Magnetic Combined Cross-Linked Enzyme Aggregates of Ketoreductase and Alcohol Dehydrogenase: An Efficient and Stable Biocatalyst for Asymmetric Synthesis of (R)-3-Quinuclidinol with Regeneration of Coenzymes In Situ
Abstract
:1. Introduction
2. Results
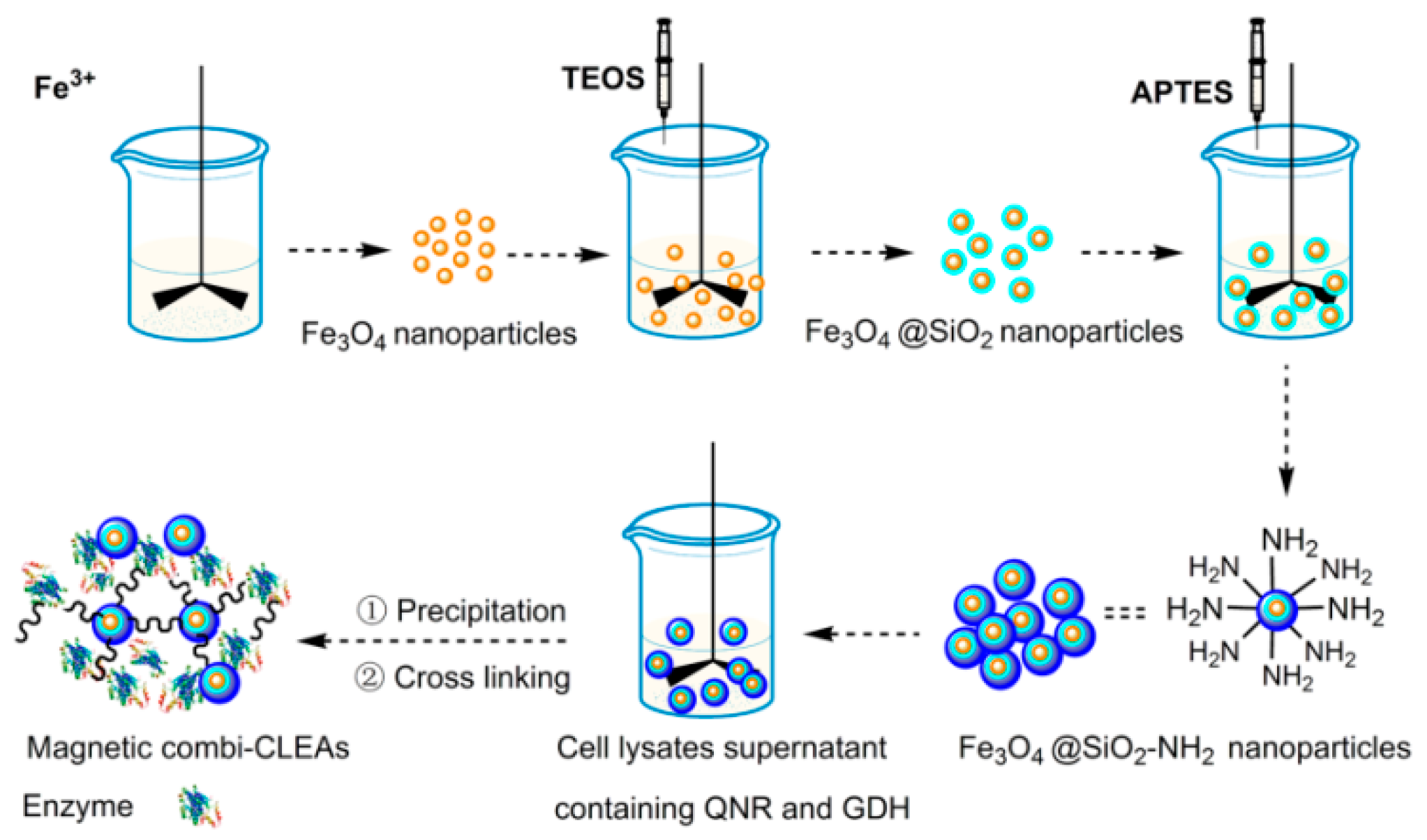
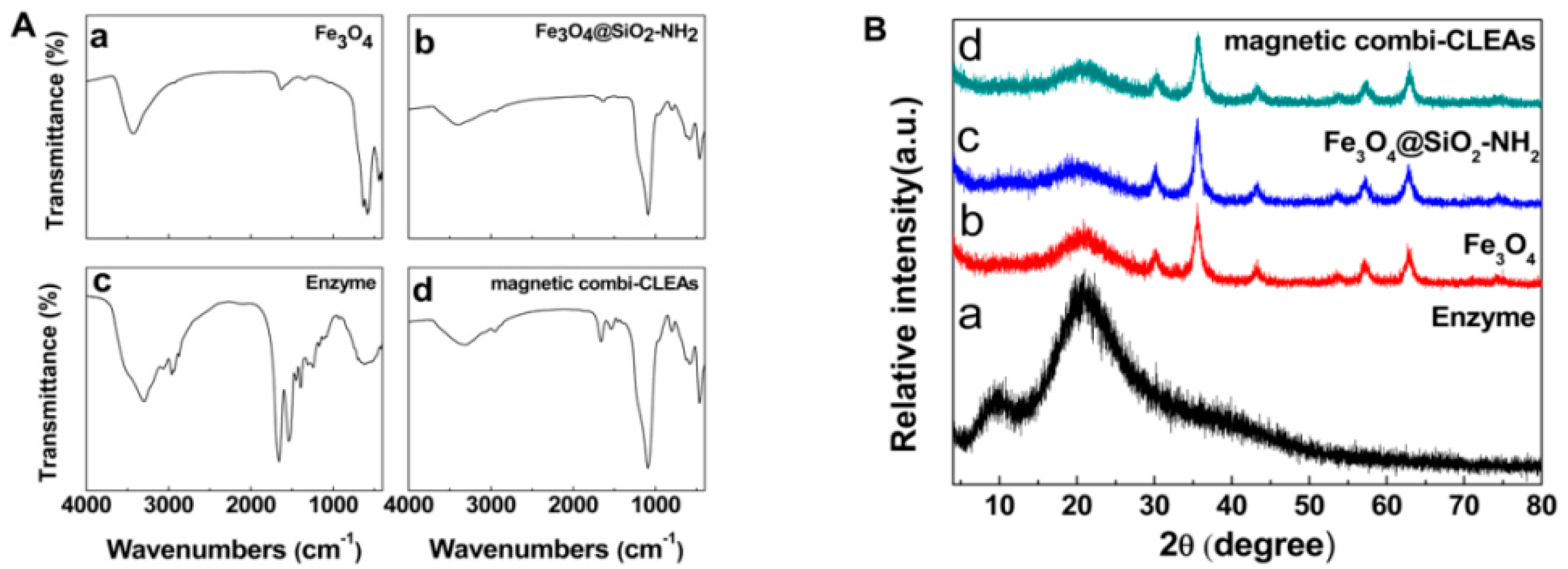
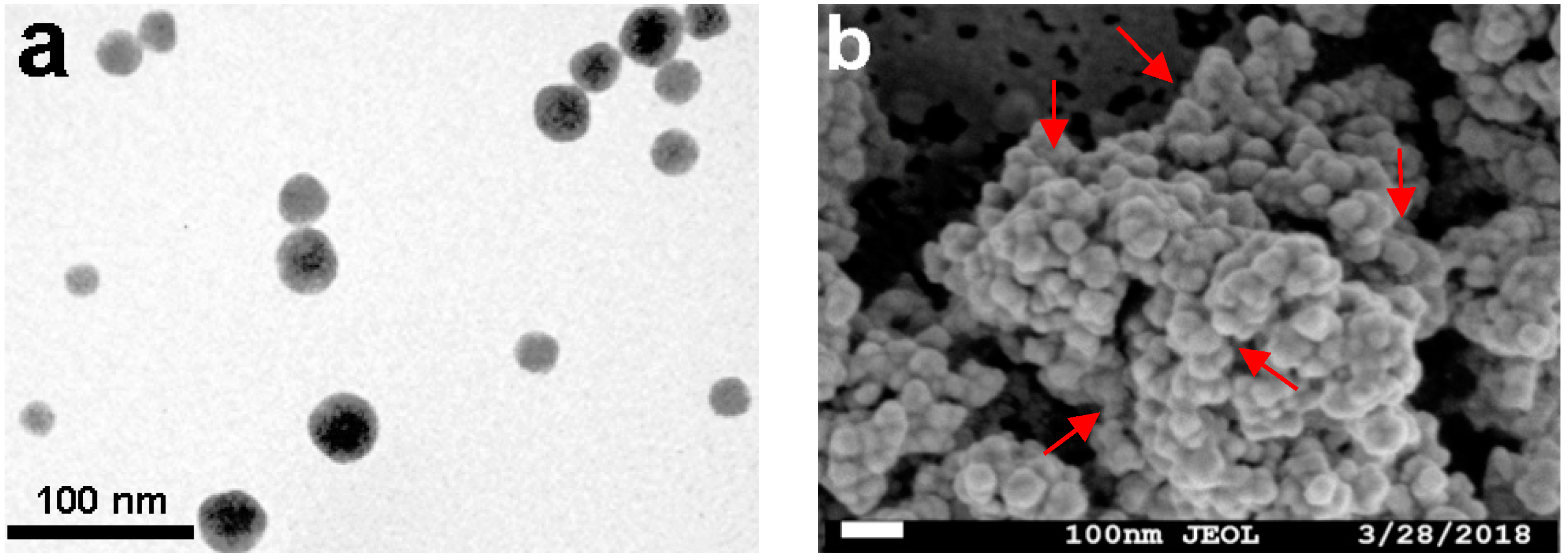
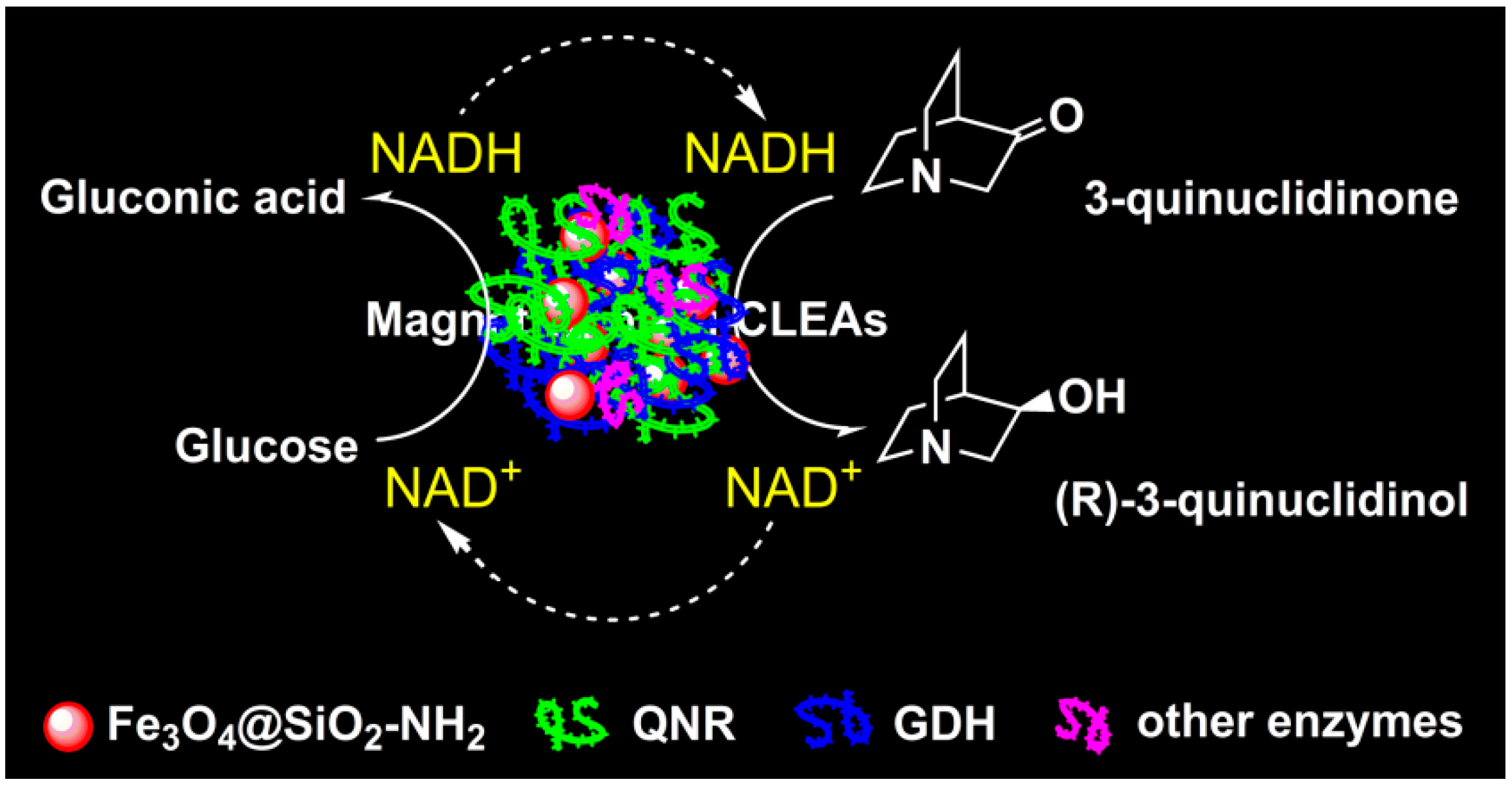
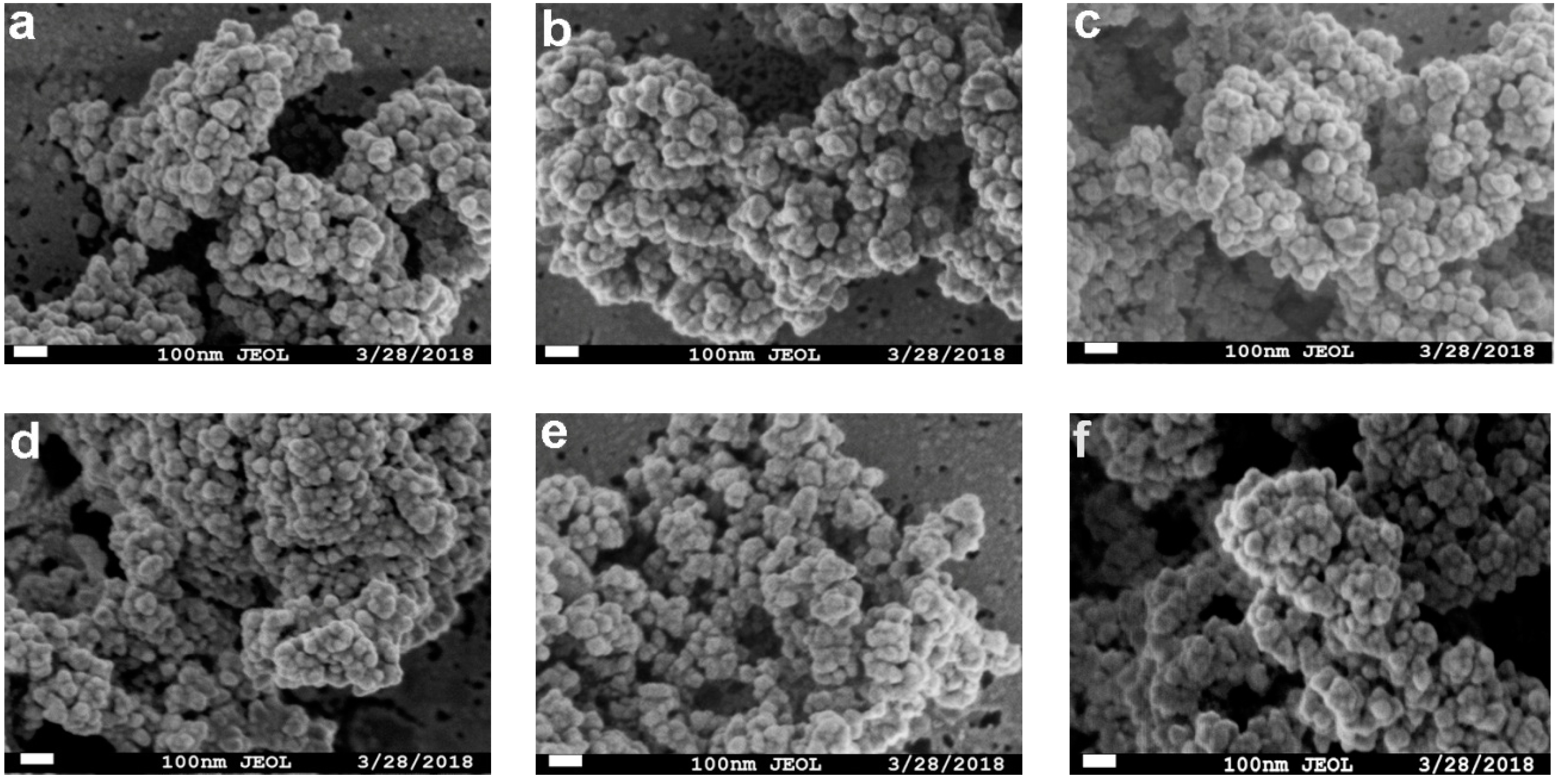
2.1. Preparation and Characterization of the Magnetic Combi-CLEAs of QNR and GDH
