Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism
Abstract
| Outline |
| 1. Introduction |
| 2. The mechanism of Hydrogen Absorption/Desorption and the Need for a Catalyst |
| 3. Catalysts for MgH2 |
| 3.1 Transition Metal Catalysts |
| 3.2 Carbon and Other Elements as Additive |
| 3.3 Metal Oxide Catalysts |
| 3.4 Metal Halide Catalysts |
| 3.5 Hydride, Hydride Forming Alloys and Sulfide as Catalyst |
| 4. Catalysts for Complex Hydrides |
| 4.1 Catalysts for Alanates |
| 4.2 Catalysts for Borohydrides |
| 4.3 Catalysts for Amides |
| 4.4 Catalysts for Silanides |
| 5. Concluding Remarks and Future Prospective |
| References |
1. Introduction
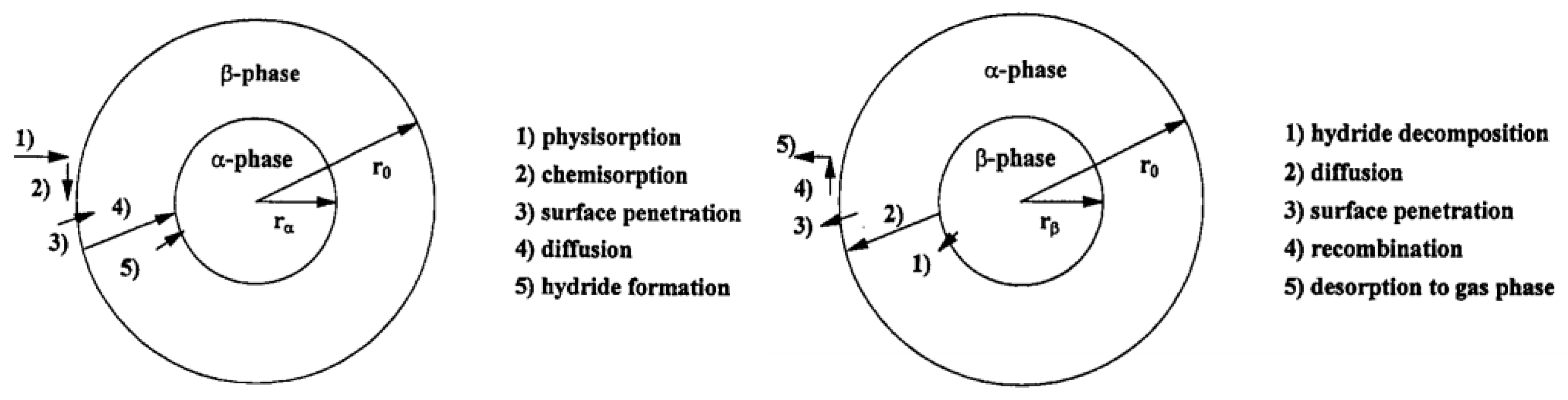
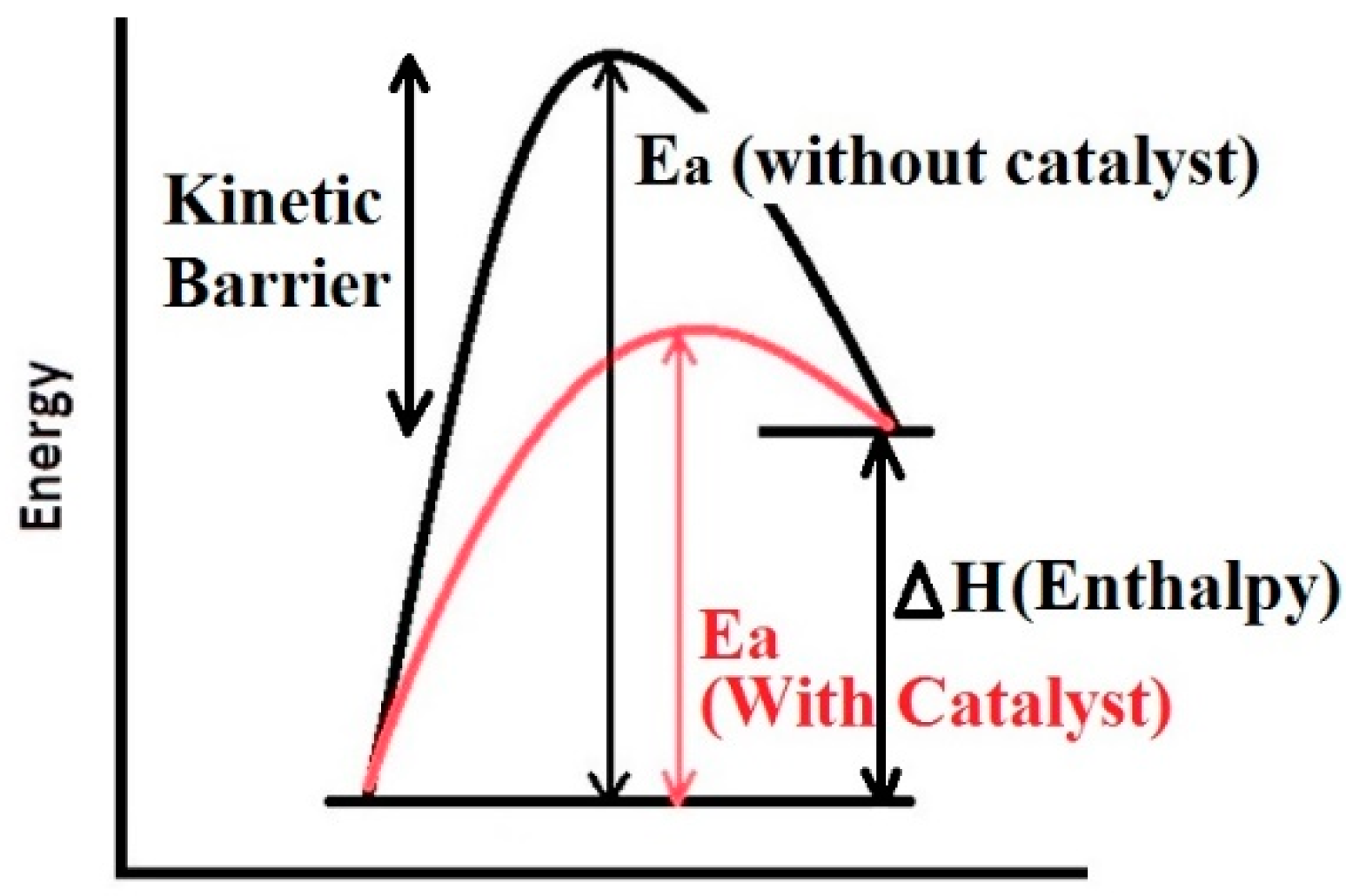
2. The Mechanism of Hydrogen Absorption/Desorption and the Need of Catalyst
- Physisorption of H2 molecule;
- Chemisorption of H atoms;
- Surface penetration of H atoms;
- Diffusion of hydrogen atoms;
- Hydride formation at metal/hydride interface.
3. Catalysts for MgH2
3.1. Transition Metal Catalysts
3.2. Carbon and Other Elements as Additive
3.3. Metal Oxide Catalysts
3.4. Metal Halide Catalysts
3.5. Hydride, Hydride Forming Alloys and Sulfide as Catalyst
4. Catalysts for Complex hydrides
4.1. Catalysts for Alanates
4.2. Catalysts for Borohydrides
4.3. Catalysts for Amides
4.4. Catalysts for Silanides
5. Concluding Remark & Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: https://webbook.nist.gov/chemistry/ (accessed on 12 October 2018).
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The use of metal hydrides in fuel cell applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef]
- Sreedhar, I.; Kamani, K.M.; Kamani, B.M.; Reddy, B.M.; Venugopal, A. A Bird’s Eye view on process and engineering aspects of hydrogen storage. Renew. Sustain. Energy Rev. 2018, 91, 838–860. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 12 October 2018).
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Jain, A.; Kawasako, E.; Miyaoka, H.; Ma, T.; Isobe, S.; Ichikawa, T.; Kojima, Y. Destabilization of LiH by Li Insertion into Ge. J. Phys. Chem. C 2013, 117, 5650–5657. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloy. Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Jain, A.; Ichikawa, T.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y. Catalytic modification in dehydrogenation properties of KSiH3. Phys. Chem. Chem. Phys. 2014, 16, 26163–26167. [Google Scholar] [CrossRef] [PubMed]
- Klerke, A.; Christensen, C.H.; Norskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Hu, M.G.; Geanangel, R.A.; Wendlandt, W.W. The thermal decomposition of ammonia borane. Thermochim. Acta 1978, 23, 249–255. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Cresswell, D.; Garforth, A. Hydrogen Storage in Liquid Organic Hydride: Producing Hydrogen Catalytically from Methylcyclohexane. Energy Fuels 2011, 25, 4217–4234. [Google Scholar] [CrossRef]
- Selvaraj, S.; Jain, A.; Kumar, S.; Zhang, T.; Isobe, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Study of cyclic performance of V-Ti-Cr alloys employed for hydrogen compressor. Int. J. Hydrogen Energy 2018, 43, 2881–2889. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements: A review. Int. J. Hydrogen Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Pick, M.A. The kinetics of hydrogen absorption-desorption by Metals. In Metal Hydrides; Bambakidis, G., Ed.; NATO Advanced Study Institute Series; Springer: Boston, MA, USA, 1981; Volume 76. [Google Scholar]
- Martin, M.; Gommel, C.; Borkhart, C.; Fromm, E. Absorption and desorption kinetics of hydrogen storage alloys. J. Alloy. Compd. 1996, 238, 193–201. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloy. Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Gao, M.; Pan, H. Tailoring Thermodynamics and Kinetics for Hydrogen Storage in Complex Hydrides towards Applications. Chem. Rec. 2016, 16, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Khafidz, N.Z.A.K.; Yaakob, Z.; Lim, K.L.; Timmiati, S.N. The kinetics of lightweight solid-state hydrogen storage materials: A review. Int. J. Hydrogen Energy 2016, 41, 13131–13151. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Baldé, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; de Jong, K.P. Sodium Alanate Nanoparticles—Linking Size to Hydrogen Storage Properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Ma, H.; Chen, J. Magnesium Nanowires: Enhanced Kinetics for Hydrogen Absorption and Desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef] [PubMed]
- Mushnikov, N.V.; Ermakov, A.E.; Uimin, M.A.; Gaviko, V.S.; Terent’ev, P.B.; Skripov, A.V.; Tankeev, A.P.; Soloninin, A.V.; Buzlukov, A.L. Kinetics of interaction of Mg-based mechanically activated alloys with hydrogen. Phys. Met. Metall. 2006, 102, 421–431. [Google Scholar] [CrossRef]
- Stampfer, J.F., Jr.; Holley, C.E., Jr.; Suttle, J.F. The Magnesium-Hydrogen System. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef]
- Stander, C.M. Kinetics of decomposition of magnesium hydride. J. Inorg. Nucl. Chem. 1977, 39, 221–223. [Google Scholar] [CrossRef]
- Grant, D. Magnesium Hydride for Hydrogen Storage. In Solid State Hydrogen Storage; Gavin, W., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 357–380. [Google Scholar]
- Schlapbach, L.; Shaltiel, D.; Oelhafen, P. Catalytic effect in the hydrogenation of Mg and Mg compounds: Surface analysis of Mg–Mg2Ni and Mg2Ni. Mater. Res. Bull. 1979, 14, 1235–1246. [Google Scholar] [CrossRef]
- Stioui, M.; Grayevski, A.; Resnik, A.; Shaltiel, D.; Kaplan, N. Macroscopic and microscopic kinetics of hydrogen in magnesium-rich compounds. J. Less-Common Met. 1986, 123, 9–24. [Google Scholar] [CrossRef]
- Krozer, A.; Kasemo, B. Eqilibrium hydrogen uptake and associated kinetics for the Mg–H2 system at low pressures. J. Phys. Condens. Matter 1989, 1, 1533–1538. [Google Scholar] [CrossRef]
- Luz, Z.; Genossar, J.; Rudman, P.S. Identification of the diffusing atom in MgH2. J. Less-Common Met. 1980, 73, 113–118. [Google Scholar] [CrossRef]
- Vigeholm, B.; Kjoller, J.; Larsen, B.; Pedersen, A.S. Formation and decomposition of magnesium hydride. J. Less-Common Met. 1983, 89, 135–144. [Google Scholar] [CrossRef]
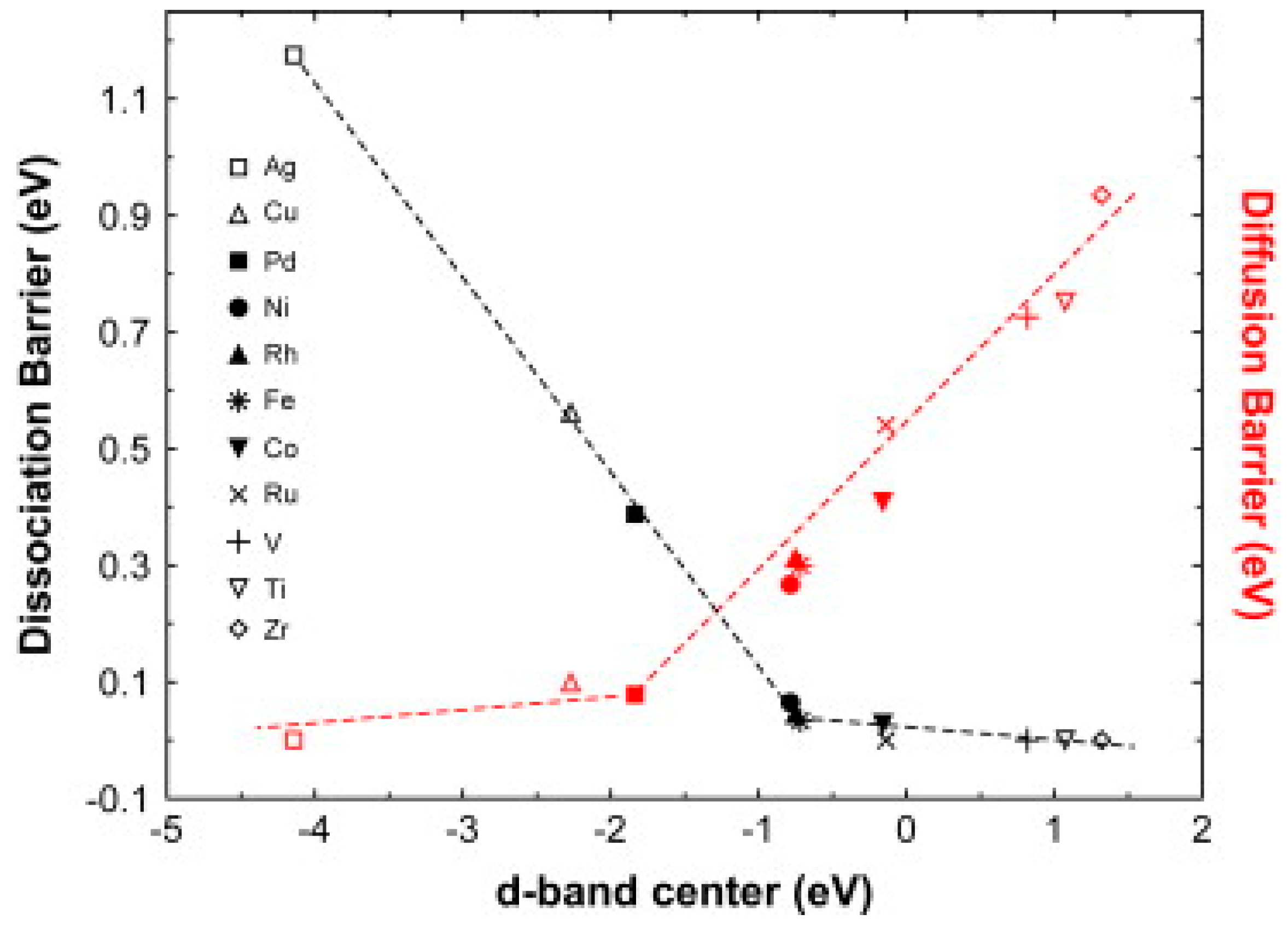
- Kecik, D.; Aydinol, M.K. Density functional and dynamics study of the dissociative adsorption of hydrogen on Mg (0001) surface. Surf. Sci. 2009, 603, 304–310. [Google Scholar] [CrossRef]
- Pozzo, M.; Alfè, D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Fe, Ru, Co, Rh, Ni, Pd, Cu, Ag)-doped Mg(0001) surfaces. Int. J. Hydrogen Energy 2009, 34, 1922–1930. [Google Scholar] [CrossRef]
- Mamula, B.P.; Novaković, J.G.; Radisavljević, I.; Ivanović, N.; Novaković, N. Electronic structure and charge distribution topology of MgH2 doped with 3d transition metals. Int. J. Hydrogen Energy 2014, 39, 5874–5887. [Google Scholar] [CrossRef]
- German, E.; Gebauer, R. Improvement of Hydrogen Vacancy Diffusion Kinetics in MgH2 by Niobium- and Zirconium-Doping for Hydrogen Storage Applications. J. Phys. Chem. C 2016, 120, 4806–4812. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Zhao, X.; Mi, Y.; Wang, L. First-Principles Investigation of Energetics and Electronic Structures of Ni and Sc Co-Doped MgH2. Am. J. Anal. Chem. 2016, 7, 34–42. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloy. Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Neste, A.V.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloy. Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Schulz, R. Hydrogen desorption kinetics of a mechanically milled MgH2 + 5at.%V nanocomposite. J. Alloy. Compd. 2000, 305, 239–245. [Google Scholar] [CrossRef]
- Bobet, J.-L.; Akiba, E.; Darriet, B. Study of Mg-M (M = Co, Ni and Fe) mixture elaborated by reactive mechanical alloying: Hydrogen sorption properties. Int. J. Hydrogen Energy 2001, 26, 493–501. [Google Scholar] [CrossRef]
- Xu, X.; Song, C. Improving hydrogen storage/release properties of magnesium with nano-sized metal catalysts as measured by tapered element oscillating microbalance. Appl. Catal. A Gen. 2006, 300, 130–138. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Denis, A.; Sellier, E.; Aymonier, C.; Bobet, J.-L. Hydrogen sorption properties of magnesium particles decorated with metallic nanoparticles as catalyst. J. Alloy. Compd. 2009, 476, 152–159. [Google Scholar] [CrossRef]
- Yu, H.; Bennici, S.; Auroux, A. Hydrogen storage and release: Kinetic and thermodynamic studies of MgH2 activated by transition metal nanoparticles. Int. J. Hydrogen Energy 2014, 39, 11633–11641. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Zhang, X.; Qu, J.; Wang, Y.; Li, X. Catalytic effect of Ni nanoparticles on the desorption kinetics of MgH2 nanoparticles. J. Alloy. Compd. 2009, 482, 388–392. [Google Scholar] [CrossRef]
- Zou, J.; Long, S.; Chen, X.; Zeng, X.; Ding, W. Preparation and hydrogen sorption properties of a Ni decorated Mg based Mg@Ni nano-composite. Int. J. Hydrogen Energy 2015, 40, 1820–1828. [Google Scholar] [CrossRef]
