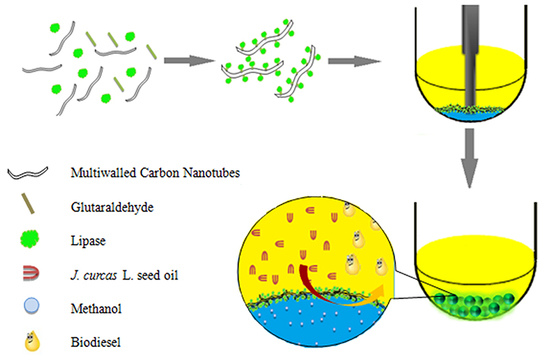
Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production
Abstract
:1. Introduction
2. Results and Discussion
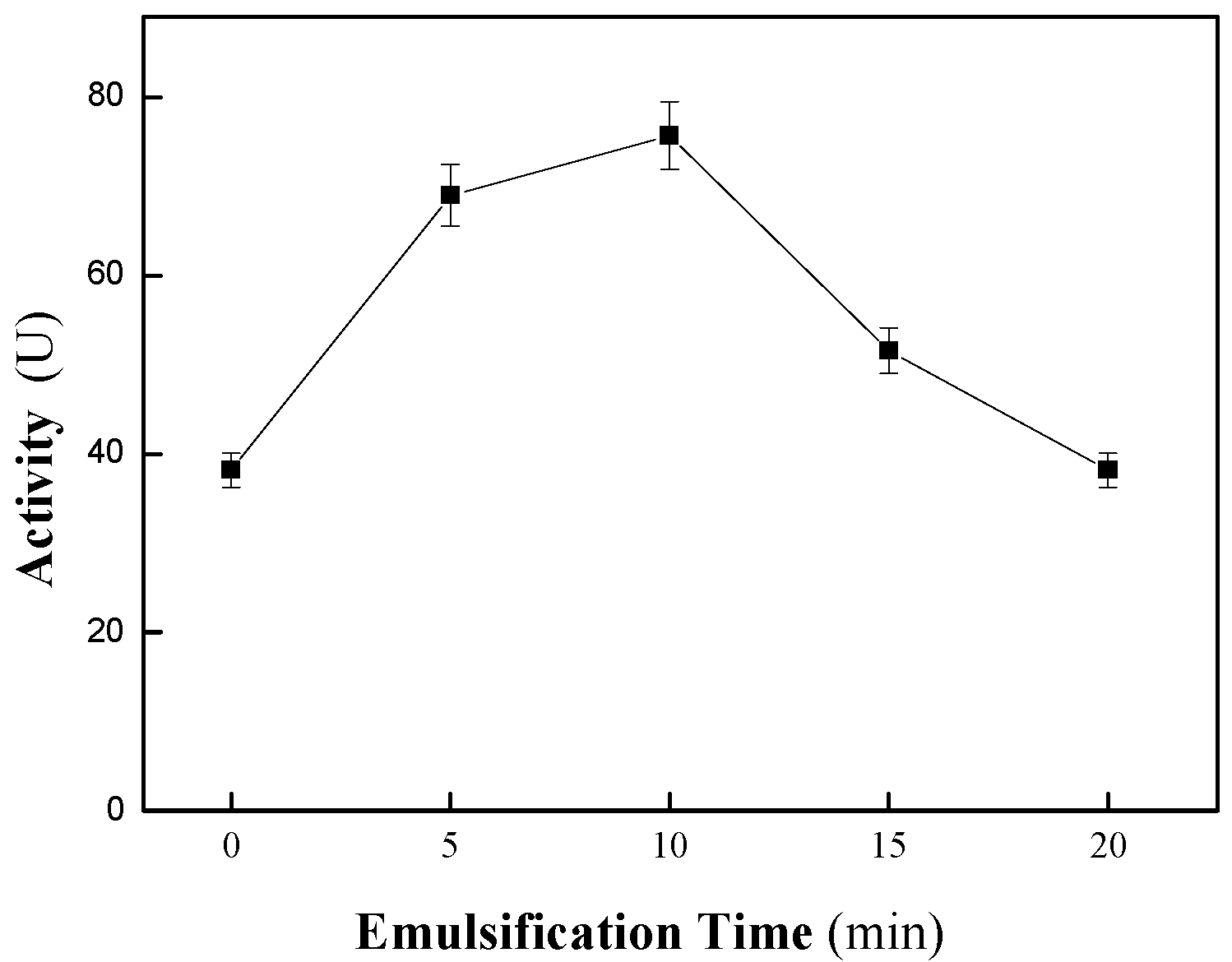
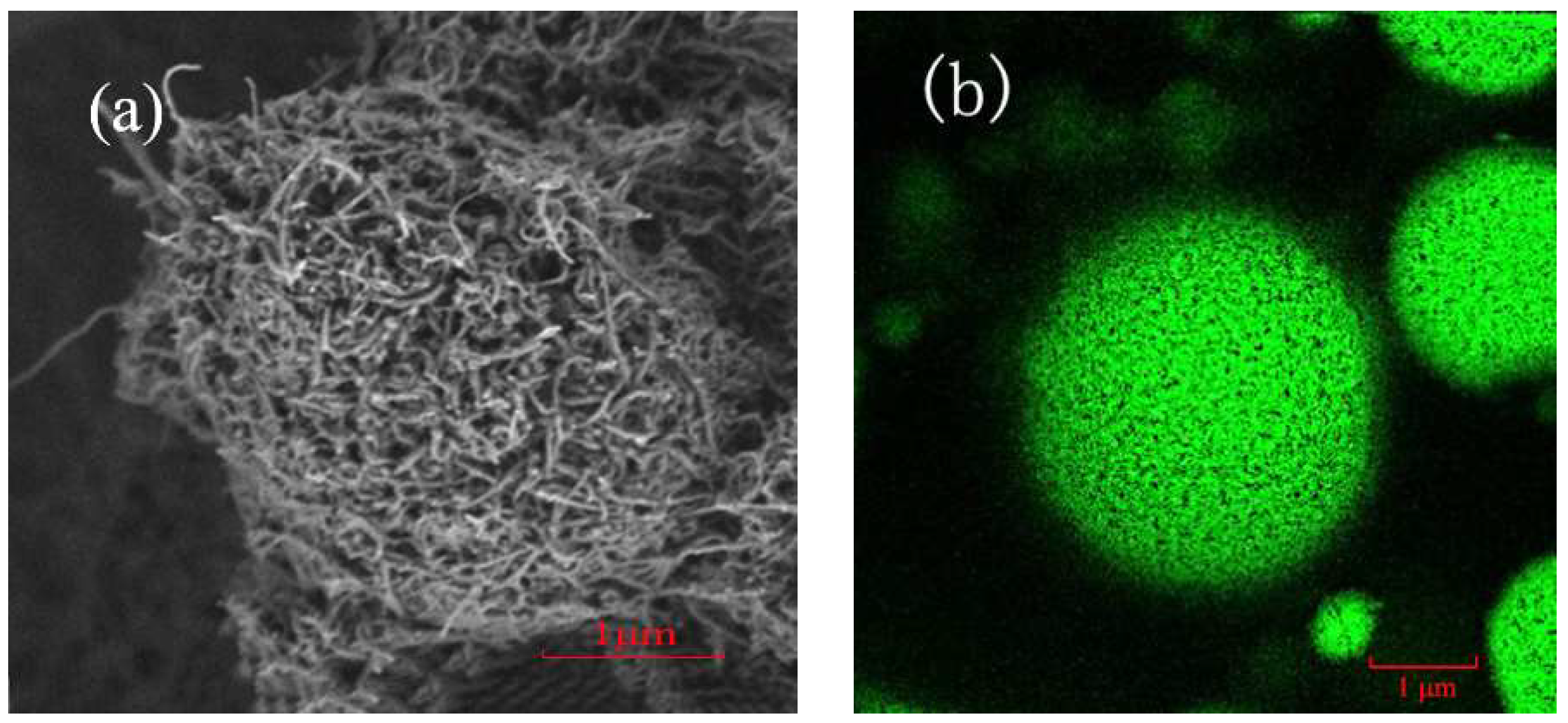
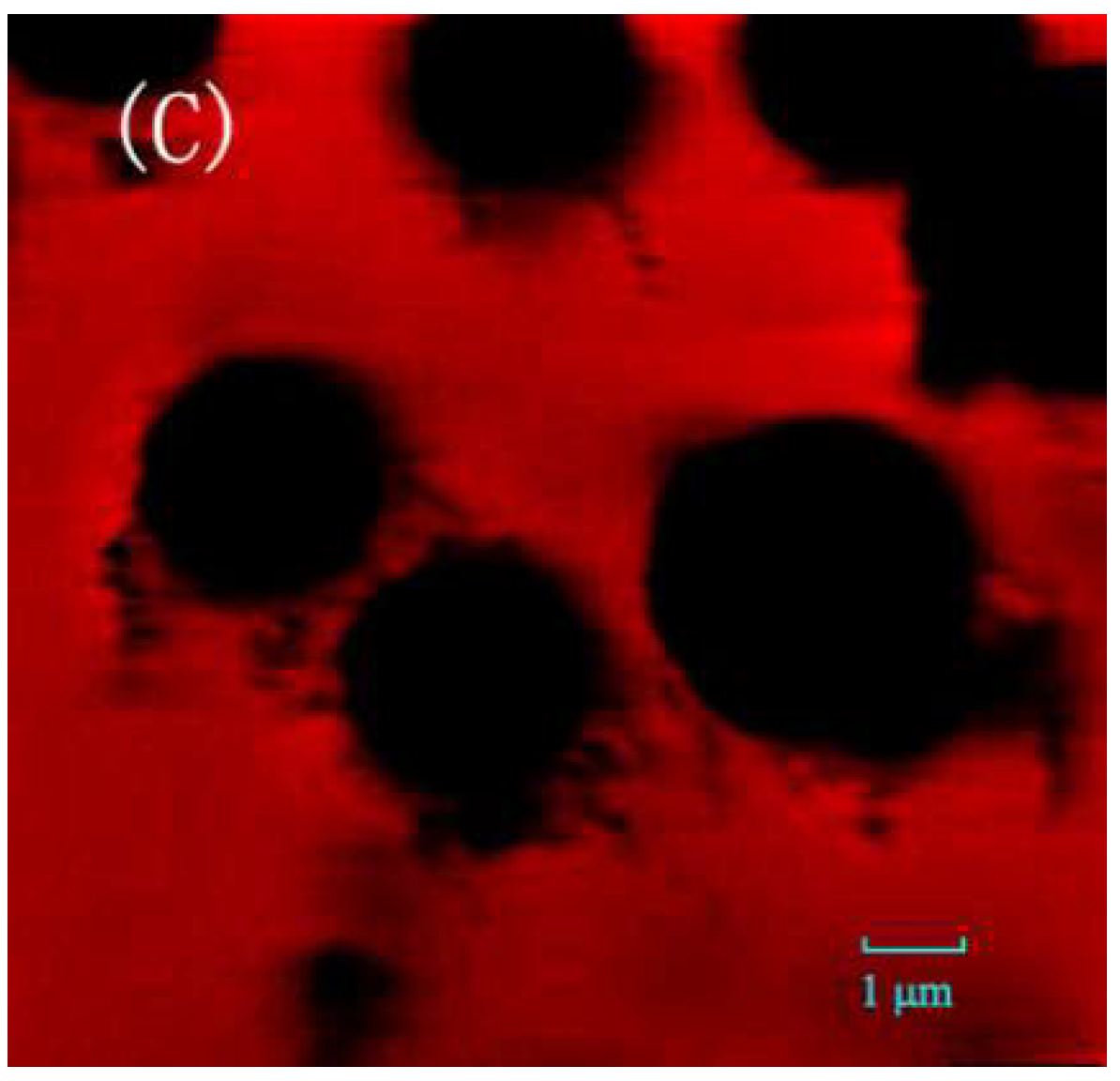
2.1. Optimization of CALB@PE Construction
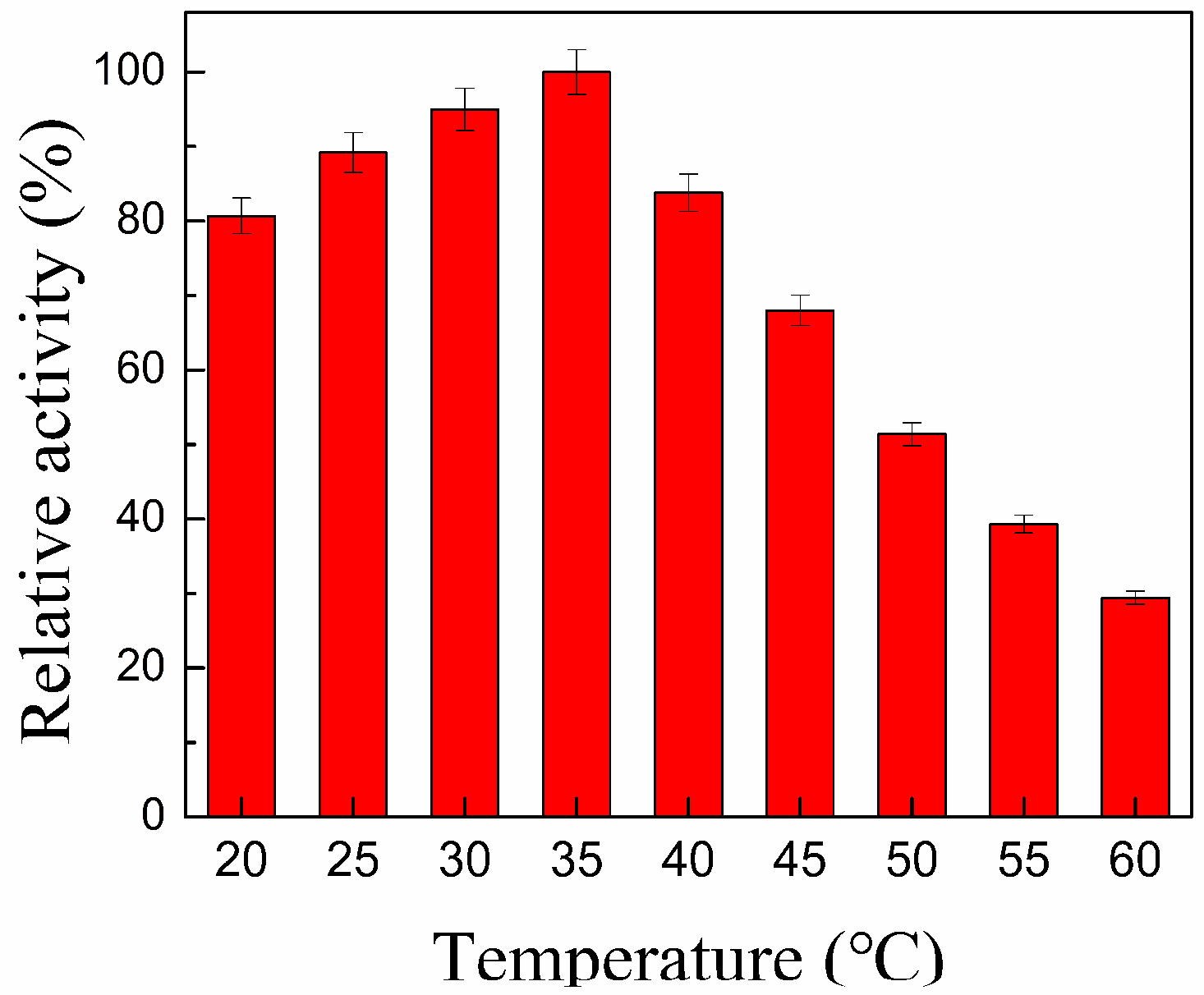
2.2. Optimization of the Biodiesel Production Catalyzed by CALB@PE
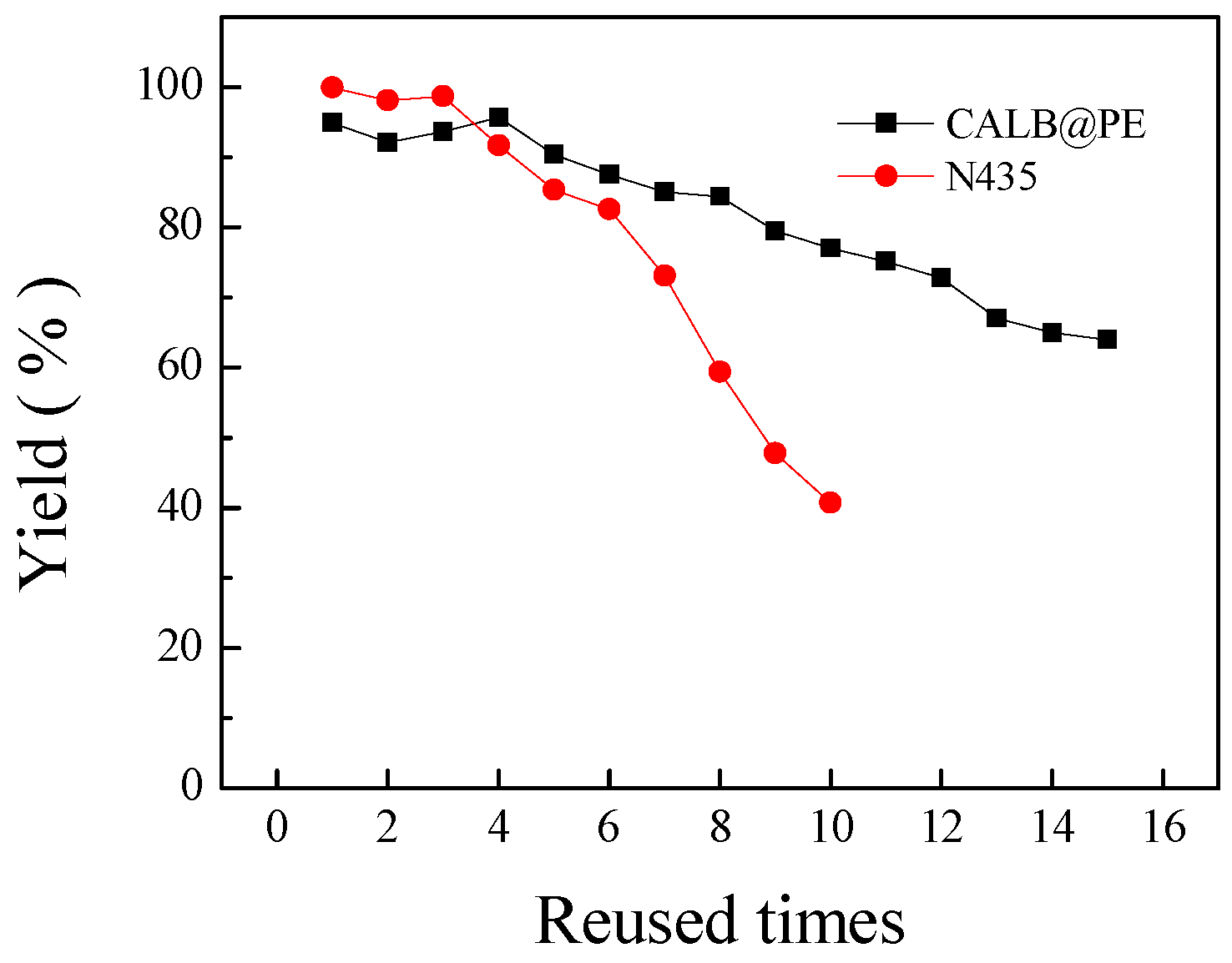
2.3. Operational Stability of CALB@PE
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pickering Emulsions
3.3. Characterization