2.2. Screening of Precipitant Agents for Magnetic Combi-CLEAs Preparation
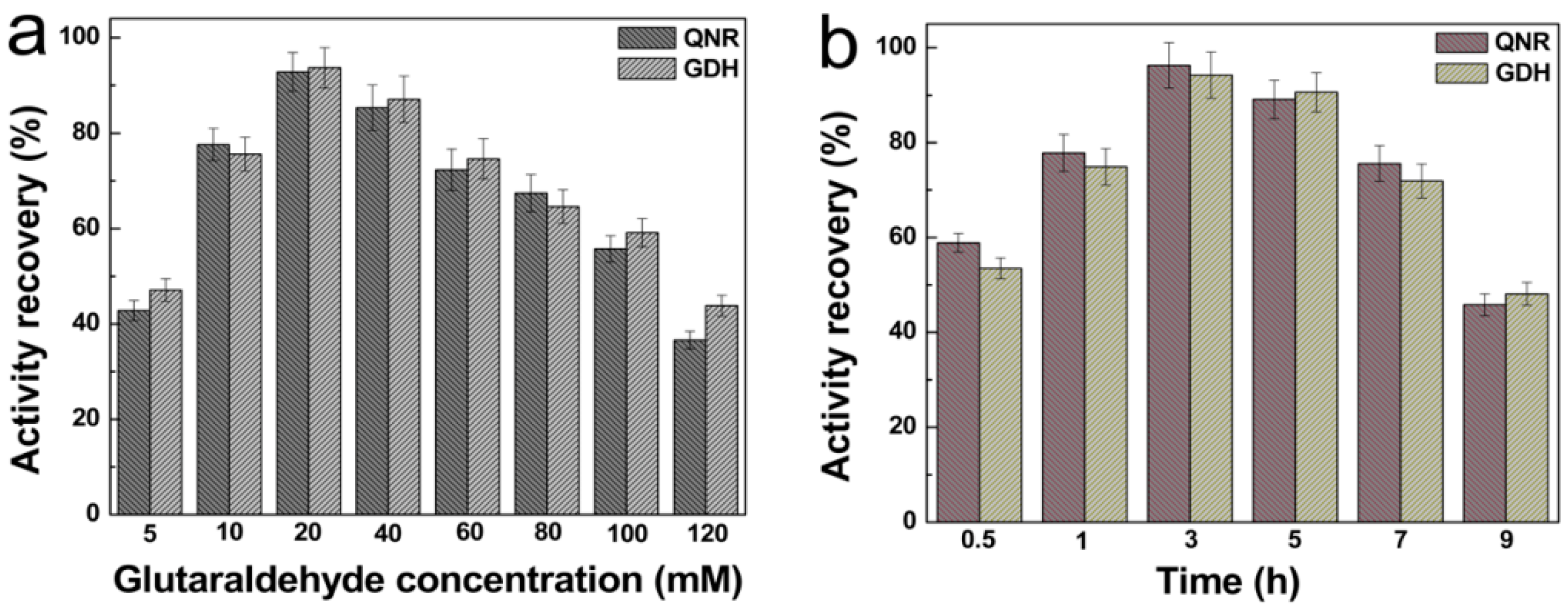
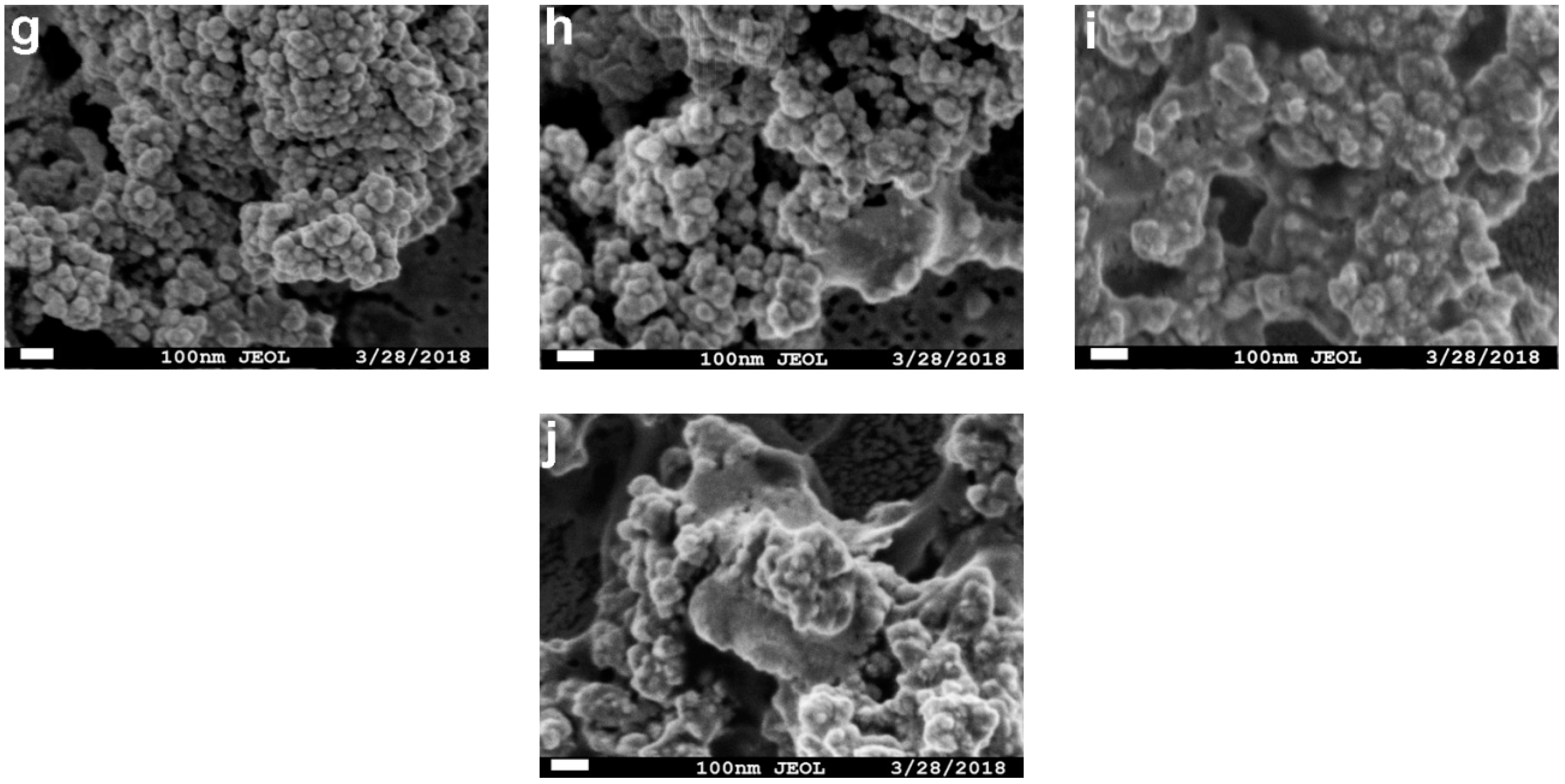
2.3. Effect of Cross-Linker Concentration and Cross-linking Time on Activity Recovery of Magnetic Combi-CLEAs
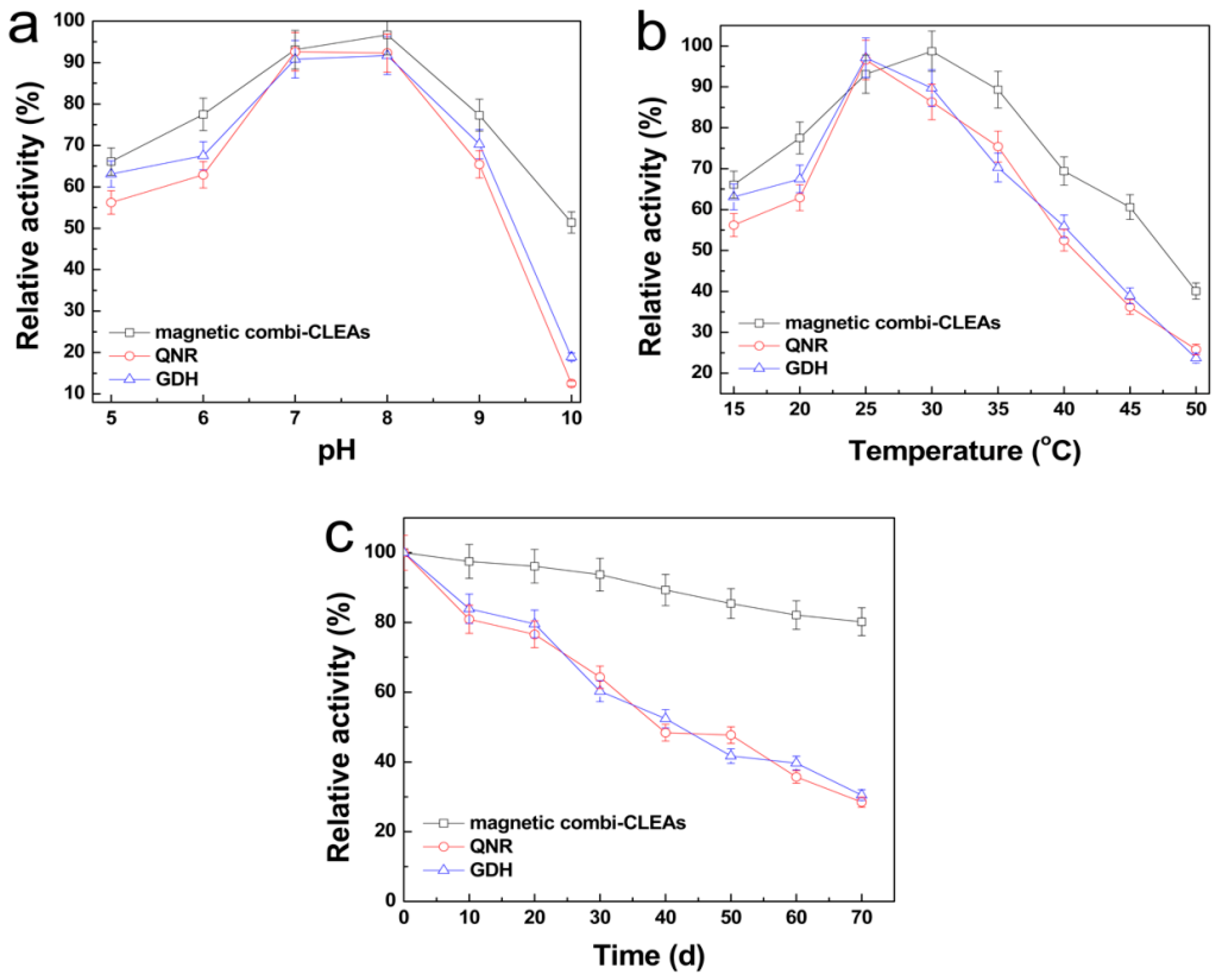
2.4. Optimal pH and Temperature for Magnetic Combi-CLEAs Activity Recovery
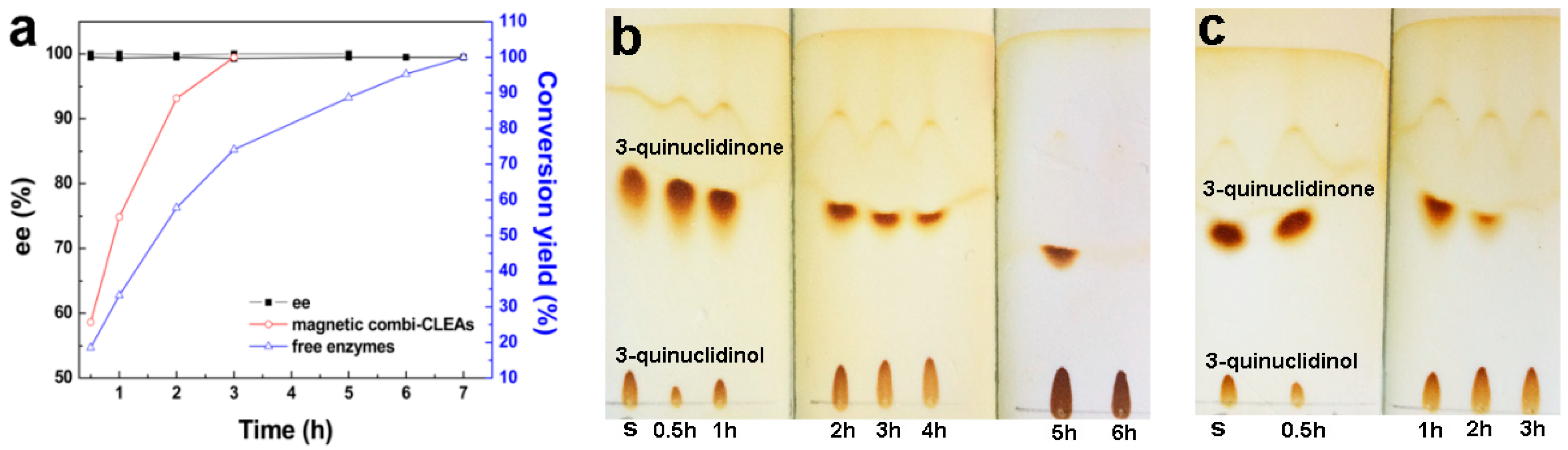
2.5. Time-Course of Biosynthesis of (R)-3-quinuclidinol
3. Materials and Methods
3.1. Materials
