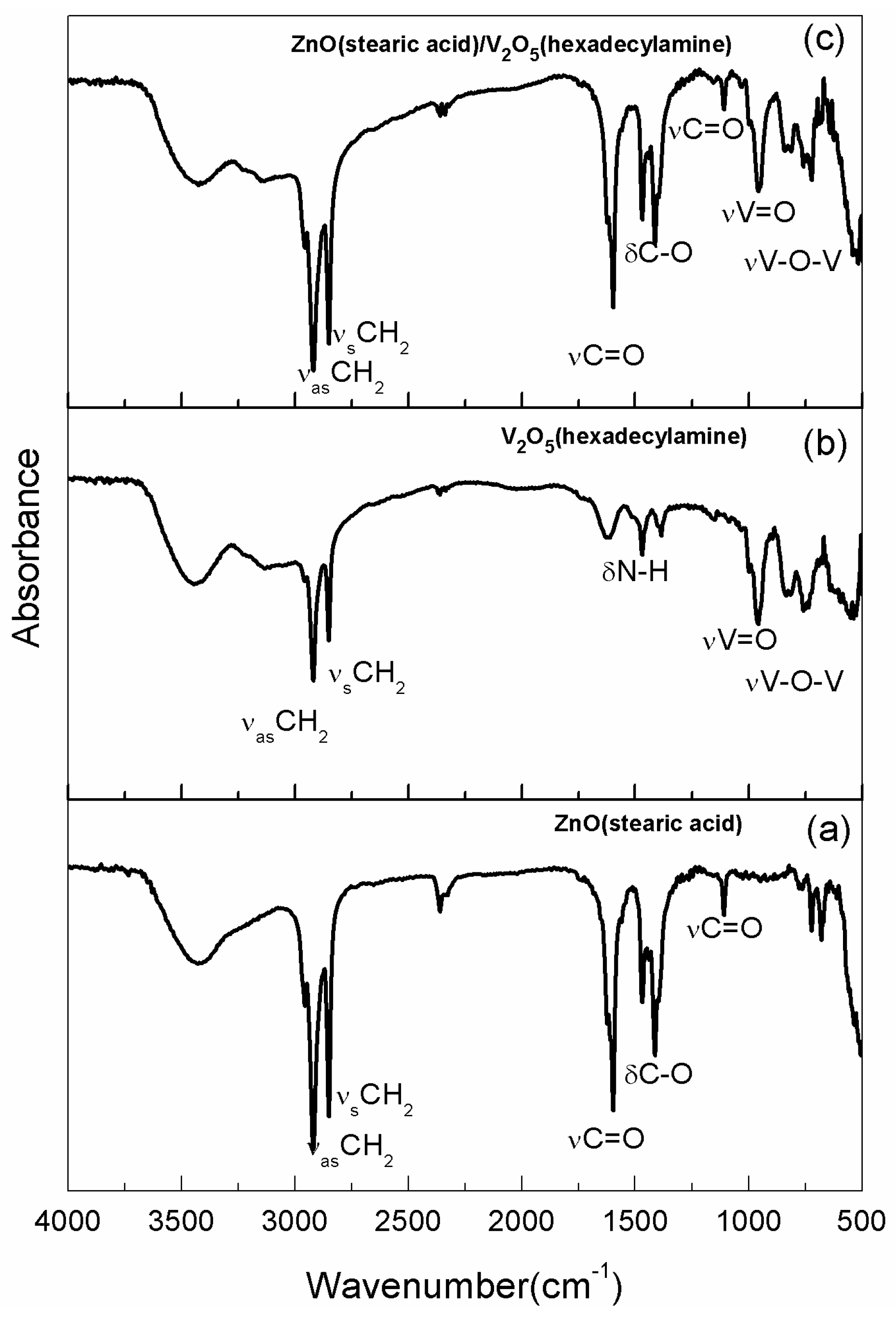
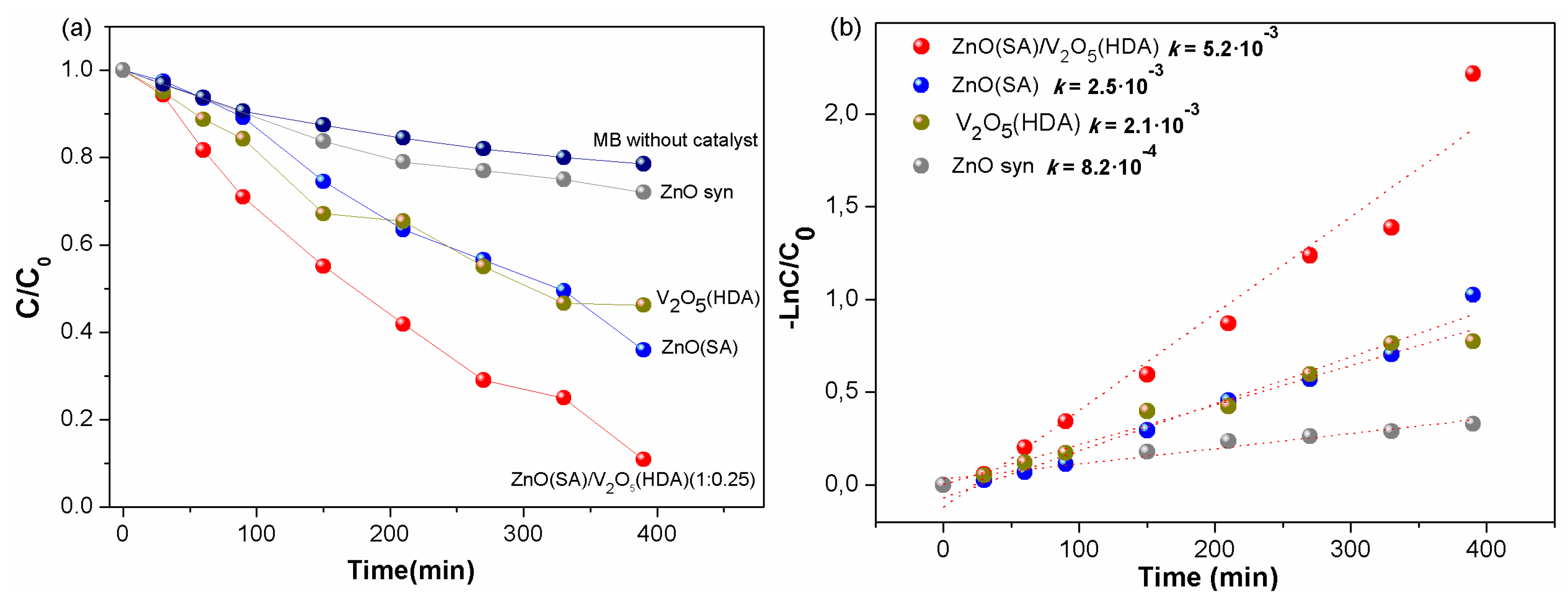
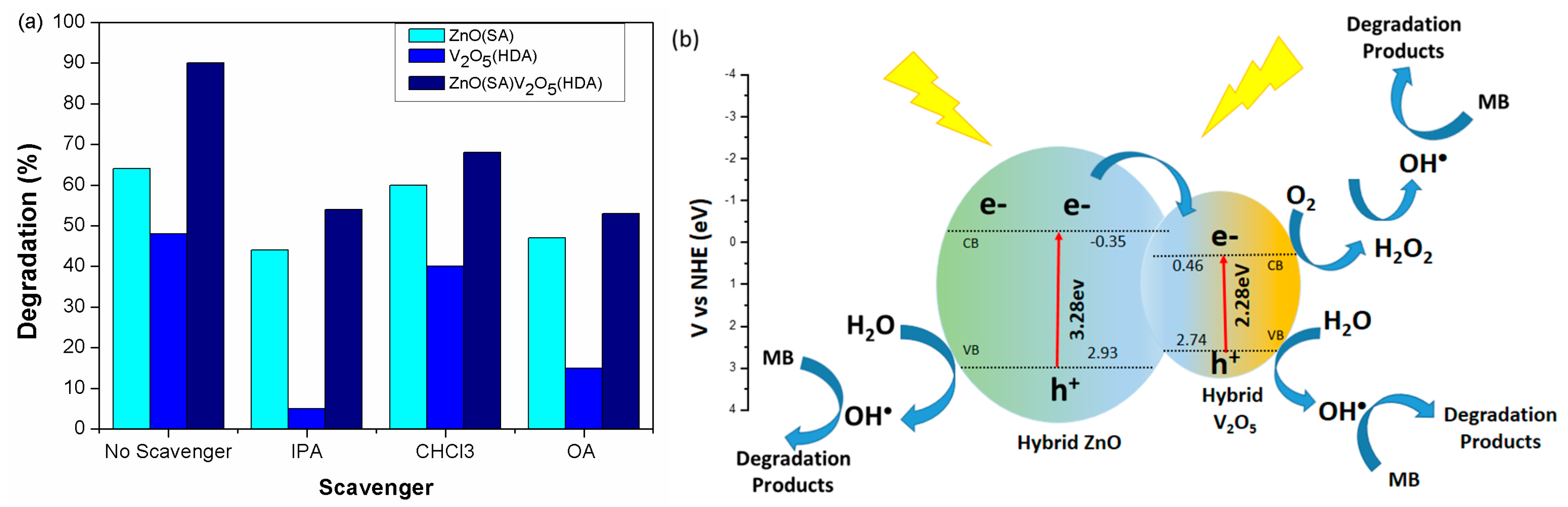
2. Results and Discussion
Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Experimental-Synthesis Nanocomposites
3.2.1. Synthesis of ZnO(Stearic Acid)-ZnO(SA)
3.2.2. Synthesis of Compounds Vanadium Pentoxide
3.3. Photocatalytic Experiments
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chan, S.H.; Wu, T.Y.; Juan, J.C.; The, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Ray, C.; Pal, T. Recent advances of metal–metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–219. [Google Scholar] [CrossRef]
- Hernandez, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tresso, E.; Saracco, G. Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, J.; Chen, R.; Zhou, D.; Xiang, L. A Review on the Fabrication of Hierarchical ZnO Nanostructures for Photocatalysis Application. Crystals 2016, 6, 148. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-Cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. App. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of Azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. App. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Dong, D.; Yang, P. Formation of nanoplate-based clew-like ZnO mesocrystals and their photocatalysis application. RSC Adv. 2016, 6, 51544–51551. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xu, P.; Zhu, Y.; Chen, X.; Yu, W. Decoration of ZnO nanowires with Pt nanoparticles and their improved gas sensing and photocatalytic performance. Nanotechnology 2010, 21, 285501–285508. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Mendoza, E.; Nuñez-Briones, A.G.; García-Cerda, L.A.; Peralta-Rodríguez, R.D.; Montes-Luna, A.J. One-step synthesis of ZnO and Ag/ZnO heterostructures and their photocatalytic activity. Ceram. Int. 2018, 44, 6176–6180. [Google Scholar] [CrossRef]