- Chen, J.; Xia, G.; Guo, Z.; Huang, Z.; Liu, H.; Yu, X. Porous Ni nanofibers with enhanced catalytic effect on the hydrogen storage performance of MgH2. J. Mater. Chem. A 2015, 3, 15843–15848. [Google Scholar] [CrossRef]
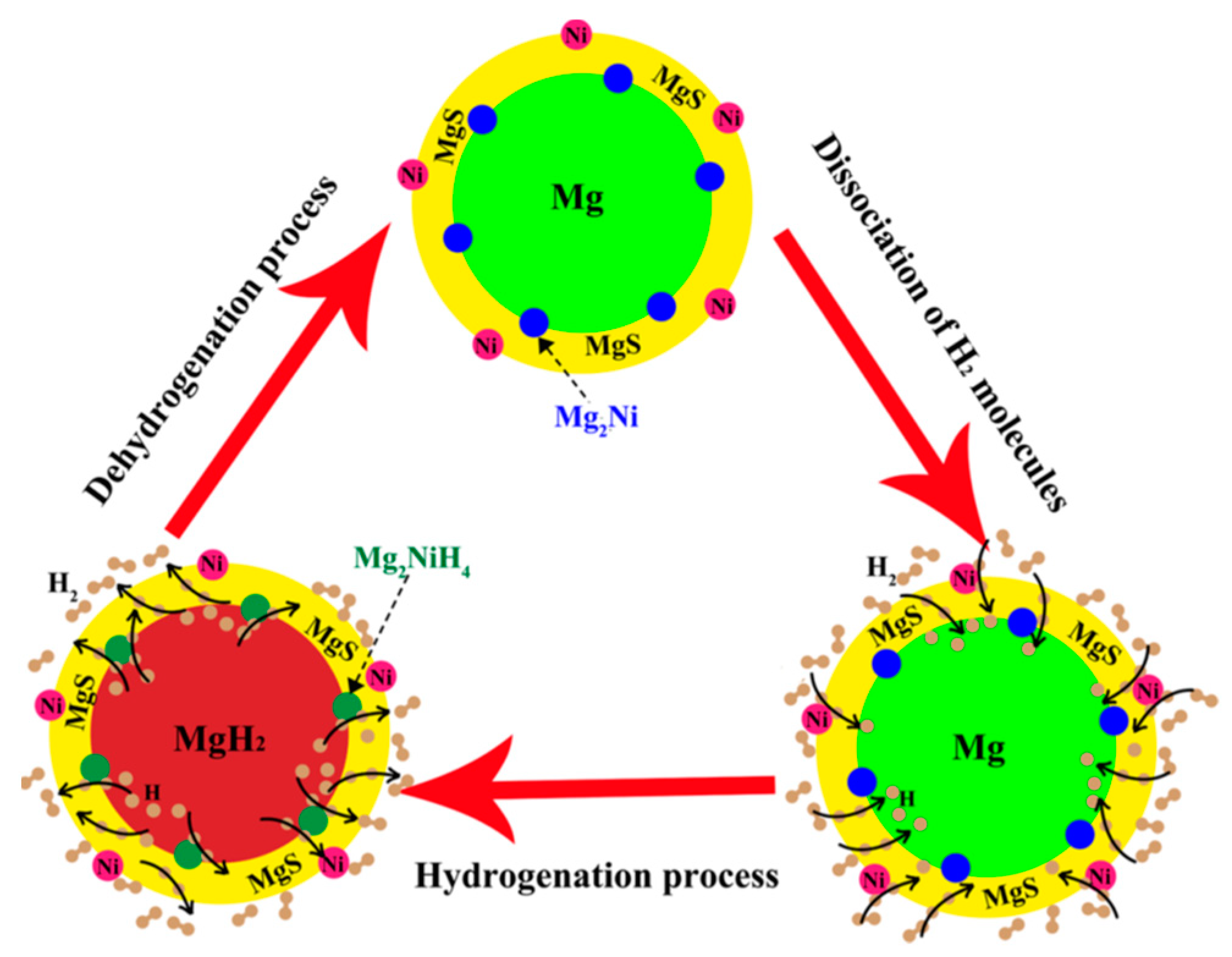
- El-Eskandarany, M.S.; Shaban, E.; Ali, N.; Aldakheel, F.; Alkandary, A. In-situ catalyzation approach for enhancing the hydrogenation/dehydrogenation kinetics of MgH2 powders with Ni particles. Sci. Rep. 2016, 6, 37335. [Google Scholar] [CrossRef]
- Lu, C.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of core-shell structured Mg@TM (TM = Co, V) composites. Int. J. Hydrogen Energy 2017, 42, 15246–15255. [Google Scholar] [CrossRef]
- Imamura, H.; Kusuhara, M.; Minami, S.; Matsumoto, M.; Masanari, K.; Sakata, Y.; Keiji, I.; Toshiharu, F. Carbon nanocomposites synthesized by high-energy mechanical milling of graphite and magnesium for hydrogen storage. Acta Mater. 2003, 51, 6407–6414. [Google Scholar] [CrossRef]
- Shang, C.X.; Guo, Z.X. Effect of carbon on hydrogen desorption and absorption of mechanically milled MgH2. J. Power Sources 2004, 129, 73–80. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride. J. Alloy. Compd. 2006, 414, 259–264. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Guo, Z.X.; Aguey-Zinsou, K.F.; Cazorla-Amorós, D.; Linares-Solano, A. Effects of different carbon materials on MgH2 decomposition. Carbon 2008, 46, 126–137. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, Y.; Zou, J.; Yao, X. Hydrogenation/dehydrogenation in MgH2-activated carbon composites prepared by ball milling. Int. J. Hydrogen Energy 2012, 37, 7579–7585. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.M.; Beregovsky, M.; Sezen, M.; Amouyal, Y.; Rabkin, E. Hydrogen storage and thermal transport properties of pelletized porous Mg-2 wt.% multiwall carbon nanotubes and Mg-2 wt.% graphite composites. Int. J. Hydrogen Energy 2016, 41, 14461–14474. [Google Scholar] [CrossRef]
- Liu, W.; Setijadi, E.; Crema, L.; Bartali, R.; Laidani, N.; Aguey-Zinsou, K.F.; Speranza, G. Carbon nanostructures/Mg hybrid materials for hydrogen storage. Diam. Relat. Mater. 2018, 82, 19–24. [Google Scholar] [CrossRef]
- Fuster, V.; Castro, F.J.; Troiani, H.; Urretavizcaya, G. Characterization of graphite catalytic effect in reactively ball-milled MgH2–C and Mg–C composites. Int. J. Hydrogen Energy 2011, 36, 9051–9061. [Google Scholar] [CrossRef]
- Lototskyy, M.; Sibanyoni, J.M.; Denys, R.V.; Williams, M.; Pollet, B.G.; Yartys, V.A. Magnesium–carbon hydrogen storage hybrid materials produced by reactive ball milling in hydrogen. Carbon 2013, 57, 146–160. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Ström-Olsen, J.O. Nanocrystalline metal hydrides. J. Alloy. Compd. 1997, 253–254, 70–79. [Google Scholar] [CrossRef]
- Shang, C.X.; Guo, Z.X. Structural and desorption characterisations of milled (MgH2 + Y,Ce) powder mixtures for hydrogen storage. Int. J. Hydrogen Energy 2007, 32, 2920–2925. [Google Scholar] [CrossRef]
- Zhu, X.; Pei, L.; Zhao, Z.; Liu, B.; Han, S.; Wang, R. The catalysis mechanism of La hydrides on hydrogen storage properties of MgH2 in MgH2 + xwt.% LaH3 (x = 0,10,20, and 30) composites. J. Alloy. Compd. 2013, 577, 64–69. [Google Scholar] [CrossRef]
- Song, J.; Zhao, Z.; Zhao, X.; Fu, R.; Han, S. Hydrogen storage properties of MgH2 co-catalyzed by LaH3 and NbH. Int. J. Miner. Metall. Mater. 2017, 24, 1183–1191. [Google Scholar] [CrossRef]
- Zou, J.; Zeng, X.; Ying, Y.; Chen, X.; Guo, H.; Zhou, S.; Ding, W. Study on the hydrogen storage properties of core–shell structured Mg–RE (RE = Nd, Gd, Er) nano-composites synthesized through arc plasma method. Int. J. Hydrogen Energy 2013, 38, 2337–2346. [Google Scholar] [CrossRef]
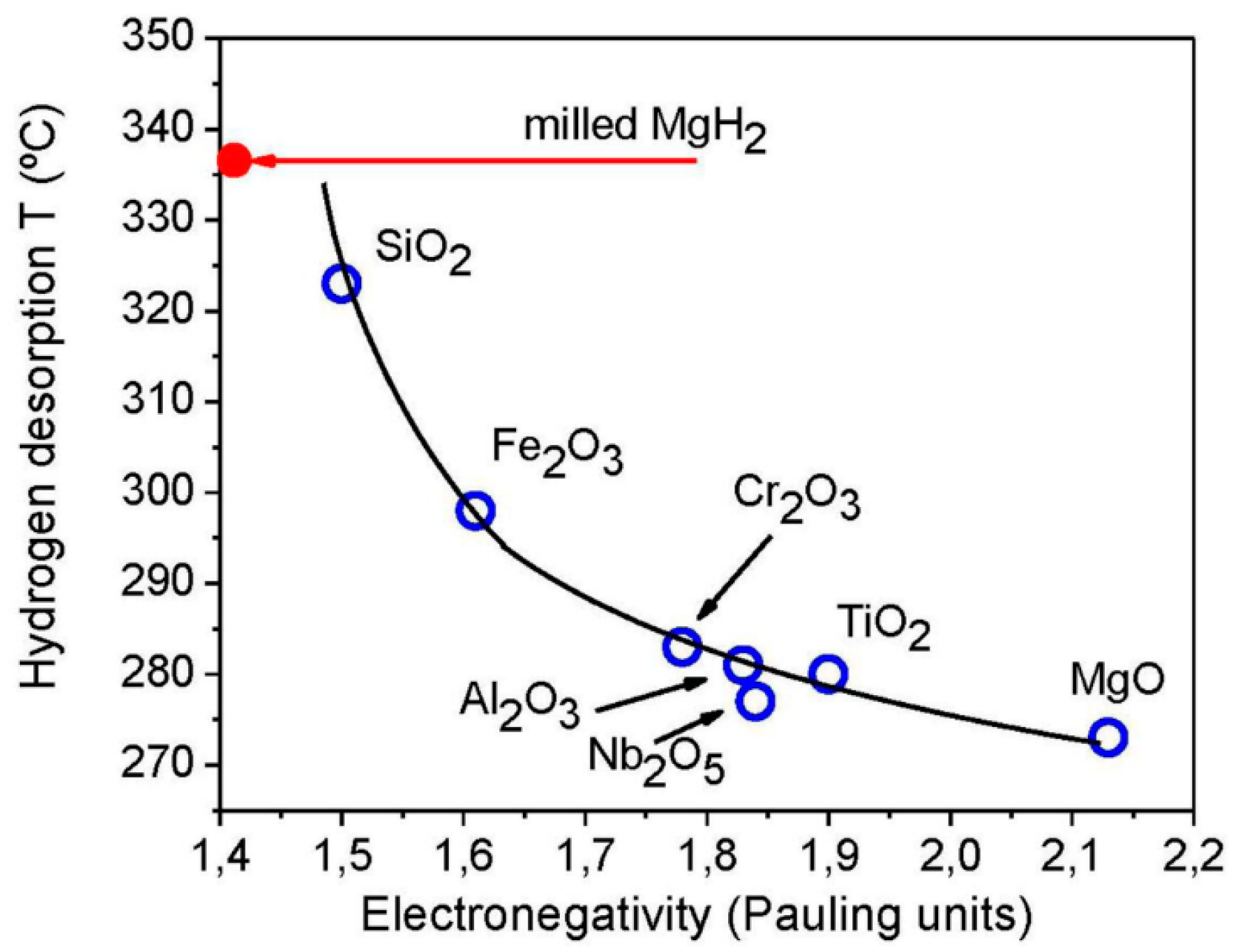
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloy. Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Song, M.Y.; Bobet, J.-L.; Darriet, B. Improvement in hydrogen sorption properties of Mg by reactive mechanical grinding with Cr2O3, Al2O3 and CeO2. J. Alloy. Compd. 2002, 340, 256–262. [Google Scholar] [CrossRef]
- Jung, K.S.; Lee, E.Y.; Lee, K.S. Catalytic effects of metal oxide on hydrogen absorption of magnesium metal hydride. J. Alloy. Compd. 2006, 421, 179–184. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic Mechanism of Transition-Metal Compounds on Mg Hydrogen Sorption Reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride. J. Alloy. Compd. 2009, 486, 697–701. [Google Scholar] [CrossRef]
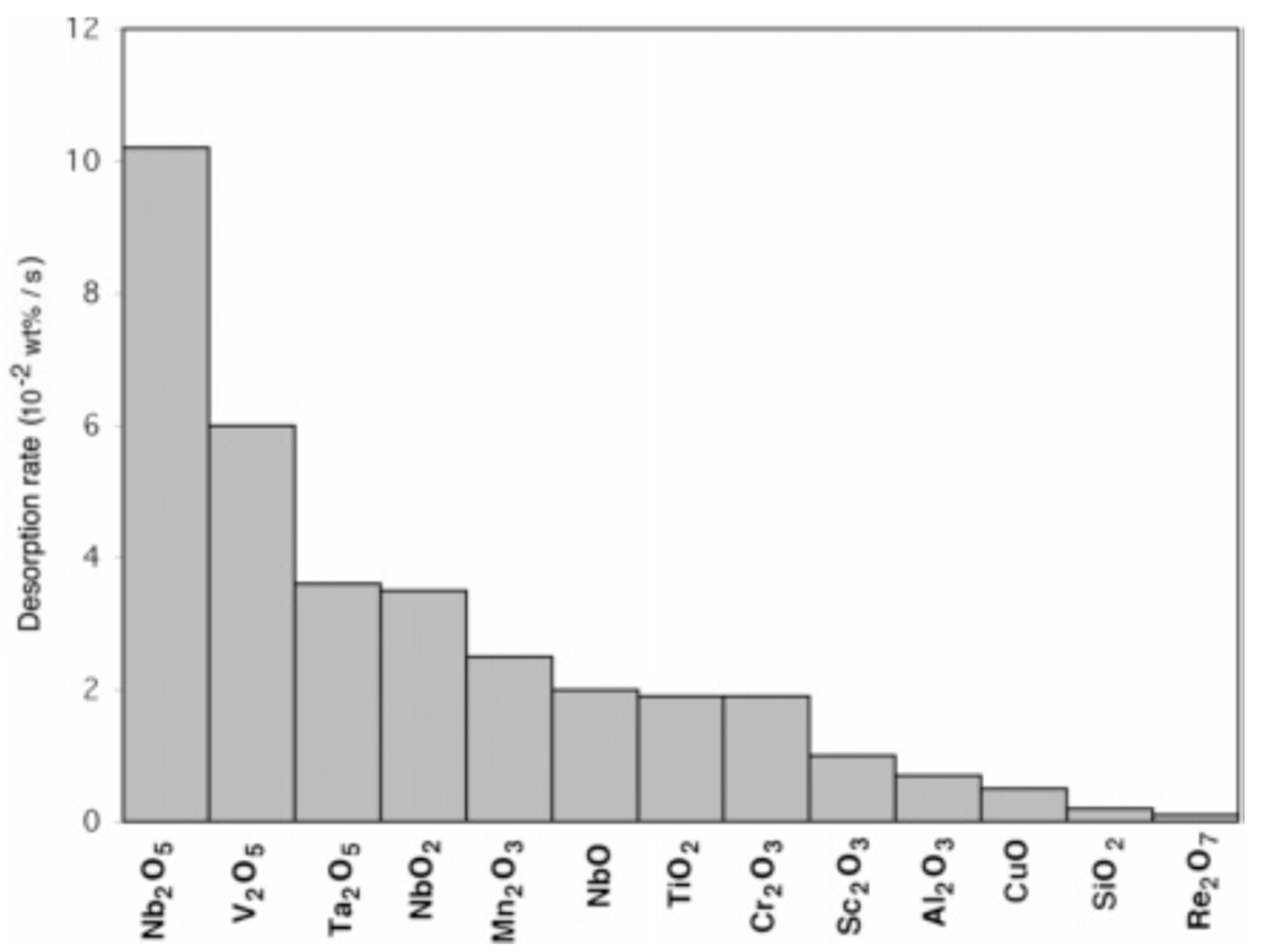
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloy. Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
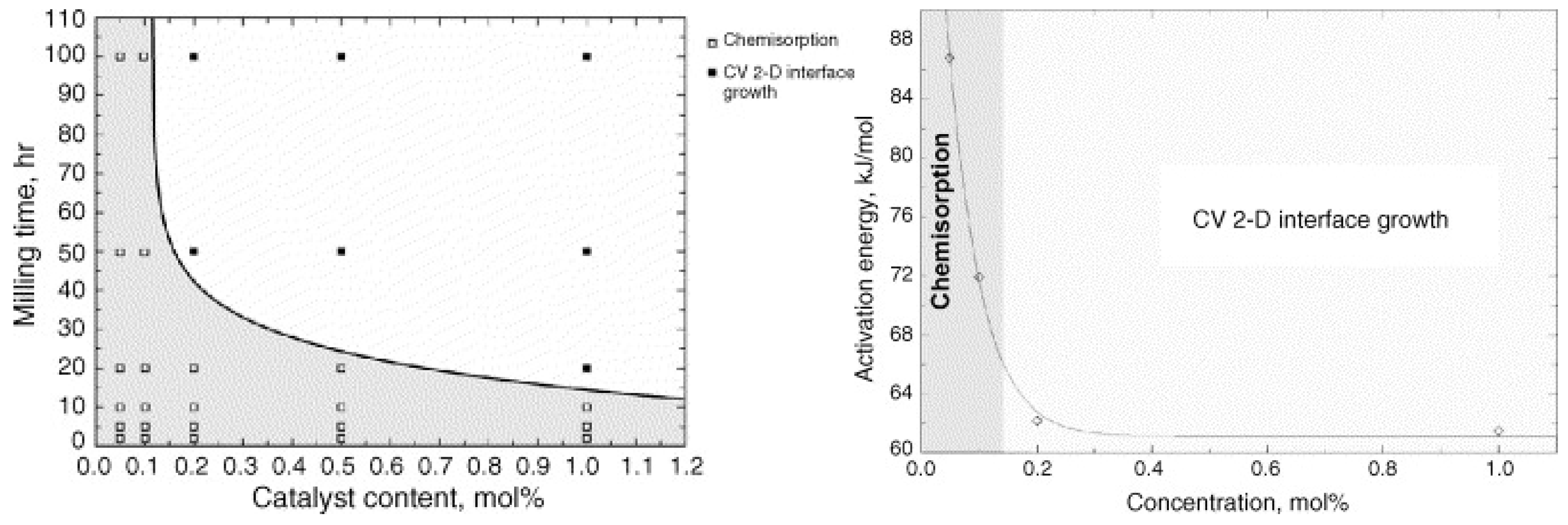
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloy. Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of Ni nano-particle and Nb oxide on H-desorption properties in MgH2 prepared by ball milling. J. Alloy. Compd. 2005, 404–406, 716–719. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. J. Alloy. Compd. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Kimura, T.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Hydrogen absorption of catalyzed magnesium below room temperature. Int. J. Hydrogen Energy 2013, 38, 13728–13733. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Isobe, S.; Nakagawa, T.; Tokoyoda, K.; Honma, T.; Fujii, H.; Kojima, Y. X-ray Absorption Spectroscopic Study on Valence State and Local Atomic Structure of Transition Metal Oxides Doped in MgH2. J. Phys. Chem. C 2009, 113, 13450–13455. [Google Scholar] [CrossRef]
- Friedrichs, O.; Aguey-Zinsou, F.K.; Fernández, J.R.A.; Sánchez-López, J.C.; Justo, J.; Klasssen, T.; Bormann, R.; Fernández, A. MgH2 with Nb2O5 as additive, for hydrogen storage: Chemical, structural and kinetic behavior with heating. Acta Mater. 2006, 54, 105–110. [Google Scholar] [CrossRef]
- Friedrichs, O.; Klasssen, T.; Sánchez-López, J.C.; Bormann, R.; Fernández, A. Hydrogen sorption improvement of nanocrystalline MgH2 by Nb2O5 nanoparticles. Scr. Mater. 2006, 54, 1293–1297. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Fernandez, J.R.A.; Klassen, T.; Bormann, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrogen Energy 2007, 42, 2400–2407. [Google Scholar] [CrossRef]
- Conceição, M.O.T.; Brum, M.C.; Santos, D.S.; Dias, M.L. Hydrogen sorption enhancement by Nb2O5 and Nb catalysts combined with MgH2. J. Alloy. Compd. 2013, 550, 179–184. [Google Scholar] [CrossRef]
- Ma, T.; Isobe, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S. Nb-Gateway for Hydrogen Desorption in Nb2O5 Catalyzed MgH2Nanocomposite. J. Phys. Chem. C 2013, 117, 10302–10307. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Antunes, I.; Russo, S.L.; Perez, J.; Fagg, D.P. Synthesis of catalytically active rock salt structured MgxNb1−xO nanoparticles for MgH2 system. Int. J. Hydrogen Energy 2014, 39, 18984–18988. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Otero-Irurueta, G.; Pérez, J.; Singh, B.; Fagg, D.P. Crystal structure, phase stoichiometry and chemical environment of MgxNbyOx+y nanoparticles and their impact on hydrogen storage in MgH2. Int. J. Hydrogen Energy 2016, 41, 11709–11715. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Perez, J.; Nasani, N.; Bdikin, I.; Kovalevsky, A.V.; Fagg, D.P. Formation of MgxNbyOx+y through the Mechanochemical Reaction of MgH2 and Nb2O5, and Its Effect on the Hydrogen-Storage Behavior of MgH2. ChemPhysChem 2016, 17, 178–183. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Comparison of the catalytic effects of V, V2O5, VN, and VC on the hydrogen sorption of nanocrystalline Mg. J. Alloy. Compd. 2001, 322, L5–L9. [Google Scholar] [CrossRef]
- Milošević, S.; Rašković-Lovre, Ž.; Kurko, S.; Vujasin, R.; Cvjetićanin, N.; Matović, L.; Novaković, J.G. Influence of VO2 nanostructured ceramics on hydrogen desorption properties from magnesium hydride. Ceram. Int. 2013, 39, 51–56. [Google Scholar] [CrossRef]
- Milošević, S.; Kurko, S.; Pasquini, L.; Matović, L.; Vujasin, R.; Novaković, N.; Novaković, J.G. Fast hydrogen sorption from MgH2–VO2(B) composite materials. J. Power Sources 2016, 307, 481–488. [Google Scholar] [CrossRef]
- Dehouche, Z.; Klassen, T.; Oelerich, W.; Goyette, J.; Bose, T.K.; Schulz, R. Cycling and thermal stability of nanostructured MgH2–Cr2O3 composite for hydrogen storage. J. Alloy. Compd. 2002, 347, 319–323. [Google Scholar] [CrossRef]
- Vijay, R.; Sundaresan, R.; Maiya, M.P.; Murthy, S.S. Hydrogen storage properties of Mg–Cr2O3 nanocomposites: The role of catalyst distribution and grain size. J. Alloy. Compd. 2006, 424, 289–293. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Plocinski, T. The effect of milling conditions on microstructure and hydrogen absorption/desorption properties of magnesium hydride (MgH2) without and with Cr2O3 nanoparticles. Int. J. Hydrogen Energy 2008, 33, 1859–1867. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Varin, R.A.; Plocinski, T.; Pisarek, M. The effect of chromium (III) oxide (Cr2O3) nanopowder on the microstructure and cyclic hydrogen storage behavior of magnesium hydride (MgH2). J. Alloy. Compd. 2011, 509, 2386–2391. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.M.; Zhang, H.F.; Ding, B.Z.; Hu, Z.Q. Hydrogenation characteristics of Mg–TiO2 (rutile) composite. J. Alloy. Compd. 2000, 313, 218–223. [Google Scholar] [CrossRef]