3.2. Expression of Recombinant Enzyme QNR and GDH
3.3. Enzyme Activity Assay
3.4. Immobilization of QNR and GDH
3.4.1. Preparation of NH2-Functionalized Fe3O4 Nanoparticles (Fe3O4@SiO2-NH2)
3.4.2. Preparation of Magnetic Combi-CLEAs of QNR and GDH
3.5. Application of Magnetic Combi-CLEAs for Biosynthesis (R)-3-quinuclidinol
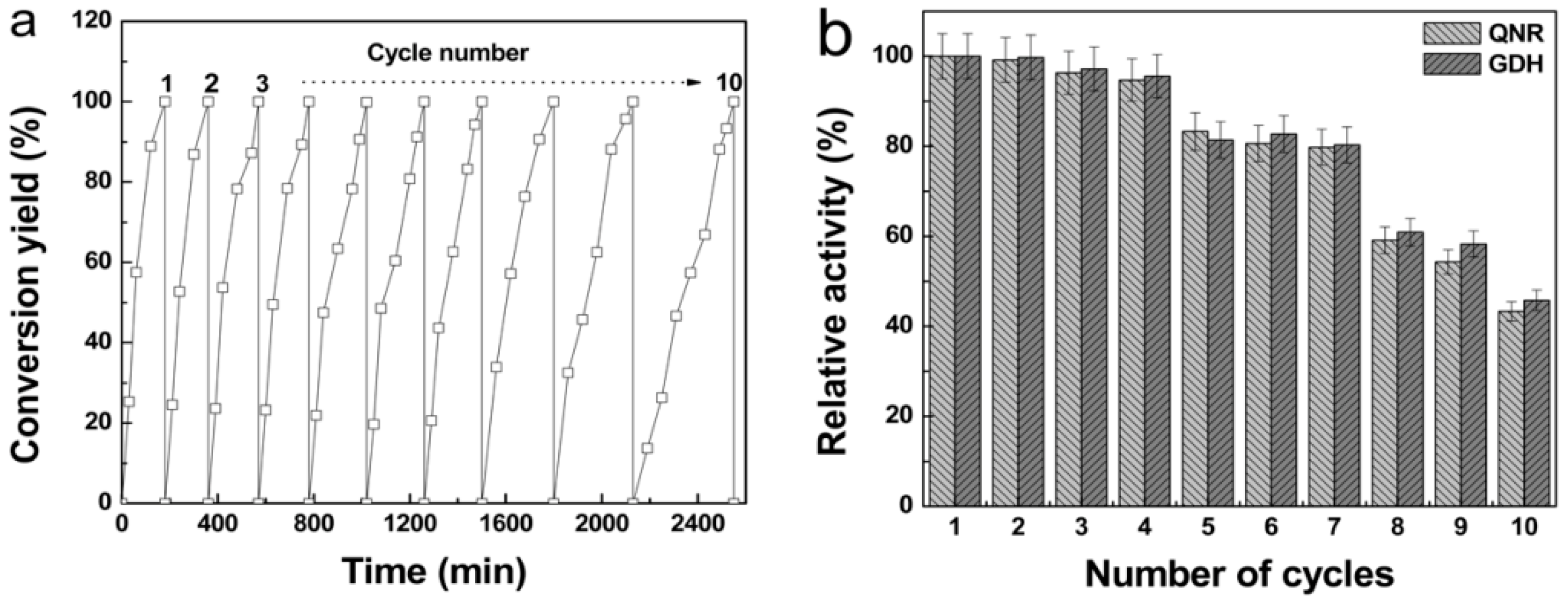
3.6. Reusability of Enzymes in Magnetic Combi-CLEAs
3.7. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Keßeler, M.; Stürmer, R.; Zelinski, T. Industrial Methods for the Production