- Mani, J.; Sakeek, H.; Habouti, S.; Dietze, M.; Es-Souni, M. Macro–meso-porous TiO2, ZnO and ZnO-TiO2 composite thick films. Properties and application to photocatalysis. Catal. Sci. Technol. 2012, 2, 379–385. [Google Scholar] [CrossRef]
- Balachandran, S.; Prakash, N.; Thirumalai, K.; Muruganandham, M.; Sillanpää, M.; Swaminathan, M. Facile construction of heterostructured BiVO4–ZnO and its dual application of greater solar photocatalytic activity and self-cleaning property. Ind. Eng. Chem. Res. 2014, 53, 8346–8356. [Google Scholar] [CrossRef]
- Sun, C.; Fu, Y.; Wang, Q.; Xing, L.; Liu, B.; Xue, X. Ultrafast piezo-photocatalytic degradation of organic pollutions by Ag2O/tetrapod-ZnO nanostructures under ultrasonic/UV exposure. RSC Adv. 2016, 6, 87446–87453. [Google Scholar] [CrossRef]
- Kandjani, K.; Sabri, Y.; Periasamy, S.; Zohora, N.; Amin, M.; Nafady, A.; Bhargava, S. Controlling core/shell formation of nanocubic p-Cu2O/n-ZnO toward enhanced photocatalytic performance. Langmuir 2015, 39, 10922–10930. [Google Scholar] [CrossRef] [PubMed]
- Vikas, L.S.; Vanaja, K.A.; Subha, P.P.; Jayaraj, M.K. Fast UV sensing properties of n-ZnO nanorods/p-GaN heterojunction. Sens. Actuators A 2016, 242, 116–122. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarkar, D.; Madras, G. Highly efficient WO3–ZnO mixed oxides for photocatalysis. RSC Adv. 2015, 5, 11895–11904. [Google Scholar] [CrossRef]
- Ahmeda, L.T.; Ma, M.; Edvinsson, T.; Zhua, J. A facile approach to ZnO/CdS nanoarrays and their photocatalytic and photoelectrochemical properties. App. Catal. B Environ. 2013, 138–139, 175–183. [Google Scholar]
- Zaera, F. New challenges in heterogeneous catalysis for the 21st century. Catal. Lett. 2012, 142, 501–516. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Samadi, M.; Yousefzadeh, S.; Soltani, M.; Rahimi, A.; Chou, T.; Chen, L.; Chen, K.; Moshfegh, A. Improved solar-driven photocatalytic activity of hybrid graphene quantum dots/ZnO nanowires: A direct Z-scheme mechanism. ACS Sustain. Chem. Eng. 2017, 5, 367–375. [Google Scholar] [CrossRef]