- Jung, K.S.; Kim, D.H.; Lee, E.Y.; Lee, K.S. Hydrogen sorption of magnesium hydride doped with nano-sized TiO2. Catal. Today 2007, 120, 270–275. [Google Scholar] [CrossRef]
- Jardim, P.M.; Conceição, M.O.T.; Brum, M.C.; dos Santos, D.S. Hydrogen sorption kinetics of ball-milled MgH2–TiO2 based 1D nanomaterials with different morphologies. Int. J. Hydrogen Energy 2015, 40, 17110–17117. [Google Scholar] [CrossRef]
- Chen, B.-H.; Chuang, Y.-S.; Chen, C.-K. Improving the hydrogenation properties of MgH2 at room temperature by doping with nano-size ZrO2 catalyst. J. Alloy. Compd. 2016, 655, 21–27. [Google Scholar] [CrossRef]
- Gupta, R.; Agresti, F.; Russo, S.L.; Maddalena, A.; Palade, P.; Principi, G. Structure and hydrogen storage properties of MgH2 catalysed with La2O3. J. Alloy. Compd. 2008, 450, 310–313. [Google Scholar] [CrossRef]
- Singh, R.K.; Sadhasivam, T.; Sheeja, G.I.; Singh, P.; Srivastava, O.N. Effect of different sized CeO2 nano particles on decomposition and hydrogen absorption kinetics of magnesium hydride. Int. J. Hydrogen Energy 2013, 38, 6221–6225. [Google Scholar] [CrossRef]
- Lin, H.-J.; Tang, J.-J.; Yu, Q.; Wang, H.; Ouyang, L.-Z.; Zhao, Y.-J.; Liu, J.-W.; Wang, W.-H.; Zhu, M. Symbiotic CeH2.73/CeO2 catalyst: A novel hydrogen pump. Nano Energy 2014, 9, 80–87. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Hydrogen sorption improvement of MgH2 catalyzed by CeO2 nanopowder. J. Alloy. Compd. 2017, 695, 2532–2538. [Google Scholar] [CrossRef]
- Shan, J.; Li, P.; Wan, Q.; Zhai, F.; Zhang, J.; Li, Z.; Liu, Z.; Volinsky, A.A.; Qu, X. Significantly improved dehydrogenation of ball-milled MgH2 doped with CoFe2O4 nanoparticles. J. Power Sources 2014, 268, 778–786. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Shan, J.; Zhai, F.; Li, Z.; Qu, X. Superior Catalytic Effect of Nickel Ferrite Nanoparticles in Improving Hydrogen Storage Properties of MgH2. J. Phys. Chem. C 2015, 119, 2925–2934. [Google Scholar] [CrossRef]
- Juahir, N.; Mustafa, N.S.; Sinin, A.M.; Ismail, M. Improved hydrogen storage properties of MgH2 by addition of Co2NiO nanoparticles. RSC Adv. 2015, 5, 60983–60989. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Sulaiman, N.N.; Ismail, M. Effect of SrFe12O19 nanopowder on the hydrogen sorption properties of MgH2. RSC Adv. 2016, 6, 110004–110010. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Jain, A.; Wang, Y.; Yamaguchi, S.; Miyaoka, H.; Ichikawa, T.; Kojima, Y.; Hashimoto, N. Enhancement of hydrogen desorption kinetics in magnesium hydride by doping with lithium metatitanate. J. Alloy. Compd. 2017, 711, 400–405. [Google Scholar] [CrossRef]
- Idris, N.H.; Mustafa, N.S.; Ismail, M. MnFe2O4 nanopowder synthesised via a simple hydrothermal method for promoting hydrogen sorption from MgH2. Int. J. Hydrogen Energy 2017, 42, 21114–21120. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Fan, X.; Xiao, X.; Zheng, J.; Huang, X. Enhanced hydrogen storage properties of MgH2 with numerous hydrogen diffusion channels provided by Na2Ti3O7 nanotubes. J. Mater. Chem. A 2017, 5, 6178–6185. [Google Scholar] [CrossRef]
- Xu, G.; Shen, N.; Chen, L.; Chen, Y.; Zhang, W. Effect of BiVO4 additive on the hydrogen storage properties of MgH2. Mater. Res. Bull. 2017, 89, 197–203. [Google Scholar] [CrossRef]
- Ares-Fernández, J.-R.; Aguey-Zinsou, K.-F. Superior MgH2 Kinetics with MgO Addition: A Tribological Effect. Catalysts 2012, 2, 330–343. [Google Scholar] [CrossRef]
- Bhat, V.V.; Rougier, A.; Aymard, L.; Darok, X.; Nazri, G.; Tarascon, J.M. Catalytic activity of oxides and halides on hydrogen storage of MgH2. J. Power Sources 2006, 159, 107–110. [Google Scholar] [CrossRef]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with magnesium hydride. Int. J. Hydrogen Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Malka, I.E.; Pisarek, M.; Czujko, T.; Bystrzycki, J. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH2 sorption properties. Int. J. Hydrogen Energy 2011, 36, 12909–12917. [Google Scholar] [CrossRef]
- Jin, S.-A.; Shim, J.-H.; Cho, Y.W.; Yi, K.-W. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides. J. Power Sources 2007, 172, 859–862. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, Y.; Erde, W. Hydrogen storage properties of the Mg–Ni–CrCl3 nanocomposite. J. Alloy. Compd. 2002, 333, 207–214. [Google Scholar]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Ismail, M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2. Energy 2015, 79, 177–182. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Juahir, N.; Yap, F.A.H. Catalytic effect of CeCl3 on the hydrogen storage properties of MgH2. Mater. Chem. Phys. 2016, 170, 77–82. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Yamaguchi, S.; Miyaoka, H.; Ichikawa, T.; Mukherjee, A.; Dey, G.K.; Kojima, Y. Surface modification of MgH2 by ZrCl4 to tailor the reversible hydrogen storage performance. Int. J. Hydrogen Energy 2017, 42, 6152–6159. [Google Scholar] [CrossRef]
- Ma, L.-P.; Kang, X.-D.; Dai, H.-B.; Liang, Y.; Fang, Z.-Z.; Wang, P.-J.; Wang, P.; Cheng, H.-M. Superior catalytic effect of TiF3 over TiCl3 in improving the hydrogen sorption kinetics of MgH2: Catalytic role of fluorine anion. Acta Mater. 2009, 57, 2250–2258. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wang, P.; Cheng, H.-M. Hydrogen sorption kinetics of MgH2 catalyzed with titanium compounds. Int. J. Hydrogen Energy 2010, 35, 3046–3050. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Sun, L.; Li, X. Effects of F and Cl on the stability of MgH2. Int. J. Hydrogen Energy 2014, 39, 877–883. [Google Scholar] [CrossRef]
- Jain, P.; Dixit, V.; Jain, A.; Srivastava, O.N.; Huot, J. Effect of Magnesium Fluoride on Hydrogenation Properties of Magnesium Hydride. Energies 2015, 8, 12546–12556. [Google Scholar] [CrossRef]
- Lin, H.-J.; Matsuda, J.; Li, H.-W.; Zhu, M.; Akiba, E. Enhanced hydrogen desorption property of MgH2 with the addition of cerium fluorides. J. Alloy. Compd. 2015, 645, S392–S396. [Google Scholar] [CrossRef]
- Recham, N.; Bhat, V.V.; Kandavel, M.; Aymard, L.; Rougier, A. Reduction of hydrogen desorption temperature of ball-milled MgH2 by NbF5 addition. J. Alloy. Compd. 2008, 464, 377–382. [Google Scholar] [CrossRef]
- Danaie, M.; Mitlin, D. TEM analysis of the microstructure in TiF3-catalyzed and pure MgH2 during the hydrogen storage cycling. Acta Mater. 2012, 60, 6441–6456. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Kumar, S.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. How does TiF4 affect the decomposition of MgH2 and its complex variants?—An XPS investigation. J. Mater. Chem. A 2017, 5, 15543–15551. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Influence of K2TiF6 additive on the hydrogen sorption properties of MgH2. Int. J. Hydrogen Energy 2014, 39, 15563–15569. [Google Scholar] [CrossRef]
- Yap, F.A.H.; Mustafa, N.S.; Ismail, M. A study on the effects of K2ZrF6 as an additive on the microstructure and hydrogen storage properties of MgH2. RSC Adv. 2015, 5, 9255–9260. [Google Scholar]
- Sulaiman, N.N.; Juahir, N.; Mustafa, N.S.; Yap, F.A.H.; Ismail, M. Improved hydrogen storage properties of MgH2 catalyzed with K2NiF6. J. Energy Chem. 2016, 25, 832–839. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Mustafa, N.S.; Ismail, M. Effect of Na3FeF6 catalyst on the hydrogen storage properties of MgH2. Dalton Trans. 2016, 45, 7085–7093. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lu, J.; Sohn, H.Y.; Fang, Z.Z. Hydrogen storage properties of the Mg–Ti–H system prepared by high-energy–high-pressure reactive milling. J. Power Sources 2008, 180, 491–497. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogen Storage Properties of Nanosized MgH2−0.1TiH2 Prepared by Ultrahigh-Energy−High-Pressure Milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogenation of Nanocrystalline Mg at Room Temperature in the Presence of TiH2. J. Am. Chem. Soc. 2010, 132, 6616–6617. [Google Scholar] [CrossRef]
- Sabitu, S.T.; Gallo, G.; Goudy, A.J. Effect of TiH2 and Mg2Ni additives on the hydrogen storage properties of magnesium hydride. J. Alloy. Compd. 2010, 499, 35–38. [Google Scholar] [CrossRef]