of Optically Active Intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, N.P. Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord. Chem. Rev. 2008, 252, 659–701. [Google Scholar]
- Kisukuri, C.M.; Andrade, L.H. Production of chiral compounds using immobilized cells as a source of biocatalysts. Org. Biomol. Chem. 2015, 13, 10086–10107. [Google Scholar] [CrossRef] [PubMed]
- Prat, M.; Fernández, D.; Buil, M.A.; Crespo, M.I.; Casals, G.; Ferrer, M.; Tort, L.; Castro, J.; Monleón, J.M.; Gavaldà, A.; et al. Discovery of Novel Quaternary Ammonium Derivatives of (3R)-Quinuclidinol Esters as Potent and Long-Acting Muscarinic Antagonists with Potential for Minimal Systemic Exposure after Inhaled Administration: Identification of (3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane Bromide (Aclidinium Bromide). J. Med. Chem. 2009, 52, 5076–5092. [Google Scholar] [PubMed]
- Naito, R.; Yonetoku, Y.; Okamoto, Y.; Toyoshima, A.; Ikeda, K.; Takeuchi, M. Synthesis and Antimuscarinic Properties of Quinuclidin-3-yl 1,2,3,4-Tetrahydroisoquinoline-2-carboxylate Derivatives as Novel Muscarinic Receptor Antagonists. J. Med. Chem. 2005, 48, 6597–6606. [Google Scholar] [CrossRef] [PubMed]
- Alabaster, V.A. Discovery & development of selective M3 antagonists for clinical use. Life Sci. 1997, 60, 1053–1060. [Google Scholar] [PubMed]
- Rabe, K.F.; Watz, H. Chronic Obstructive Pulmonary Disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Astellas Pharma US Inc. VESIcare Prescribing Information. 2013. Available online: http://www. astellas.us/docs/vesicare.pdf (accessed on 19 October 2016).