- Colombo, E.; Li, W.; Bhangu, S.; Ashokkumar, M. Chitosan microspheres as a template for TiO2 and ZnO microparticles: Studies on mechanism, functionalization and applications in photocatalysis and H2S removal. RSC Adv. 2017, 7, 19373–19383. [Google Scholar] [CrossRef]
- Anastasio, P.; Del Giacco, T.; Germani, R.; Spretic, N.; Tiecco, M. Structure effects of amphiphilic and non-amphiphilic quaternary ammonium salts on photodegradation of Alizarin Red-S catalysed by titanium dioxide. RSC Adv. 2017, 7, 361–368. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.L.; Sa, B.; Ahujad, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Yuan, C.; Hung, C.; Yuan, C.; Wen, H. Preparation and application of immobilized surfactant-modified PANi-CNT/TiO2 under visible-light irradiation. Materials 2017, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yuan, C.; Chen, C. Photocatalytic degradation of pesticide pyridaben. 3. In surfactant/TiO2 aqueous dispersions. Environ. Sci. Technol. 2007, 41, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Duta, M.; Perniu, D.; Duta, A. Photocatalytic zinc oxide thin films obtained by surfactant assisted spray pyrolysis deposition. App. Surf. Sci. 2014, 306, 80–88. [Google Scholar] [CrossRef]
- Hao, C.; Wang, J.; Cheng, Q.; Bai, Y.; Wang, X.; Yang, Y. Anionic surfactants-assisted solution-phase synthesis of ZnO with improved photocatalytic performance. J. Photochem. Photobiol. A Chem. 2017, 332, 384–390. [Google Scholar] [CrossRef]
- Benavente, E.; Navas, D.; Devis, S.; Segovia, M.; Sotomayor-Torres, C.; González, G. Composites of laminar nanostructured ZnO and VOx-Nanotubes hybrid as visible light active photocatalysts. Catalysts 2018, 8, 93. [Google Scholar] [CrossRef]
- Yin, H.; Yu, K.; Hu, J.; Song, C.; Guo, B.; Wang, Z.; Zhu, Z. Novel photoluminescence properties and enhanced photocatalytic activities for V2O5-loaded ZnO nanorods. Dalton Trans. 2015, 10, 4671–4678. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.W.; Rao, Y.F.; Alyamani, A.; Chu, W.; Chen, M.J.; Patterson, D.A.; Emanuelsson, E.A.; Gao, W. Heterogeneous lollipop-like V2O5/ZnO Array: A promising composite nanostructure for visible light photocatalysis. Langmuir 2010, 14, 11615–11620. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ismail, I.; Almeelbi, T.; Salah, N.; Chandrasekaran, S.; Hameed, A. Enhanced photocatalytic activity of V2O5–ZnO composites for the mineralization of nitrophenols. Chemosphere 2014, 117, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Segovia, M.; Lemus, K.; Moreno, M.; Santa Ana, M.A.; González, G.; Ballesteros, B.; Sotomayor, C.; Benavente, E. Zinc oxide/carboxylic acid lamellar structures. Mater. Res. Bull. 2011, 46, 2191–2195. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, C.; Navas, D.; Lavayen, V.; Benavente, E.; Santa Ana, M.A.; González, G.; Newcomb, S.B.; Sotomayor Torres, C.M. Nano-Urchin: The formation and structure of high-density spherical clusters of vanadium oxide nanotubes. Chem. Mater. 2006, 13, 3016–3022. [Google Scholar] [CrossRef]
- Asim, N.; Radiman, S.; Yarmo, M.A. Preparation and characterization of core–shell polyaniline/V2O5 nanocomposite via microemulsion method. Mater. Lett. 2008, 62, 1044–1047. [Google Scholar] [CrossRef]
- Martha, S.; Das, D.P.; Biswal, N.; Parida, K.M. Facile synthesis of visible light responsive V2O5/N,S–TiO2 composite photocatalyst: Enhanced hydrogen production and phenol degradation. J. Mater. Chem. 2012, 22, 10695–10703. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Mendive, C.B.; Bahnemann, D.W.; Blesa, M.A. Microscopic characterization of the photocatalytic oxidation of oxalic acid adsorbed onto TiO2 by FTIR-ATR. Catal. Today 2005, 101, 237–244. [Google Scholar] [CrossRef]
- O’Dwyer, C.; Lavayen, V.; Newcomb, S.B.; Santa Ana, M.A.; Benavente, E.; González, G.; Sotomayor-Torres, C.M. Vanadate conformation variations in vanadium pentoxide nanostructures. J. Electrochem. Soc. 2007, 154, K29–K35. [Google Scholar] [CrossRef]