- Cuevas, F.; Korablova, D.; Latroche, M. Synthesis, structural and hydrogenation properties of Mg-rich MgH2–TiH2 nanocomposites prepared by reactive ball milling under hydrogen gas. Phys. Chem. Chem. Phys. 2012, 14, 1200–1211. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Agarwal, S.; Zhang, T.; Kumar, S.; Selvaraj, S.; Ichikawa, T.; Jain, I.P. The enhanced de/re-hydrogenation performance of MgH2 with TiH2 additive. Int. J. Energy Res. 2018, 42, 1139–1147. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Johnson, J.K.; Shaz, M.A.; Srivastava, O.N. TiH2 as a Dynamic Additive for Improving the De/Rehydrogenation Properties of MgH2: A Combined Experimental and Theoretical Mechanistic Investigation. J. Phys. Chem. C 2018, 122, 21248–21261. [Google Scholar] [CrossRef]
- Yavari, A.R.; de Castro, J.F.R.; Vaughan, G.; Heunen, G. Structural evolution and metastable phase detection in MgH2–5%NbH nanocomposite during in-situ H-desorption in a synchrotron beam. J. Alloy. Compd. 2003, 353, 246–251. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liu, Y.; Dong, Z.; Ge, H.; Li, S.; Yan, M. Hydrogen Desorption Properties of the MgH2–AlH3 Composites. J. Phys. Chem. C 2014, 118, 37–45. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Enhanced hydrogen storage properties of 4MgH2 + LiAlH4 composite system by doping with Fe2O3 nanopowder. Int. J. Hydrogen Energy 2014, 39, 7834–7841. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Mao, J.F.; Dou, S.X. The hydrogen storage properties and reaction mechanism of the MgH2–NaAlH4 composite system. Int. J. Hydrogen Energy 2011, 36, 9045–9050. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Meethom, S.; Utke, R. Dehydrogenation kinetics, reversibility, and reaction mechanisms of reversible hydrogen storage material based on nanoconfined MgH2−NaAlH4. J. Phys. Chem. Solids 2015, 87, 16–22. [Google Scholar] [CrossRef]
- Johnson, S.R.; Anderson, P.A.; Edwards, P.P.; Gameson, I.; Prendergast, J.W.; Al-Mamouri, M.; Book, D.; Harris, I.R.; Speight, J.D.; Walton, A. Chemical activation of MgH2; a new route to superior hydrogen storage materials. Chem. Commun. 2005, 22, 2823–2825. [Google Scholar] [CrossRef] [PubMed]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Torben, R.J.; Yngve, C.; Oliver, G.; Thomas, K.; et al. Hydrogen sorption properties of MgH2–LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Pan, Y.; Leng, H.; Wei, J.; Li, Q. Effect of LiBH4 on hydrogen storage property of MgH2. Int. J. Hydrogen Energy 2013, 38, 10461–10469. [Google Scholar] [CrossRef]
- Czujko, T.; Varin, R.A.; Wronski, Z.; Zaranski, Z.; Durejko, T. Synthesis and hydrogen desorption properties of nanocomposite magnesium hydride with sodium borohydride (MgH2 + NaBH4). J. Alloy. Compd. 2007, 427, 291–299. [Google Scholar] [CrossRef]
- Pan, Y.-B.; Wu, Y.-F.; Li, Q. Modeling and analyzing the hydriding kinetics of Mg–LaNi5 composites by Chou model. Int. J. Hydrogen Energy 2011, 36, 12892–12901. [Google Scholar] [CrossRef]
- Vijay, R.; Sundaresan, R.; Maiya, M.P.; Murthy, S.S.; Fu, Y.; Klein, H.-P.; Groll, M. Characterisation of Mg–x wt.% FeTi (x = 5–30) and Mg–40wt.% FeTiMn hydrogen absorbing materials prepared by mechanical alloying. J. Alloy. Compd. 2004, 384, 283–295. [Google Scholar] [CrossRef]
- Amirkhiz, B.S.; Zahiri, B.; Kalisvaart, P.; Mitlin, D. Synergy of elemental Fe and Ti promoting low temperature hydrogen sorption cycling of magnesium. Int. J. Hydrogen Energy 2011, 36, 6711–6722. [Google Scholar] [CrossRef]
- Yu, X.B.; Yang, Z.X.; Liu, H.K.; Grant, D.M.; Walker, G.S. The effect of a Ti-V-based BCC alloy as a catalyst on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2010, 35, 6338–6344. [Google Scholar] [CrossRef]
- Laversenne, L.; Andrieux, J.; Plante, D.; Lyard, L.; Miraglia, S. In operando study of TiVCr additive in MgH2 composites. Int. J. Hydrogen Energy 2013, 38, 11937–11945. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Zhou, C.; Lu, J.; Ren, Y.; Zhang, X. Hydrogen Storage Properties of Magnesium Hydride with V-Based Additives. J. Phys. Chem. C 2014, 118, 21778–21784. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti Intermetallic Catalysts on Hydrogen Storage Properties of Magnesium Hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, A.; Jain, P.; Jangir, M.; Jain, I.P. Kinetic Enhancement in the Sorption Properties by Forming Mg–x wt % ZrCrCu Composites. J. Phys. Chem. C 2013, 117, 11953–11959. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-H.; Hwang, K.-T.; Kang, Y.-M. Hydrogen storage in magnesium based-composite hydride through hydriding combustion synthesis. Int. J. Hydrogen Energy 2010, 35, 9641–9645. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Jain, I.P.; Montone, A. Catalytic effect of ZrCrNi alloy on hydriding properties of MgH2. Int. J. Hydrogen Energy 2009, 34, 9157–9162. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.F.; Ding, B.Z.; Hu, Z.Q. Direct hydrogenation of Mg and decomposition behavior of the hydride formed. J. Alloy. Compd. 2000, 313, 209–213. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.; Zhang, H.; Ding, B.; Hu, Z. Hydriding properties of a mechanically milled Mg–50 wt.% ZrFe1.4Cr0.6 composite. J. Alloy. Compd. 2000, 297, 240–245. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Montone, A. Structural and H2 sorption properties of MgH2–10 wt%ZrCrM (M = Cu, Ni) nano-composites. J. Nanopart. Res. 2011, 13, 5719–5726. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Jain, P.; Gislon, P.; Prosini, P.P.; Jain, I.P. Hydriding behavior of Mg-50 wt% ZrCrFe composite Prepared by high energy ball milling. Int. J. Hydrogen Energy 2012, 37, 3665–3670. [Google Scholar] [CrossRef]
- Jain, A.; Jain, P.; Agarwal, S.; Gislon, P.; Prosini, P.P.; Jain, I.P. Structural and Hydrogen Storage Properties Of Mg-x Wt% ZrCrMn Composites. Adv. Mater. Lett. 2014, 5, 692–698. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, A.; Jain, P.; Jangir, M.; Vyas, D.; Jain, I.P. Effect of ZrCrCo alloy on hydrogen storage properties of Mg. J. Alloy. Compd. 2015, 645, S518–S523. [Google Scholar] [CrossRef]
- Molinas, B.; Ghilarducci, A.A.; Melnichuk, M.; Corso, H.L.; Peretti, H.A.; Agresti, F.; Bianchin, A.; Russo, S.L.; Maddalena, A.; Principi, G. Scaled-up production of a promising Mg-based hydride for hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 4597–4601. [Google Scholar] [CrossRef]
- Pighin, S.A.; Capurso, G.; Russo, S.L.; Peretti, H.A. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloy. Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- Jia, Y.; Han, S.; Zhang, W.; Zhao, X.; Wang, J. Hydrogen absorption and desorption kinetics of MgH2 catalyzed by MoS2 and MoO2. Int. J. Hydrogen Energy 2013, 38, 2352–2356. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, Y.; Han, D.; Han, S. The hydrogen storage properties of MgH2–Fe3S4 composites. Energy 2015, 93, 625–630. [Google Scholar] [CrossRef]
- Xie, X.; Chen, M.; Liu, P.; Shang, J.; Liu, T. High hydrogen desorption properties of Mg-based nanocomposite at moderate temperatures: The effects of multiple catalysts in situ formed by adding nickel sulfides/graphene. J. Power Sources 2017, 371, 112–118. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Liu, P.; Shang, J.; Li, X.; Liu, T. Formation of Multiple-Phase Catalysts for the Hydrogen Storage of Mg Nanoparticles by Adding Flowerlike NiS. ACS Appl. Mater. Interfaces 2017, 9, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, G.; Cheng, Y.; Chen, L.; Huo, Q.; Liu, S. Improved hydrogen storage properties of MgH2 by the addition of FeS2 micro-spheres. Dalton Trans. 2018, 47, 5217–5225. [Google Scholar] [CrossRef] [PubMed]
- Milanese, C.; Garroni, S.; Gennari, F.; Marini, A.; Klassen, T.; Dornheim, M.; Pistidda, C. Solid State Hydrogen Storage in Alanates and Alanate-Based Compounds: A Review. Metals 2018, 8, 567. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Zhang, X.; Jian, N.; Yang, Y.; Gao, M.; Pan, H. Development of Catalyst-Enhanced Sodium Alanate as an Advanced Hydrogen-Storage Material for Mobile Applications. Energy Technol. 2018, 6, 487–500. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloy. Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Bogdanović, B.; Sandrock, G. Catalyzed Complex Metal Hydrides. MRS Bull. 2002, 27, 712–716. [Google Scholar] [CrossRef]
- Leon, A.; Kircher, O.; Rothe, J.; Fichtner, M. Chemical state and local structure around titanium atoms in NaAlH4 doped with TiCl3 using X-ray absorption spectroscopy. J. Phys. Chem. B 2004, 108, 16372–16376. [Google Scholar] [CrossRef]
- Luo, W.; Gross, K.J. A kinetics model of hydrogen absorption and desorption in Ti-doped NaAlH4. J. Alloy. Compd. 2004, 385, 224–231. [Google Scholar] [CrossRef]
- Gross, K.J.; Majzoub, E.H.; Spangler, S.W. The effects of titanium precursors on hydriding properties of alanates. J. Alloy. Compd. 2003, 356–357, 423–428. [Google Scholar] [CrossRef]
- von Colbe, J.M.B.; Felderhoff, M.; Bogdanovic, B.; Schuth, F.; Weidenthaler, C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material. Chem. Commun. 2005, 37, 4732–4734. [Google Scholar] [CrossRef]
- Onkawa, M.; Zhang, S.; Takeshita, H.T.; Kuriyama, N.; Kiyobayashi, T. Dehydrogenation kinetics of Ti-doped NaAlH4—Influence of Ti precursors and preparation methods. Int. J. Hydrogen Energy 2008, 33, 718–721. [Google Scholar] [CrossRef]
