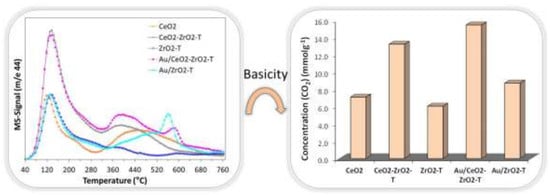
The acid-base properties of metal oxides play an important role in catalysis. They can be characterized by test-reactions like 2-propanol or 2-butanol decomposition. The dehydration of the alcohols into propene and butene, respectively, proves the acid character of the catalyst, whereas their dehydrogenation to acetone or butanone proves the basic character of the catalyst.
2.3.2. Conversion of 2-Propanol
The catalytic activity of various catalysts synthesized by co-precipitation using templates or impregnation methods was examined in a down-stream reactor described in the experimental section. The catalytic activities were compared at every temperature in terms of acetone, and propene production rates, 2-propanol conversion, activation energies Ea and selectivities for acetone.
Figure 11 shows the comparison on the dehydrogenation (acetone) (A), and dehydration (propene) (B) production rates of the catalysts as a function of the reaction temperature.
The results show a similar behavior with respect to the course of the reaction; the activity of all the samples increased with the rise of the reaction temperature measured in the down-stream reactor. For all samples, deactivation was not observed (even not during the reaction at 225 °C). CeO2 (N600) showed a high initial activity at 150 °C. The initial rates of acetone formation were similar for templated zirconia and CeO2-ZrO2 prepared by the co-precipitation method. A significant difference in the dehydrogenation rate was observed for the co-precipitated and templated ceria-zirconia mixed oxides. The rate of dehydrogenation for the templated CeO2-ZrO2-T catalyst at 225 °C was 62 µmol/g∙s and the rate of dehydration was only 2.39 µmol/g∙s. This indicates the presence of basic sites on the catalyst surface, whereas the rates of acetone and propene formation for the co-precipitated sample CeO2-ZrO2_NH3 were almost the same at 225 °C (15.7 µmol/g∙s for acetone and 14.16 µmol/g∙s for propene). The dehydrogenation rate was at least four times higher than the dehydrogenation rate of the co-precipitated catalyst. For all catalysts, no activity in dehydration was observed at temperatures lower than 200 °C.
Figure 12 presents the reaction rates of acetone for catalyst supports plotted against the reciprocal of the temperature.
Notably, templated CeO2-ZrO2-T and CeO2 (N600) have quite low apparent activation energies (Ea = 18 and 23 kJ/mol, respectively). The activation energies are half as small as the activation energies of templated zirconias (Ea = 68 and 53 kJ/mol) or co-precipitated ceria-zirconia (Ea = 58 kJ/mol).
In general, an increase in the conversion was observed with an increase of temperature. The activity of co-precipitated CeO2-ZrO2_NH3 (3.6% at 225 °C) was half as small as the activity of templated ceria-zirconia (8% at 225 °C). On the other hand, the newly synthesized templated zirconia exhibited a conversion of 0.17% at 150 °C, which increased to 7.36% at 225 °C. The increase in the conversion in case of the templated zirconia and ceria-zirconia may be due to the better balance of acid-base properties.
It is known that the activities of gold catalysts can be greatly influenced by the particle size of gold, the nature of the support, and the reaction conditions. However, there are general agreements that small gold particle sizes and redox interactions between gold and the reducible support are very important for its high catalytic activity.
The rates for acetone and propene formation in the conversion of 2-propanol for various gold supported catalysts at temperatures between 150 and 225 °C are shown in
Figure 13A (acetone production) and B (propene production).
The comparison of the rates of acetone and propene formation for all of the prepared catalysts showed that the dehydrogenation was hundred orders of magnitude higher than that of the dehydration (see
Figure 13A,B). At all temperatures, acetone was formed as main product. However, Au/CeO
2_reference prepared from commercial ceria showed a significantly lower rate of acetone formation of 65 µmol/g∙s at 225 °C compared to the other self-prepared catalysts Au/CeO
2-ZrO
2-T (549 µmol/g∙s), Au/N600 (543 µmol/g∙s) and Au/ZrO
2-T (424 µmol/g∙s).
The rates for dehydration were similar for Au loaded on ceria or ceria zirconia, whereas for Au/ZrO2-T the highest rate of propene formation (5 µmol/g∙s) was observed. Au/CeO2 reference prepared from commercial ceria was the only catalyst that showed deactivation with time on stream.
Assuming an Arrhenius type temperature dependence of the rate of acetone, the slope of the logarithm of the rate as a function of the inverse temperature provides the activation energy
Ea. The activation energies for each gold catalyst were calculated, and are presented in
Figure 14.
For the sample Au/CeO2_reference, an Ea of ∼22 kJ/mol was determined in this way. Nevertheless, the values of the measured activation energies were found to be at least three times higher in the range from 61 to 75 kJ/mol for self-prepared ceria, zirconia, and ceria-zirconia gold supported catalysts.
A comparison of the conversion of 2-propanol with other gold supported catalysts under the same conditions reveals that the Au/CeO2-ZrO2-T catalyst (69% conversion at 225 °C) appears to be the most active catalyst. Even though the sample Au/N600 exhibited similar activities (66% conversion at 225 °C) and higher conversions at lower temperatures, Au supported on ceria-zirconia forms barely any propene during the reaction, showing that almost only basic sites are involved in the reaction.