3.4. Synthesis of Biodiesel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silitonga, A.S.; Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-Immobilized Biocatalysts for Biodiesel Production from Renewable and Sustainable Resources. Catalysts 2018, 8, 68. [Google Scholar] [CrossRef]
- Qian, J.; Wang, F.; Liu, S.; Yun, Z. In situ alkaline transesterification of cottonseed oil for production of biodiesel and nontoxic cottonseed meal. Bioresour. Technol. 2008, 99, 9009–9012. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Wang, X.L.; Zhang, S.H.; Tan, L.; Jiang, Z.Y. Enzyme-conjugated ZIF-8 particles as efficient and stable Pickering interfacial biocatalysts for biphasic biocatalysis. J. Mater. Chem. B 2016, 4, 2654–2661. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Tong, J.; Wang, Y.; Chen, S. Research Development on Lipase-catalyzed Biodiesel. Energy Procedia 2012, 16, 1014–1021. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, L.; Wu, D.; Lei, Q.; Guo, Y.; Fang, W. Ionic strength-response hyperbranched polyglycerol/polyacrylic acid hydrogel for the reversible immobilization of enzyme and the synthesis of biodiesel. Energy Convers. Manag. 2017, 144, 303–311. [Google Scholar] [CrossRef]
- Zhou, Z.; Inayat, A.; Schwieger, W.; Hartmann, M. Improved activity and stability of lipase immobilized in cage-like large pore mesoporous organosilicas. Microporous Mesoporous Mater. 