- Paskevicius, M.; Filsø, U.; Karimi, F.; Puszkiel, J.; Pranjas, P.K.; Pistidda, C.; Armin, H.; Edmund, W.; Andreas, S.; Thomas, K.; et al. Cyclic stability and structure of nanoconfined Ti-doped NaAlH4. Int. J. Hydrogen Energy 2016, 41, 4159–4167. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.D.; Lomness, J.K.; Slattery, D.K. Effect of TixAly catalysts on hydrogen storage properties of LiAlH4 and NaAlH4. Int. J. Hydrogen Energy 2005, 30, 1417–1421. [Google Scholar] [CrossRef]
- Lee, G.-J.; Kim, J.W.; Shim, J.-H.; Cho, Y.W.; Lee, K.S. Synthesis of ultrafine titanium aluminide powders and their catalytic enhancement in dehydrogenation kinetics of NaAlH4. Scr. Mater. 2007, 56, 125–128. [Google Scholar] [CrossRef]
- Li, L.; Qiu, F.; Wang, Y.; Liu, G.; Yan, C.; An, C.; Xu, Y.; Wang, Y.; Song, D.; Jiao, L.; et al. Improved dehydrogenation performances of TiB2-doped sodium alanate. Mater. Chem. Phys. 2012, 134, 1197–1202. [Google Scholar] [CrossRef]
- Chen, L.-X.; Fan, X.-L.; Xiao, X.-Z.; Xue, J.-W.; Li, S.-Q.; Ge, H.-W.; Chen, C.-P. Influence of TiC catalyst on absorption/desorption behaviors and microstructures of sodium aluminum hydride. Trans. Nonferr. Met. Soc. China 2011, 21, 1297–1302. [Google Scholar] [CrossRef]
- Wu, R.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Remarkably improved hydrogen storage properties of NaAlH4 doped with 2D titanium carbide. J. Power Sources 2016, 327, 519–525. [Google Scholar] [CrossRef]
- Xiong, R.; Sang, G.; Zhang, G.; Yan, X.; Li, P.; Yao, Y.; Luo, D.; Chen, C.; Tang, T. Evolution of the active species and catalytic mechanism of Ti doped NaAlH4 for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 6088–6095. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Cao, Y.; Gao, M.; Pan, H.; Wang, Q. Mechanisms for the enhanced hydrogen desorption performance of the TiF4-catalyzed Na2LiAlH6 used for hydrogen storage. Energy Environ. Sci. 2010, 3, 645–653. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Jiao, L.; Yuan, H. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst. J. Alloy. Compd. 2003, 356–357, 423–428. [Google Scholar] [CrossRef]
- Lee, G.-J.; Shim, J.-H.; Cho, Y.W.; Lee, K.S. Improvement in desorption kinetics of NaAlH4 catalyzed with TiO2 nanopowder. Int. J. Hydrogen Energy 2008, 33, 3748–3753. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, K.; Gao, M.; Pan, H. Remarkably improved hydrogen storage properties of nanocrystalline TiO2-modified NaAlH4 and evolution of Ti-containing species during dehydrogenation/hydrogenation. Nano Res. 2015, 8, 533–545. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sterlin, M.; Hudson, L.; Sinha, A.S.K.; Srivastava, O.N. Studies on metal oxide nanoparticles catalyzed sodium aluminum hydride. Energy 2010, 35, 5037–5042. [Google Scholar] [CrossRef]
- Wang, P.; Jensen, C.M. Method for preparing Ti-doped NaAlH4 using Ti powder: Observation of an unusual reversible dehydrogenation behavior. J. Alloy. Compd. 2004, 379, 99–102. [Google Scholar] [CrossRef]
- Wang, P.; Jensen, C.M. Preparation of Ti-Doped Sodium Aluminum Hydride from Mechanical Milling of NaH/Al with Off-the-Shelf Ti Powder. J. Phys. Chem. B 2004, 108, 15827–15829. [Google Scholar] [CrossRef]
- Zidan, R.A.; Takara, S.; Hee, A.G.; Jensen, C.M. Hydrogen cycling behavior of zirconium and titanium–zirconium-doped sodium aluminum hydride. J. Alloy. Compd. 1999, 285, 119–122. [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Zidan, R.; Ritter, J.A. Synergistic effects of co-dopants on the dehydrogenation kinetics of sodium aluminum hydride. J. Alloy. Compd. 2005, 391, 245–255. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N. Advanced Hydrogen-Storage Materials Based on Sc-, Ce-, and Pr-Doped NaAlH4. Adv. Mater. 2006, 18, 1198–1201. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N.; Stark, A. Cycling properties of Sc- and Ce-doped NaAlH4 hydrogen storage materials prepared by the one-step direct synthesis method. J. Alloy. Compd. 2005, 391, 245–255. [Google Scholar] [CrossRef]
- Rongeat, C.; Scheerbaum, N.; Schultz, L.; Gutfleisch, O. Catalysis of H2 sorption in NaAlH4: General description and new insights. Acta Mater. 2011, 59, 1725–1733. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Chen, L.; Yu, K.; Wu, Z.; Li, S.; Wang, Q. Active species of CeAl4 in the CeCl3-doped sodium aluminium hydride and its enhancement on reversible hydrogen storage performance. Chem. Commun. 2009, 44, 6857–6859. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiao, X.; Chen, L.; Li, S.; Ge, H.; Wang, Q. Enhanced Hydriding−Dehydriding Performance of CeAl2-Doped NaAlH4 and the Evolvement of Ce-Containing Species in the Cycling. J. Phys. Chem. C 2011, 115, 2537–2543. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Chen, L.; Li, S.; Ge, H.; Wang, Q. Thermodynamics, Kinetics, and Modeling Investigation on the Dehydrogenation of CeAl4-Doped NaAlH4 Hydrogen Storage Material. J. Phys. Chem. C 2011, 115, 22680–22687. [Google Scholar] [CrossRef]
- Balema, V.P.; Wiench, J.W.; Dennis, K.W.; Pruski, M.; Pecharsky, V.K. Titanium catalyzed solid-state transformations in LiAlH4 during high-energy ball-milling. J. Alloy. Compd. 2001, 329, 108–114. [Google Scholar] [CrossRef]
- Liu, X.; McGrady, G.S.; Langmi, H.W.; Jensen, C.M. Facile cycling of Ti-doped LiALH4 for high performance hydrogen storage. J. Am. Chem. Soc. 2009, 131, 5032–5033. [Google Scholar] [CrossRef]
- Blanchard, D.; Brinks, H.W.; Hauback, B.C.; Norby, P. Desorption of LiAlH4 with Ti- and V-based additives. Mater. Sci. Eng. B 2004, 108, 54–59. [Google Scholar] [CrossRef]
- Zang, L.; Cai, J.; Zhao, L.; Gao, W.; Liu, J.; Wang, Y. Improved hydrogen storage properties of LiAlH4 by mechanical milling with TiF3. J. Alloy. Compd. 2015, 647, 756–762. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.D.; Lomness, J.K.; Slattery, D.K. Effects of various catalysts on hydrogen release and uptake characteristics of LiAlH4. Int. J. Hydrogen Energy 2008, 33, 3748–3753. [Google Scholar]
- Fernandez, J.R.A.; Aguey-Zinsou, F.; Elsaesser, M.; Ma, X.Z.; Bormann, R. Mechanical and thermal decomposition of LiAlH4 with metal halides. Int. J. Hydrogen Energy 2007, 32, 1033–1040. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Matsumoto, M.; Haga, T. Hydrogen release of catalyzed lithium aluminum hydride by a mechanochemical reaction. J. Alloy. Compd. 2008, 462, 275–278. [Google Scholar] [CrossRef]
- Cai, J.; Zang, L.; Zhao, L.; Liu, J.; Wang, Y. Dehydrogenation characteristics of LiAlH4 improved by in-situ formed catalysts. J. Energy Chem. 2016, 25, 868–873. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X.; Wang, H.; Ouyang, L. Catalytic effect of ScCl3 on the dehydrogenation properties of LiAlH4. J. Alloy. Compd. 2018, 762, 73–79. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Dou, S.X. Effects of NbF5 addition on the hydrogen storage properties of LiAlH4. Int. J. Hydrogen Energy 2010, 35, 2361–2367. [Google Scholar] [CrossRef]
- Sun, T.; Huang, C.K.; Wang, H.; Sun, L.X.; Zhu, M. The effect of doping NiCl2 on the dehydrogenation properties of LiAlH4. Int. J. Hydrogen Energy 2008, 33, 6216–6221. [Google Scholar] [CrossRef]
- Ismail, M.; Sinin, A.M.; Sheng, C.K.; Nik, W.B.W. Desorption Behaviours of Lithium Alanate with Metal Oxide Nanopowder Additives. Int. J. Electrochem. Sci. 2014, 9, 4959–4973. [Google Scholar]
- Qu, X.; Li, P.; Zhang, L.; Ahmad, M. Hydrogen Sorption Improvement of LiAlH4 Catalyzed by Nb2O5 and Cr2O3 Nanoparticles. J. Phys. Chem. C 2011, 115, 13088–13099. [Google Scholar]
- Liu, S.; Ma, Q.; Zheng, X.; Fang, X.; Guo, X.; Zheng, X. Influences of Y2O3 Doping on Hydrogen Release Property of LiAlH4. Rare Metal Mater. Eng. 2014, 43, 0287–0290. [Google Scholar]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Ranjbar, A.; Dou, S.X. Improved hydrogen desorption in lithium alanate by addition of SWCNT–metallic catalyst composite. Int. J. Hydrogen Energy 2011, 36, 3593–3599. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Yang, C.-H.; Tsai, W.-T. Catalytic effect of MWCNTs on the dehydrogenation behavior of LiAlH4. Int. J. Hydrogen Energy 2014, 39, 927–933. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, C.; Zhou, H.; Gao, M.; Pan, H.; Wang, Q. A novel catalyst precursor K2TiF6 with remarkable synergetic effects of K, Ti and F together on reversible hydrogen storage of NaAlH4. Chem. Commun. 2011, 47, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, S.; Si, X.; Zhang, J.; Jiao, C.; Wang, S. Significantly improved dehydrogenation of LiAlH4 destabilized by K2TiF6. Int. J. Hydrogen Energy 2012, 37, 3261–3267. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Li, Z.; Zhao, K.; Liu, Z.; Wang, L. NaAlH4 dehydrogenation properties enhanced by MnFe2O4 nanoparticles. J. Power Sources 2014, 248, 388–395. [Google Scholar] [CrossRef]