The sample Au/CeO2_reference shows a constant conversion of ∼5% at 150, 175, and 200 °C. The highest conversion for this catalyst at 225 °C was found to be 8.8%, which is only the tenth part of the conversion rate of the other synthesized gold catalysts.
A comparison of the activity of Au/CeO2-ZrO2 catalysts prepared by different methods shows lower conversion rates of the catalysts Au/CeO2-ZrO2-NH3 (35% at 225 °C) prepared by the co-precipitation method in comparison to the templated catalysts Au/CeO2-ZrO2-T (69% at 225 °C).
Au supported ceria-zirconia catalysts are highly coke-resistant and exhibit promising stable activities without deactivation during time-on-stream runs. This was mainly attributed to the availability of lattice oxygen, due to the ability of the ceria-based mixed oxides to store and release oxygen [
30].
The selectivities of acetone in the conversion of 2-propanol at 225 °C and 10 mL min
−1 helium gas flow for various catalysts are presented in
Figure 15.
With regard to the selectivities, it can be noted that all catalysts tend to favor the formation of acetone with some traces of propene. Au/CeO2-ZrO2-T and Au/N600 showed the highest selectivity for acetone 99.7%.
2.3.3. Conversion of 2-Butanol
The decomposition of 2-butanol was carried out to test the catalytic activity and investigate the acid-base sites of the catalysts. Briefly, butane is formed on acid sites via dehydration and butanone is formed on dominating basic sites butanone via dehydrogenation of 2-butanol.
The catalytic activities were compared at every temperature in terms of butanone and butene production rates, 2-butanol conversion, and selectivities to butanone.
The rates of butanone and butene formation for various supports are shown in
Figure 16A (butanone) and
Figure 16B (butene).
CeO2-ZrO2-T shows a significant butanone formation at 225 °C with a rate of 79.9 µmol/g·s, whereas for the templated zirconias a maximal rate of butanone of 24.2 and 30 µmol/g·s was observed for ZrO2-T (NO3) and ZrO2-T (propox.), respectively. Non-templated CeO2 (N600) prepared by precipitation with ammonia exhibits a rate for butanone formation of 65 µmol/g·s at 225 °C and a rate of dehydration (butene formation) of 79 µmol/g·s. All catalysts show a slight deactivation during the reaction at each temperature. At temperatures higher than 200 °C, ZrO2-T (propox.) favors the formation of butene with a rate of 70.5 µmol/g·s, while ZrO2 (NO3) had a selectivity of 100% for butanone at 150 °C. With increasing temperatures, the selectivity for butanone was decreased to 31% at 225 °C, whereas butene was formed as main product with a rate of formation of 41.3 µmol/g·s.
From all catalysts, the template mixed oxide CeO2-ZrO2-T was the only sample that showed a preferred formation of butanone. The other catalysts failed in the reaction of 2-butanol decomposition compared to the conversion of 2-propanol. The main product of these catalysts at temperatures higher than 200 °C was butene.
From all the measured supports, CeO2 (N600) showed the greatest conversion of 19.9% at 225 °C, whereas for ZrO2-T (NO3) only 9% of the 2-butanol introduced is being catalytically converted. At temperatures lower than 175 °C, zirconia prepared from the nitrate precursor showed almost no conversion of 2-butanol (0.39%).
The rates of butanone formation (A) and butene formation (B and C) for various gold supported catalysts between 150 and 225 °C are presented in
Figure 17.
Au/CeO2-ZrO2-T, like its homologous support, yielded butanone as main product at all temperatures with 98.5% at 150 °C and 97.4% at 225 °C. The highest butanone formation (548 µmol/g·s) was observed for this catalyst at 225 °C.
In contrast to the 2-propanol decomposition reaction, all catalysts were subject to deactivation at each single temperature during the reaction. At 225 °C, Au supported on zirconia and ceria-zirconia, synthesized with CTAB template, shows a similar rate of butanone formation with 351 and 356 µmol/g·s, respectively. Au/CeO2 (N600) exhibits a constant rate of butanone formation of 220 µmol/g·s at 175 and 200 °C, while a significant decrease (61 µmol/g·s) was observed at 225 °C.
The non-templated samples Au/N600 and Au/CeO2_reference yield butene as main product at temperatures higher than 200 °C, with a rate of 785.5 and 528.5 µmol/g·s, whereas Au supported on zirconia and ceria-zirconia only show a rate of butene formation with a maximum value of 29.7 µmol/g·s.
For Au/N600, the conversion increased at each temperature step from 21.5% at the lowest temperature (150 °C) to 98.7% at the highest temperature (225 °C). In contrast to Au supported on ceria-zirconia, Au/ceria favors butene formation at temperatures higher than 175 °C. At 150 °C, the conversion of 2-propanol reaches up to 27% for the Au supported catalyst templated P-123® and Brij®56. Au/CeO2-ZrO2-T exhibits 65% conversion when the temperature is raised to 225 °C. The gold catalyst prepared by the co-precipitation method showed the lowest conversion when compared to the templated catalysts from 4% at 150 °C to 25% at 225 °C.
Figure 18 presents the calculated selectivities for butanone formation for the synthesized catalysts at 225 °C, and helium carrier-gas flow of 10 mL min
−1.
In contrast to the 2-propanol decomposition, the selectivities for butanone are lower than those for acetone formation. More investigations are needed to clear the increased deactivation during the butanol conversion. In general, Au supported on ceria-zirconia and on templated zirconia showed high butanone selectivities up to 97%, while Au supported on ceria shows very low selectivities in the order Au/N600 (11%) < Au/N600 (6%) < Au/CeO2 reference (3.7%).