- Marsh, K.; Zaiser, E.; Orfanos, P.; Salverda, S.; Wilcox, T.; Sun, S.; Dixit, S. Evaluation of COPD Treatments: A Multicriteria Decision Analysis of Aclidinium and Tiotropium in the United States. Value Health 2017, 20, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Katayama, T.; Utsumi, N.; Murata, K.; Arai, N.; Kurono, N.; Ohkuma, T. Practical Asymmetric Hydrogenation of 3-Quinuclidinone Catalyzed by the XylSkewphos/PICA−Ruthenium(II) Complex. Org. Process Res. Dev. 2009, 13, 625–628. [Google Scholar] [CrossRef]
- Arai, N.; Akashi, M.; Sugizaki, S.; Ooka, H.; Inoue, T.; Ohkuma, T. Asymmetric Hydrogenation of Bicyclic Ketones Catalyzed by BINAP/IPHAN−Ru(II) Complex. Org. Lett. 2010, 12, 3380–3383. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, F.; Hirayama, Y.; Ikunaka, M.; Inoue, T.; Otsuka, K. A practical chemoenzymatic process to access (R)-quinuclidin-3-ol on scale. Tetrahedron Asymmetry 2003, 14, 1871–1877. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalysis: Synthesis of chiral intermediates for drugs. Curr. Opin. Drug Discov. Dev. 2006, 9, 741–764. [Google Scholar]
- Zhao, H. Highlights of Biocatalysis and Biomimetic Catalysis. ACS Catal. 2011, 1, 1119–1120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wu, Q.; Zhu, D. Microbial stereospecific reduction of 3-quinuclidinone with newly isolated Nocardia sp. and Rhodococcus erythropolis. J. Mol. Catal. B Enzym. 2013, 88, 14–19. [Google Scholar] [CrossRef]
- Kolet, S.P.; Jadhav, D.D.; Priyadarshini, B.; Swarge, B.N.; Thulasiram, H.V. Fungi mediated production and practical purification of (R)-(−)-3-quinuclidinol. Tetrahedron Lett. 2014, 55, 5911–5914. [Google Scholar] [CrossRef]
- Isotani, K.; Kurokawa, J.; Suzuki, F.; Nomoto, S.; Negishi, T.; Matsuda, M.; Itoh, N. Gene Cloning and Characterization of Two NADH-Dependent 3-Quinuclidinone Reductases from Microbacterium luteolum JCM 9174. Appl. Environ. Microbiol. 2012, 79, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Xu, G.-C.; Huang, L.; Pan, J.; Yu, H.-L.; Xu, J.-H. Highly Efficient Synthesis of (R)-3-Quinuclidinol in a Space–Time Yield of 916 g L−1 d−1 Using a New Bacterial Reductase Ar QR. Org. Lett. 2013, 15, 4917–4919. [Google Scholar] [CrossRef] [PubMed]
- Wildeman, S.M.A.D.; Sonke, T.; Schoemaker, H.E.; May, O. Biocatalytic Reductions: From Lab Curiosity to “First Choice”. Acc. Chem. Res. 2007, 40, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Biocatalysis in Organic Chemistry and Biotechnology: Past, Present, and Future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.P.N.; Li, A.; Tiew, K.W.; Li, Z. Efficient transformation of grease to biodiesel using highly active and easily recyclable magnetic nanobiocatalyst aggregates. Bioresour. Technol. 2013, 145, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.P.N.; Zhang, W.; Wang, W.; Li, Z. Reversible clustering of magnetic nanobiocatalysts for high-performance biocatalysis and easy catalyst recycling. Chem. Commun. 2012, 48, 4585. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Y.; Wang, D.I.C.; Li, Z. Recyclable Nanobiocatalyst for Enantioselective Sulfoxidation: Facile Fabrication and High Performance of Chloroperoxidase-Coated Magnetic Nanoparticles with Iron Oxide Core and Polymer Shell. J. Am. Chem. Soc. 2009, 131, 12892–12893. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, D.I.C.; Li, Z. Facile fabrication of recyclable and active nanobiocatalyst: Purification and immobilization of enzyme in one pot with Ni-NTA functionalized magnetic nanoparticle. Chem. Commun. 2011, 47, 8115–8117. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, K.E.; Kourist, R.; Lindberg, D.; Larsen, M.W.; Thanh, N.H.; Widersten, M.; Bornscheuer, U.T.; Berglund, P. One-step enzyme extraction and immobilization for biocatalysis applications. Biotechnol. J. 2011, 6, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Herdt, A.R.; Kim, B.-S.; Taton, T.A. Encapsulated Magnetic Nanoparticles as Supports for Proteins and Recyclable Biocatalysts. Bioconjug. Chem. 2007, 18, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, A.K.; Yang, Y.; Ngo, T.P.N.; Li, Z. Simple and Efficient Immobilization of Extracellular His-Tagged Enzyme Directly from Cell Culture Supernatant as Active and Recyclable Nanobiocatalyst: High-Performance Production of Biodiesel from Waste Grease. ACS Catal. 2015, 5, 3157–3161. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, S.; Liu, Q.; Yan, D.; Wang, Y.; Gao, L.; Han, J.; Shi, H. Synchronized purification and immobilization of his-tagged β-glucosidase via Fe3O4/PMG core/shell magnetic nanoparticles. Sci. Rep. 2017, 7, 41741. [Google Scholar] [CrossRef] [PubMed]