- Zhai, F.; Li, P.; Sun, A.; Wu, S.; Wan, Q.; Zhang, W.; Li, Y.; Cui, L.; Qu, X. Significantly Improved Dehydrogenation of LiAlH4 Destabilized by MnFe2O4 Nanoparticles. J. Phys. Chem. C 2012, 116, 11939–11945. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, X.; Qu, X. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles. J. Alloy. Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]
- Li, L.; An, C.; Wang, Y.; Xu, Y.; Qiu, F.; Wang, Y.; Jiao, L.; Yuan, H. Enhancement of the H2 desorption properties of LiAlH4 doping with NiCo2O4 nanorods. Int. J. Hydrogen Energy 2014, 39, 4414–4420. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Wang, Y.; Oka, H.; Hashimoto, N.; Ohnuki, S. A metal-oxide catalyst enhanced the desorption properties in complex metal hydrides. J. Mater. Chem. A 2014, 2, 4361–4365. [Google Scholar] [CrossRef]
- Frankcombe, T.J. Proposed Mechanisms for the Catalytic Activity of Ti in NaAlH4. Chem. Rev. 2012, 112, 2164–2178. [Google Scholar] [CrossRef]
- Ivanov, E.; Konstanchuk, I.; Stepanov, A.; Boldyrev, V. Magnesium mechanical alloys for hydrogen storage. J. Less Common Met. 1987, 131, 25–29. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O.; Kircher, O.; Rothe, J. Small Ti clusters for catalysis of hydrogen exchange in NaAlH4. Nanotechnology 2003, 14, 778. [Google Scholar] [CrossRef]
- Fichtner, M.; Engel, J.; Fuhr, O.; Kircher, O.; Rubner, O. Nanocrystalline aluminium hydrides for hydrogen storage. Mater. Sci. Eng. B 2004, 108, 42–47. [Google Scholar] [CrossRef]
- Kircher, O.; Fichtner, M. Hydrogen exchange kinetics in NaAlH4 catalyzed in different decomposition states. J. Appl. Phys. 2014, 95, 7748. [Google Scholar] [CrossRef]
- Sun, D.; Srinivasan, S.S.; Chen, G.; Jensen, C.M. Rehydrogenation and cycling studies of dehydrogenated NaAlH4. J. Alloy. Compd. 2004, 373, 265–269. [Google Scholar] [CrossRef]
- Singh, S.; Eijt, S.W.H.; Huot, J.; Kockelmann, W.A.; Wagemaker, M.; Mulder, F.M. The TiCl3 catalyst in NaAlH4 for hydrogen storage induces grain refinement and impacts on hydrogen vacancy formation. Acta Mater. 2007, 55, 5549–5557. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible Hydrogen Storage via Titanium-Catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Takeshita, H.T.; Sakai, T. Nanocrystalline Ti-doped Li3AlH6 as a Reversible Hydrogen Storage Material. Adv. Eng. Mater. 2001, 3, 695–698. [Google Scholar] [CrossRef]
- Sandrock, G.; Gross, K.; Thomas, G. Effect of Ti-catalyst content on the reversible hydrogen storage properties of the sodium alanates. J. Alloy. Compd. 2002, 339, 299–308. [Google Scholar] [CrossRef]
- Wang, P.; Kang, X.-D.; Cheng, H.-M. Exploration of the Nature of Active Ti Species in Metallic Ti-Doped NaAlH4. J. Phys. Chem. B 2005, 109, 20131–20136. [Google Scholar] [CrossRef]
- Blomqvist, A.; Araújo, C.M.; Jena, P.; Ahuja, R. Dehydrogenation from 3d-transition-metal-doped NaAlH4: Prediction of catalysts. Appl. Phys. Lett. 2007, 90, 141904. [Google Scholar] [CrossRef]
- Bai, K.; Wu, P. Role of Ti in the reversible dehydrogenation of Ti-doped sodium alanate. Appl. Phys. Lett. 2006, 89, 201904. [Google Scholar] [CrossRef]
- Araújo, C.M.; Ahuja, R.; Guillén, J.M.O. Role of titanium in hydrogen desorption in crystalline sodium alanate. Appl. Phys. Lett. 2005, 86, 251913. [Google Scholar] [CrossRef]
- Gross, K.J.; Guthrie, S.; Takara, S.; Thomas, G. In-situ X-ray diffraction study of the decomposition of NaAlH4. J. Alloy. Compd. 2000, 297, 270–281. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Chen, G.; Sun, D.; He, B.; Wei, Z.; Wei, S. Nature and Role of Ti Species in the Hydrogenation of a NaH/Al Mixture. J. Phys. Chem. C 2007, 111, 3476–3479. [Google Scholar]
- Fu, Q.J.; Ramirez-Cuesta, A.J.; Tsang, S.C. Molecular Aluminum Hydrides Identified by Inelastic Neutron Scattering during H2 Regeneration of Catalyst-Doped NaAlH4. J. Phys. Chem. B 2006, 110, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.T.; Scogin, J.H. A reversible hydrogen storage mechanism for sodium alanate: The role of alanes and the catalytic effect of the dopant. J. Alloy. Compd. 2004, 379, 135–142. [Google Scholar] [CrossRef]
- Ivancic, T.M.; Hwang, S.-J.; Bowman, R.C., Jr.; Birkmire, D.S.; Jensen, C.M.; Udovic, T.J.; Conradi, M.S. Discovery of A New Al Species in Hydrogen Reactions of NaAlH4. J. Phys. Chem. Lett. 2010, 1, 2412–2416. [Google Scholar] [CrossRef]
- Gunaydin, H.; Houk, K.N.; Ozoliņš, V. Vacancy-mediated dehydrogenation of sodium alanate. Proc. Natl. Acad. Sci. USA 2008, 105, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Borgschulte, A.; Züttel, A.; Hug, P.; Barkhordarian, G.; Eigen, N.; Dornheim, M.; Bormann, R.; Ramirez-Cuesta, A.J. Hydrogen–deuterium exchange experiments to probe the decomposition reaction of sodium alanate. Phys. Chem. Chem. Phys. 2008, 10, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Peles, A.; Van de Walle, C.G. Role of charged defects and impurities in kinetics of hydrogen storage materials: A first-principles study. Phys. Rev. B 2007, 76, 214101. [Google Scholar] [CrossRef]
- Marashdeh, A.; Olsen, R.A.; Løvvik, O.M.; Kroes, G.-J. Density Functional Theory Study of the TiH2 Interaction with a NaAlH4 Cluster. J. Phys. Chem. C 2008, 112, 15759–15764. [Google Scholar] [CrossRef]
- Atakli, Z.Ö.K.; Callini, E.; Kato, S.; Mauron, P.; Orimo, S.-I.; Züttel, A. The catalyzed hydrogen sorption mechanism in alkali alanates. Phys. Chem. Chem. Phys. 2015, 17, 20932–20940. [Google Scholar] [CrossRef] [PubMed]
- Ohba, N.; Miwa, K.; Aoki, M.; Noritake, T.; Towata, S.; Nakamori, Y.; Orimo, S.; Züttel, A. First-principles study on the stability of intermediate compounds of LiBH4. Phys. Rev. B 2006, 74, 075110. [Google Scholar] [CrossRef]
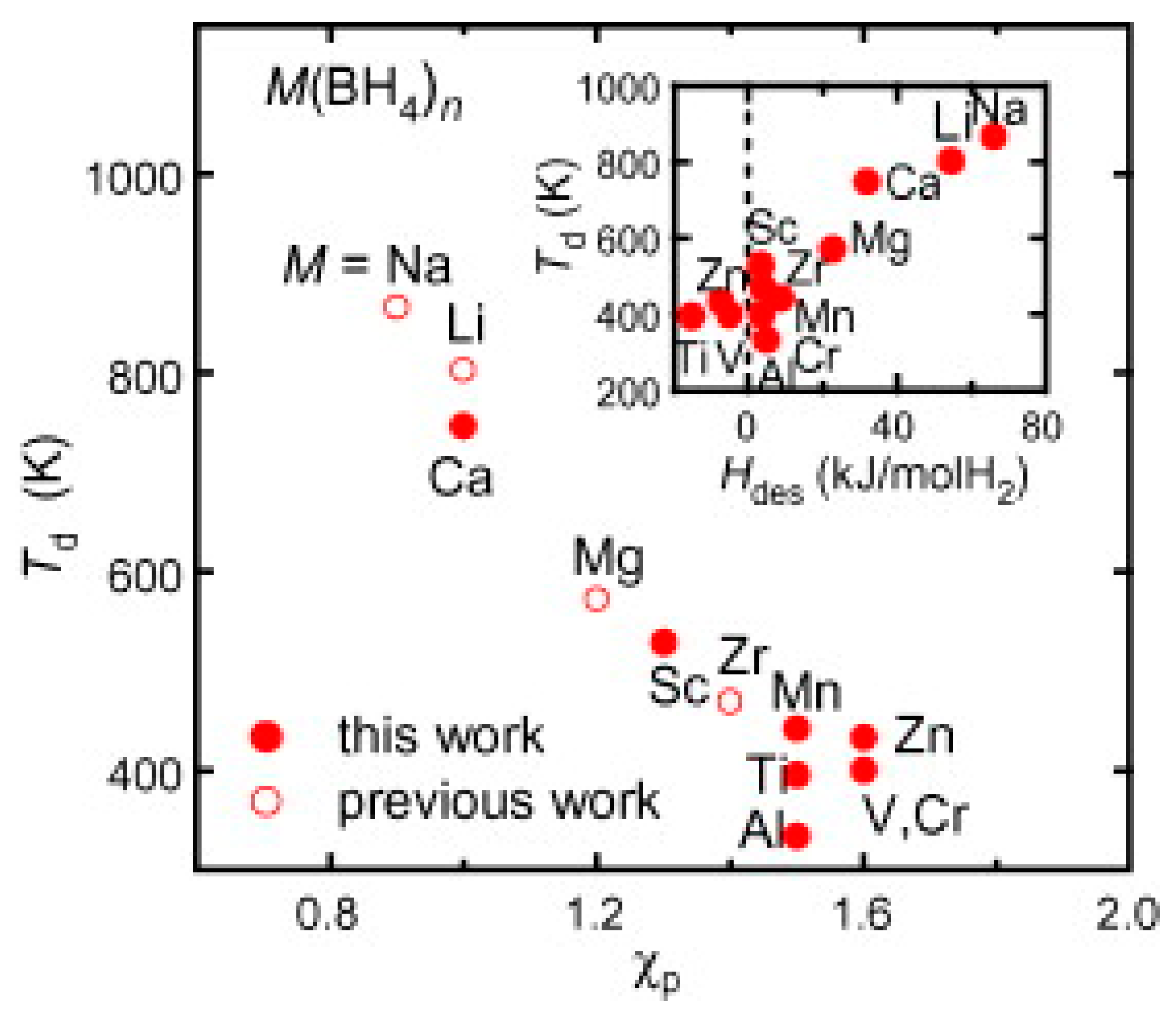
- Nakamori, Y.; Li, H.-W.; Kikuchi, K.; Aoki, M.; Miwa, K.; Towata, S.; Orimo, S. Thermodynamical stabilities of metal-borohydrides. J. Alloy. Compd. 2007, 446–447, 296–300. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Martin, D.; Flemming, B.; Torben, R.J. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Wu, Z.; Chen, Q.R.; Li, Z.L.; Weng, B.C.; Huang, T.S. Improved hydrogen storage properties of LiBH4 destabilized by carbon. Appl. Phys. Lett. 2007, 90, 034106. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, J.; Han, H.; Wu, Y. Effect of carbon nanotubes on the microstructural evolution and hydrogen storage properties of Mg(BH4)2. J. Alloy. Compd. 2018, 743, 11–16. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Kang, X.-D.; Wang, P. Improved hydrogen storage properties of LiBH4 by mechanical milling with various carbon additives. Int. J. Hydrogen Energy 2010, 35, 8247–8252. [Google Scholar] [CrossRef]
- Xu, J.; Meng, R.; Cao, J.; Gu, X.; Qi, Z.; Wang, W.; Chen, Z. Enhanced dehydrogenation and rehydrogenation properties of LiBH4 catalyzed by graphene. Int. J. Hydrogen Energy 2013, 38, 2796–2803. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, X.; Fan, X.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Fast hydrogen release under moderate conditions from NaBH4 destabilized by fluorographite. RSC Adv. 2014, 4, 2550–2556. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, G.; Chen, L.; Pan, S.; Jing, X.; Wang, J.; Han, S. Enhanced hydrogen storage performances of LiBH4 modified with three-dimensional porous fluorinated graphene. Int. J. Hydrogen Energy 2017, 42, 15262–15270. [Google Scholar] [CrossRef]
- Guo, L.; Li, Y.; Ma, Y.; Liu, Y.; Peng, D.; Zhang, L.; Han, S. Enhanced hydrogen storage capacity and reversibility of LiBH4 encapsulated in carbon nanocages. Int. J. Hydrogen Energy 2017, 42, 2215–2222. [Google Scholar] [CrossRef]
- Xua, J.; Meng, R.; Cao, J.; Gu, X.; Song, W.-L.; Qi, Z.; Wang, W.; Chen, Z. Graphene-supported Pd catalysts for reversible hydrogen storage in LiBH4. J. Alloy. Compd. 2013, 564, 84–90. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, F.; Tang, R.; Wang, Y.; Dong, H.; Li, Z. Hydrogen storage performance of nano Ni decorated LiBH4 on activated carbon prepared through organic solvent. J. Alloy. Compd. 2014, 612, 287–292. [Google Scholar] [CrossRef]
- Wahab, M.A.; Young, D.J.; Karim, A.; Fawzia, S.; Beltramini, J.N. Low-temperature hydrogen desorption from Mg(BH4)2 catalysed by ultrafine Ni nanoparticles in a mesoporous carbon matrix. Int. J. Hydrogen Energy 2016, 41, 20573–20582. [Google Scholar] [CrossRef]
- Xia, G.L.; Guo, Y.H.; Wu, Z.; Yu, X.B. Enhanced hydrogen storage performance of LiBH4–Ni composite. J. Alloy. Compd. 2009, 479, 545–548. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A.; Zeigler, K. Modified Lithium Borohydrides for Reversible Hydrogen Storage (2). J. Phys. Chem. B 2006, 110, 26482–26487. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with a Metal (M = Mg, Al, Ti, V, Cr, or Sc) or Metal Hydride (MH2 = MgH2, TiH2, or CaH2). J. Phys. Chem. C 2007, 111, 19134–19140. [Google Scholar] [CrossRef]