- Liu, H.R.; Shao, G.X.; Zhao, J.F.; Zhang, Z.X.; Zhang, Y.; Liang, J.; Liu, X.G.; Jia, H.S.; Xu, B.S. Worm-like Ag/ZnO core-shell heterostructural composites: Fabrication, characterization, and photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.; Bhattacharyya, K.; Krishnana, V. N-doped ZnO-MoS2 binary heterojunctions: Dual role of 2D MoS2 in the enhancement of photostability and photocatalytic activity under visible light irradiation for tetracycline degradation. Mater. Chem. Front. 2017, 1, 1093–1106. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Zhu, F.; Hua, Y.; Shi, W. The fabrication of a novel Ag3VO4/WO3 heterojunction with enhanced visible light efficiency in the photocatalytic degradation of TC. Phys. Chem. Chem. Phys. 2016, 18, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.H.; Yang, D.; Sun, Y.Y.; Tian, Y.; Jiang, Z.Y. In situ fabrication of Ag3PO4/TiO2 nanotube heterojunctions with enhanced visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2015, 17, 12199–12206. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Sun, Y.; Fu, M. Enhanced visible light photocatalytic activity of V2O5 cluster modified N-Doped TiO2 for degradation of toluene in air. Int. J. Photoenergy 2012, 10, 569716. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaga, J.; Cifuentes, N.; González, G.; Sotomayor-Torres, C.; Benavente, E. Enhancement Photocatalytic Activity of the Heterojunction of Two-Dimensional Hybrid Semiconductors ZnO/V2O5. Catalysts 2018, 8, 374. https://doi.org/10.3390/catal8090374
Aliaga J, Cifuentes N, González G, Sotomayor-Torres C, Benavente E. Enhancement Photocatalytic Activity of the Heterojunction of Two-Dimensional Hybrid Semiconductors ZnO/V2O5. Catalysts. 2018; 8(9):374. https://doi.org/10.3390/catal8090374
Chicago/Turabian StyleAliaga, Juan, Nasla Cifuentes, Guillermo González, Clivia Sotomayor-Torres, and Eglantina Benavente. 2018. "Enhancement Photocatalytic Activity of the Heterojunction of Two-Dimensional Hybrid Semiconductors ZnO/V2O5" Catalysts 8, no. 9: 374. https://doi.org/10.3390/catal8090374