- Kulsharova, G.; Dimov, N.; Marques, M.P.C.; Szita, N.; Baganz, F. Simplified immobilisation method for histidine-tagged enzymes in poly(methyl methacrylate) microfluidic devices. New Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Fernández-Lorente, G.; Cortes, E.; Garcia, J.L.; Fernandez-Lafuente, R.; Guisan, J.M. One-step purification, covalent immobilization, and additional stabilization of poly-His-tagged proteins using novel heterofunctional chelate-epoxy supports. Biotechnol. Bioeng. 2001, 76, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, Y.; Yuhei, K.; Masaya, M. Techniques for Preparation of Cross-Linked Enzyme Aggregates and Their Applications in Bioconversions. Catalysts 2018, 8, 174. [Google Scholar] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Sorokina, K.N.; Piligaev, A.V.; Parmon, V.N. Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater. Catalysts 2018, 8, 154. [Google Scholar] [CrossRef]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Ning, C.; Su, E.; Tian, Y.; Wei, D. Combined cross-linked enzyme aggregates (combi-CLEAs) for efficient integration of a ketoreductase and a cofactor regeneration system. J. Biotechnol. 2014, 184, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzym. Microb. Technol. 2014, 61–62, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Wei, W.; Shuo, L.; Song, M.-H.; Yun, Y.-S. Fabrication of Stable and Regenerable Amine Functionalized Magnetic Nanoparticles as a Potential Material for Pt(IV) Recovery from Acidic Solutions. ACS Appl. Mater. Interfaces 2017, 9, 18650–18659. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.C.S.; Daniel-da-Silva, A.L.; Xavier, A.M.R.B.; Tavares, A.P.M. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Abdulla-Al-Mamun, M.; Kusumoto, Y.; Zannat, T.; Horie, Y.; Manaka, H. Au-ultrathin functionalized core–shell (Fe3O4@Au) monodispersed nanocubes for a combination of magnetic/plasmonic photothermal cancer cell killing. RSC Adv. 2013, 3, 7816–7827. [Google Scholar] [CrossRef]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Matijošytė, I.; Arends, I.W.C.E.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.-Q.; Hu, Z.-L.; Sheldon, R.A.; Yang, Z. Catalytic performance of cross-linked enzyme aggregates of Penicillium expansum lipase and their use as catalyst for biodiesel production. Process Biochem. 2012, 47, 2058–2063. [Google Scholar] [CrossRef]
- Cui, J.; Cui, L.; Jia, S.; Su, Z.; Zhang, S. Hybrid cross-linked lipase aggregates with magnetic nanoparticles: A robust and recyclable biocatalysis for epoxidation of oleic acid. J. Agric. Food. Chem. 2016, 64, 7179–7187. [Google Scholar] [CrossRef] [PubMed]
- Uzura, A.; Nomoto, F.; Sakoda, A.; Nishimoto, Y.; Kataoka, M.; Shimizu, S. Stereoselective synthesis of (R)-3-quinuclidinol through asymmetric reduction of 3-quinuclidinone with 3-quinuclidinone reductase of Rhodotorula rubra. Appl. Microbiol. Biotechnol. 2009, 83, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Chen, H.; Wang, X.; Yang, Y.Y.; Ching, C.B. Cross-linked enzyme aggregates (CLEAs) with controlled particles: Application to Candida rugosa lipase. J. Mol. Catal. B Enzym. 2006, 43, 124–127. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, D.; Sun, L.; Li, J.; Chen, Q.; Pan, L.; Shang, J.; Zhang, S.; Li, W. Facile fabrication of 3D porous hybrid sphere by co-immobilization of multi-enzyme directly from cell lysates as an efficient and recyclable biocatalyst for asymmetric reduction with coenzyme regeneration in situ. Int. J. Biol. Macromol. 2017, 103, 424–434. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Jiang, Q.; Sun, L.; Li, Q.; Zhou, L.; Chen, Q.; Li, S.; Yu, M.; Li, W. Magnetic Combined Cross-Linked Enzyme Aggregates of Ketoreductase and Alcohol Dehydrogenase: An Efficient and Stable Biocatalyst for Asymmetric Synthesis of (R)-3-Quinuclidinol with Regeneration of Coenzymes In Situ. Catalysts 2018, 8, 334. https://doi.org/10.3390/catal8080334
Chen Y, Jiang Q, Sun L, Li Q, Zhou L, Chen Q, Li S, Yu M, Li W. Magnetic Combined Cross-Linked Enzyme Aggregates of Ketoreductase and Alcohol Dehydrogenase: An Efficient and Stable Biocatalyst for Asymmetric Synthesis of (R)-3-Quinuclidinol with Regeneration of Coenzymes In Situ. Catalysts. 2018; 8(8):334. https://doi.org/10.3390/catal8080334
Chicago/Turabian StyleChen, Yuhan, Qihua Jiang, Lili Sun, Qiang Li, Liping Zhou, Qian Chen, Shanshan Li, Mingan Yu, and Wei Li. 2018. "Magnetic Combined Cross-Linked Enzyme Aggregates of Ketoreductase and Alcohol Dehydrogenase: An Efficient and Stable Biocatalyst for Asymmetric Synthesis of (R)-3-Quinuclidinol with Regeneration of Coenzymes In Situ" Catalysts 8, no. 8: 334. https://doi.org/10.3390/catal8080334