- Pendolino, F.; Mauron, P.; Borgschulte, A.; Züttel, A. Effect of Boron on the Activation Energy of the Decomposition of LiBH4. J. Phys. Chem. C 2009, 113, 17231–17234. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloy. Compd. 2003, 356–357, 515–520. [Google Scholar]
- Au, M.; Jurgensen, A. Modified Lithium Borohydrides for Reversible Hydrogen Storage. J. Phys. Chem. B 2006, 110, 7062–7067. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. Low-Temperature Dehydrogenation of LiBH4 through Destabilization with TiO2. J. Phys. Chem. C 2008, 112, 11059–11062. [Google Scholar] [CrossRef]
- Au, M.; Spencer, W.; Jurgensen, A.; Zeigler, C. Hydrogen storage properties of modified lithium borohydrides. J. Alloy. Compd. 2008, 462, 303–309. [Google Scholar] [CrossRef]
- Mosegaard, L.; Møller, B.; Jørgensen, J.-E.; Filinchuk, Y.; Cerenius, Y.; Hanson, J.C.; Dimasi, E.; Besenbacher, F.; Jensen, T.R. Reactivity of LiBH4: In Situ Synchrotron Radiation Powder X-ray Diffraction Study. J. Phys. Chem. C 2008, 112, 1299–1303. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. Dehydrogenation of LiBH4 Destabilized with Various Oxides. J. Phys. Chem. C 2009, 113, 17945–17949. [Google Scholar] [CrossRef]
- Ploysuksai, W.; Rangsunvigit, P.; Kulprathipanja, S. Effects of TiO2 and Nb2O5 on Hydrogen desorption of Mg(BH4)2. Int. J. Mater. Metall. Eng. 2012, 6, 311–315. [Google Scholar]
- Zavorotynska, O.; Saldan, I.; Hino, S.; Humphries, T.D.; Deledda, S.; Hauback, B.C. Hydrogen cycling in γ-Mg(BH4)2 with cobalt-based additives. J. Mater. Chem. A 2015, 3, 6592–6602. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H. Hydrogen storage properties of γ–Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrogen Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Xu, X.; Zang, L.; Zhao, Y.; Zhao, Y.; Wang, Y.; Jiao, L. Hydrogen storage behavior of LiBH4 improved by the confinement of hierarchical porous ZnO/ZnCo2O4 nanoparticles. J. Power Sources 2017, 359, 134–141. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Liu, Y.; Wang, Y.; Yuan, H.; Jiao, L. Synergistic effects of destabilization, catalysis and nanoconfinement on dehydrogenation of LiBH4. Int. J. Hydrogen Energy 2017, 42, 1354–1360. [Google Scholar] [CrossRef]
- Au, M.; Walters, R.T. Reversibility aspect of lithium borohydrides. Int. J. Hydrogen Energy 2010, 35, 10311–10316. [Google Scholar] [CrossRef]
- Zhang, B.J.; Liu, B.H. Hydrogen desorption from LiBH4 destabilized by chlorides of transition metal Fe, Co, and Ni. Int. J. Hydrogen Energy 2010, 35, 7288–7294. [Google Scholar] [CrossRef]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.E.; Zhang, J.Z. Reversibility and Improved Hydrogen Release of Magnesium Borohydride. J. Phys. Chem. C 2010, 114, 5224–5232. [Google Scholar] [CrossRef]
- Bardají, E.G.; Hanada, N.; Zabara, O.; Fichtner, M. Effect of several metal chlorides on the thermal decomposition behaviour of α-Mg(BH4)2. Int. J. Hydrogen Energy 2011, 36, 12313–12318. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Nevirkovets, I.P.; Liu, H.K.; Dou, S.X. Hydrogen De-/Absorption Improvement of NaBH4 Catalyzed by Titanium-Based Additives. J. Phys. Chem. C 2012, 116, 1596–1604. [Google Scholar] [CrossRef]
- Al-Kukhun, A.; Hwang, H.T.; Varma, A. NbF5 additive improves hydrogen release from magnesium borohydride. Int. J. Hydrogen Energy 2012, 37, 17671–17677. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, H.; Liu, J.W.; Zhu, M. Thermal decomposition behaviors of magnesium borohydride doped with metal fluoride additives. Thermochim. Acta 2013, 560, 82–88. [Google Scholar] [CrossRef]
- Paduani, C.; Jena, P. Role of Ti-based catalysts in the dehydrogenation mechanism of magnesium borohydride: A cluster approach. Int. J. Hydrogen Energy 2013, 38, 2357–2362. [Google Scholar] [CrossRef]
- Humphries, T.D.; Kalantzopoulos, G.N.; Llamas-Jansa, I.; Olsen, J.E.; Hauback, B.C. Reversible Hydrogenation Studies of NaBH4 Milled with Ni-Containing Additives. J. Phys. Chem. C 2013, 117, 6060–6065. [Google Scholar] [CrossRef]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Mechanisms of reversible hydrogen storage in NaBH4 through NdF3 addition. J. Mater. Chem. A 2013, 1, 3983–3991. [Google Scholar] [CrossRef]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Reversible hydrogen sorption in NaBH4 at lower temperatures. J. Mater. Chem. A 2013, 1, 13510–13523. [Google Scholar] [CrossRef]
- Zou, J.; Li, L.; Zeng, X.; Ding, W. Reversible hydrogen storage in a 3NaBH4/YF3 composite. Int. J. Hydrogen Energy 2012, 37, 17118–17125. [Google Scholar] [CrossRef]
- Saldan, I.; Hino, S.; Humphries, T.D.; Zavorotynska, O.; Chong, M.; Jensen, C.M.; Deledda, S.; Hauback, B.C. Structural Changes Observed during the Reversible Hydrogenation of Mg(BH4)2 with Ni-Based Additives. J. Phys. Chem. C 2014, 118, 23376–23384. [Google Scholar] [CrossRef]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Study on reversible hydrogen sorption behaviors of a 3NaBH4/HoF3 composite. Int. J. Hydrogen Energy 2014, 39, 14275–14281. [Google Scholar] [CrossRef]
- Tu, G.; Xiao, X.; Jiang, Y.; Qin, T.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Composite cooperative enhancement on the hydrogen desorption kinetics of LiBH4 by co-doping with NbCl5 and hexagonal BN. Int. J. Hydrogen Energy 2015, 33, 10527–10535. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, L.; Gao, S.; Liu, H.; Xu, L.; Wang, X.; Yan, M. Hydrogen storage properties of activated carbon confined LiBH4 doped with CeF3 as catalyst. Int. J. Hydrogen Energy 2017, 42, 23010–23017. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Nakajima, K.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Dey, G.K.; Kojima, Y. Improved hydrogen release from magnesium borohydride by ZrCl4 additive. Int. J. Hydrogen Energy 2017, 42, 22342–22347. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Study on the thermal decomposition of NaBH4 catalyzed by ZrCl4. Int. J. Hydrogen Energy 2017, 42, 22432–22437. [Google Scholar] [CrossRef]
- Zhao, S.X.; Wang, C.Y.; Liu, D.M.; Tan, Q.J.; Li, Y.T.; Si, T.Z. Destabilization of LiBH4 by SrF2 for reversible hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 5098–5103. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, J.; Zeng, X.; Ding, W. Mechanisms of partial hydrogen sorption reversibility in a 3NaBH4/ScF3 composite. RSC Adv. 2018, 8, 9211–9217. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Isobe, S.; Hanada, N.; Fujii, H. Lithium nitride for reversible hydrogen storage. J. Alloy. Compd. 2004, 365, 271–276. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hanada, N.; Isobe, S.; Leng, H.Y.; Fujii, H. Hydrogen storage properties in Ti catalyzed Li–N–H system. J. Alloy. Compd. 2005, 404–406, 435–438. [Google Scholar] [CrossRef]
- Matsumoto, M.; Haga, T.; Kawai, Y.; Kojima, Y. Hydrogen desorption reactions of Li–N–H hydrogen storage system: Estimation of activation free energy. J. Alloy. Compd. 2007, 439, 358–362. [Google Scholar] [CrossRef]
- Albanesi, L.F.; Larochette, P.A.; Gennari, F.C. Destabilization of the LiNH2–LiH hydrogen storage system by aluminum incorporation. Int. J. Hydrogen Energy 2013, 38, 12325–12334. [Google Scholar] [CrossRef]
- Albanesia, L.F.; Garroni, S.; Larochette, P.A.; Nolis, P.; Mulas, G.; Enzo, S.; Baró, M.D.; Gennari, F.C. Role of aluminum chloride on the reversible hydrogen storage properties of the Li–N–H system. Int. J. Hydrogen Energy 2015, 39, 13506–13517. [Google Scholar] [CrossRef]
- Lin, H.-J.; Li, H.-W.; Murakami, H.; Akiba, E. Remarkably improved hydrogen storage properties of LiNH2-LiH composite via the addition of CeF4. J. Alloy. Compd. 2018, 735, 1017–1022. [Google Scholar] [CrossRef]
- Leng, H.; Wu, Z.; Duan, W.; Xia, G.; Li, Z. Effect of MgCl2 additives on the H-desorption properties of Li–N–H system. Int. J. Hydrogen Energy 2012, 37, 903–907. [Google Scholar] [CrossRef]
- Price, C.; Gray, J.; Lascola, R.; Anton, D.L. The effects of halide modifiers on the sorption kinetics of the Li-Mg-N-H System. Int. J. Hydrogen Energy 2012, 37, 2742–2749. [Google Scholar] [CrossRef]
- Bill, R.F.; Reed, D.; Book, D.; Anderson, P.A. Effect of the calcium halides, CaCl2 and CaBr2, on hydrogen desorption in the Li–Mg–N–H system. J. Alloy. Compd. 2015, 645, S96–S99. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Aguey-Zinsou, K.F.; Guo, Z.X. Effect of nitride additives on Li–N–H hydrogen storage system. Int. J. Hydrogen Energy 2011, 36, 7920–7926. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Ternary Imides for Hydrogen Storage. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. New Metal−N−H System Composed of Mg(NH2)2 and LiH for Hydrogen Storage. J. Phys. Chem. B 2004, 108, 8763–8765. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Orimo, S. Synthesis and dehydriding studies of Mg–N–H systems. J. Power Sources 2004, 138, 309–312. [Google Scholar] [CrossRef]
- Ma, L.-P.; Dai, H.-B.; Liang, Y.; Kang, X.-D.; Fang, Z.-Z.; Wang, P.-J.; Wang, P.; Cheng, H.-M. Catalytically Enhanced Hydrogen Storage Properties of Mg(NH2)2 + 2LiH Material by Graphite-Supported Ru Nanoparticles. J. Phys. Chem. C 2008, 112, 18280–18285. [Google Scholar] [CrossRef]
- Shahi, R.R.; Yadav, T.P.; Shaz, M.A.; Srivastva, O.N. Studies on dehydrogenation characteristic of Mg(NH2)2/LiH mixture admixed with vanadium and vanadium based catalysts (V, V2O5 and VCl3). Int. J. Hydrogen Energy 2010, 35, 238–246. [Google Scholar] [CrossRef]
- Anton, D.L.; Price, C.J.; Gray, J. Affects of Mechanical Milling and Metal Oxide Additives on Sorption Kinetics of 1:1 LiNH2/MgH2 Mixture. Energies 2011, 4, 826–844. [Google Scholar] [CrossRef]
- Hu, J.; Poh, A.; Wang, S.; Rothe, J.; Fichtner, M. Additive Effects of LiBH4 and ZrCoH3 on the Hydrogen Sorption of the Li-Mg-N-H Hydrogen Storage System. J. Phys. Chem. C 2012, 116, 20246–20253. [Google Scholar] [CrossRef]
- Ulmer, U.; Hu, J.; Franzreb, M.; Fichtner, M. Preparation, scale-up and testing of nanoscale, doped amide systems for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 1439–1449. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Gu, Y.; Gao, M.; Pan, H. Improved Hydrogen-Storage Thermodynamics and Kinetics for an RbF-Doped Mg(NH2)2–2 LiH System. Chem. Asian J. 2013, 8, 2136–2143. [Google Scholar] [CrossRef]
- Demirocak, D.E.; Srinivasan, S.S.; Ram, M.K.; Kuhn, J.N.; Muralidharan, R.; Li, X.; Goswami, D.Y.; Stefanakos, E.K. Reversible hydrogen storage in the Li–Mg–N–H system—The effects of Ru doped single walled carbon nanotubes on NH3 emission and kinetics. Int. J. Hydrogen Energy 2013, 38, 10039–10049. [Google Scholar] [CrossRef]
- Yan, M.-y.; Sun, F.; Liu, X.-p.; Ye, J.-h. Effects of compaction pressure and graphite content on hydrogen storage properties of Mg(NH2)2–2LiH hydride. Int. J. Hydrogen Energy 2014, 39, 19656–19661. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Wu, G.; Li, W.; Liu, Y.; Araújo, C.M. Potassium—Modified Mg(NH2)2/2 LiH System for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 5828–5832. [Google Scholar] [CrossRef]
- Teng, Y.-L.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Improvement of hydrogen desorption kinetics in the LiH–NH3 system by addition of KH. Chem. Commun. 2011, 47, 12227–12229. [Google Scholar] [CrossRef] [PubMed]
- Durojaiye, T.; Goudy, A. Desorption kinetics of lithium amide/magnesium hydride systems at constant pressure thermodynamic driving forces. Int. J. Hydrogen Energy 2012, 37, 3298–3304. [Google Scholar] [CrossRef]
- Luo, W.; Stavila, V.; Klebanoff, L.E. New insights into the mechanism of activation and hydrogen absorption of (2LiNH2–MgH2). Int. J. Hydrogen Energy 2012, 37, 6646–6652. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Pan, H.; Xiong, Z.; Gao, M.; Wu, G.; Liang, C.; Li, C.; Li, B.; Wang, J. Solid–Solid Heterogeneous Catalysis: The Role of Potassium in Promoting the Dehydrogenation of the Mg(NH2)2/2 LiH Composite. ChemSusChem 2013, 6, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Ma, R.; Zhang, X.; Li, Y.; Gao, M.; Pan, H. Superior Dehydrogenation/Hydrogenation Kinetics and Long-Term Cycling Performance of K and Rb Cocatalyzed Mg(NH2)2-2LiH system. ACS Appl. Mater. Interfaces 2014, 6, 17024–17033. [Google Scholar] [CrossRef]
- Durojaiye, T.; Hayes, J.; Goudy, A. Rubidium Hydride: An Exceptional Dehydrogenation Catalyst for the Lithium Amide/Magnesium Hydride System. J. Phys. Chem. C 2013, 117, 6554–6560. [Google Scholar] [CrossRef]
- Hayes, J.; Durojaiye, T.; Goudy, A. Hydriding and dehydriding kinetics of RbH-doped 2LiNH2/MgH2 hydrogen storage system. J. Alloy. Compd. 2015, 645, S496–S499. [Google Scholar] [CrossRef]
- Durojaiye, T.; Hayes, J.; Goudy, A. Potassium, rubidium and cesium hydrides as dehydrogenation catalysts for the lithium amide/magnesium hydride system. Int. J. Hydrogen Energy 2015, 40, 2266–2273. [Google Scholar] [CrossRef]
- Torre, F.; Valentoni, A.; Milanese, C.; Pistidda, C.; Marini, A.; Dornheim, M. Kinetic improvement on the CaH2-catalyzed Mg(NH2)2 + 2LiH system. J. Alloy. Compd. 2015, 645, S284–S287. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, B.; Gao, M.; Pan, H. Metathesis Reaction-Induced Significant Improvement in Hydrogen Storage Properties of the KF-Added Mg(NH2)2–2LiH System. J. Phys. Chem. C 2013, 117, 866–875. [Google Scholar] [CrossRef]
- Sun, F.; Yan, M.; Ye, J.; Liu, X.; Jiang, L. Effect of CO on hydrogen storage performance of KF doped 2LiNH2 + MgH2 material. J. Alloy. Compd. 2014, 616, 47–50. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Yang, Y.; Gao, M.; Pan, H. High-temperature failure behaviour and mechanism of K-based additives in Li–Mg–N–H hydrogen storage systems. J. Mater. Chem. A 2014, 2, 7345–7353. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Gao, M.; Pan, H. Understanding the role of K in the significantly improved hydrogen storage properties of a KOH-doped Li–Mg–N–H system. J. Mater. Chem. A 2013, 1, 5031–5036. [Google Scholar]
- Liu, Y.; Yang, Y.; Zhang, X.; Li, Y.; Gao, M.; Pan, H. Insights into the dehydrogenation reaction process of a K-containing Mg(NH2)2–2LiH system. Dalton Trans. 2015, 44, 18012–18018. [Google Scholar] [CrossRef]
- Chotard, J.-N.; Tang, W.S.; Raybaud, P.; Janot, R. Potassium Silanide (KSiH3): A Reversible Hydrogen Storage Material. Chem. Eur. J. 2011, 17, 12302–12309. [Google Scholar] [CrossRef]
- Tang, W.S.; Chotard, J.-N.; Raybaud, P.; Janot, R. Hydrogenation properties of KSi and NaSi Zintl phases. Phys. Chem. Chem. Phys. 2012, 14, 13319–13324. [Google Scholar] [CrossRef]
- Tang, W.S.; Chotard, J.-N.; Janot, R. Syntheis of single phase LiSi by ball milling: Electrochemical behavior and hydrogenation properties. J. Electrochem. Soc. 2013, 160, A1232–1240. [Google Scholar] [CrossRef]
- Tang, W.S.; Chotard, J.-N.; Raybaud, P.; Janot, R. Enthalpy-entropy compensation effect in hydrogen storage materials: Striking example of alkali silanides MSiH3 (M = K, Rb, Cs). J. Phys. Chem. C 2014, 118, 3409–3419. [Google Scholar] [CrossRef]
- Kranak, V.F.; Lin, Y.C.; Karlsson, M.; Mink, J.; Norberg, S.T.; Haussermann, U. Structural and vibrational properties of Silyl (SiH3−) anion in KSiH3 and RbSiH3: New insight into Si-H interactions. Inorg. Chem. 2015, 54, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.S.; Dimitrievska, M.; Chotard, J.-N.; Zhou, W.; Janot, R.; Skripov, A.V.; Udovic, T.J. Structural and dynamical trends in alkali-metal silanides characterized by neutron-scattering methods. J. Phys. Chem. C 2016, 120, 21218–21227. [Google Scholar] [CrossRef]
- Auer, H.; Kohlmann, H. In situ investigations on the formation and decomposition of KSiH3 and CsSiH3. Z. Anorg. Allg. Chem. 2017, 643, 945–951. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H.; Yu, X.F.; Peng, P. Alkali metal silanides α-MSiH3: A family of complex hydrides for solid-state hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 12405–12413. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Tailoring the absorption-desorption properties of KSiH3 compound using nano-metals (Ni, Co, Nb) as catalyst. J. Alloy. Compd. 2015, 645, 1441–1447. [Google Scholar] [CrossRef]
- Janot, R.; Tang, W.S.; Clemencon, D.; Chotard, J.-N. Catalyzed KSiH3 as a reversible hydrogen storage material. J. Mater. Chem. A 2016, 4, 19045–19052. [Google Scholar] [CrossRef]





| Materials | Storage Capacity | Operating Temperature |
|---|---|---|
| Sorbent Systems [8] | ||
| Hydrogen is attached to the surface via physisorptionEx.—C-based materials, MOFs | 2–7 wt% | ~77 K |
| Conventional metal hydrides [9,10,11] | ||
| Hydrogen forms various bonds with metal atoms. | ||
| 1~4 wt% | RT |
| >7 wt% | >600 K |
| Complex Hydrides [12,13] | ||
| Hydrogen covalently bonded and the formed anion complex is bonded with cation via ionic bond | ||
| Alanates (Ex.—LiAlH4, NaAlH4, Mg(AlH4)2 etc.) | 5.8~10.5 wt% | ≥400 K |
| Borohydrides (Ex.—LiBH4, NaBH4, Mg(BH4)2 etc.) | 10~18.5 wt% | ≥400 K |
| Amides (Ex.—LiNH2, NaNH2, Mg(NH2)2 etc.) | 5~10 wt% | ≥400 K |
| Silanides (Ex.—KSiH3, RbSiH3, CsSiH3) | 2~4.5 wt% | RT~500 K |
| Chemical Hydrides [14,15] | ||
| Hydrogen is covalently bonded and these materials are irreversible | ||
| Ex.—NH3, NH3BH3 | 17.8~20 wt% | 373~>773 K |
| Liquid Organic Materials [16] | ||
| Ex.—methylcyclohexane-toluene-hydrogen (MTH cycle), Cyclohexane-benzene-hydrogen (CBH cycle) etc. | ~6–7 wt% | 500~750 K |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A.; Agarwal, S.; Ichikawa, T. Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts 2018, 8, 651. https://doi.org/10.3390/catal8120651
Jain A, Agarwal S, Ichikawa T. Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts. 2018; 8(12):651. https://doi.org/10.3390/catal8120651
Chicago/Turabian StyleJain, Ankur, Shivani Agarwal, and Takayuki Ichikawa. 2018. "Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism" Catalysts 8, no. 12: 651. https://doi.org/10.3390/catal8120651
APA StyleJain, A., Agarwal, S., & Ichikawa, T. (2018). Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts, 8(12), 651. https://doi.org/10.3390/catal8120651