2012, 154, 133–141. [Google Scholar] [CrossRef]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-Shaped ZIF-8 for Immobilization Rhizomucor miehei Lipase via Encapsulation and Its Application toward Biodiesel Production. Catalysts 2018, 8, 96. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhao, Y.; Zhang, Y. Biodiesel production from jatropha oil catalyzed by immobilized Burkholderia cepacia lipase on modified attapulgite. Bioresour. Technol. 2013, 148, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Liang, S.H.; Doan, T.T.; Su, C.H.; Yang, P.C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Prlainović, N.Ž.; Bezbradica, D.I.; Knežević-Jugović, Z.D.; Stevanović, S.I.; Avramov Ivić, M.L.; Uskoković, P.S.; Mijin, D.Ž. Adsorption of lipase from Candida rugosa on multi walled carbon nanotubes. J. Ind. Eng. Chem. 2013, 19, 279–285. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Liu, J.; Lan, G.J.; Peng, J.; Li, Y.; Li, C.; Yang, Q.H. Enzyme Confined in Silica-based Nanocages for Biocatalysis in Pickering Emulsion. Chem. Commun. 2013, 49, 9558–9560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; van Oers, M.C.M.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymersome Colloidosomes for Enzyme Catalysis in a Biphasic System. Angew. Chem. Int. Ed. 2012, 51, 10746–10750. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kraume, M.; Ansorge-Schumacher, M.B. Optimized Biocatalytically Active Static Emulsions for Organic Synthesis in Nonaqueous Media. ChemCatChem 2011, 3, 1314–1319. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Hong, L.; Ngai, T. Submicron Inverse Pickering Emulsions for Highly Efficient and Recyclable Enzymatic Catalysis. Chem. Asian J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, M.; Zhang, X.; Xin, H.; Yang, H. Pickering Emulsion as an Efficient Platform for Enzymatic Reactions without Stirring. ACS Sustain. Chem. Eng. 2016, 4, 6838–6843. [Google Scholar] [CrossRef]
- Binks, B.P.; Whitby, C.P. Silica Particle-Stabilized Emulsions of Silicone Oil and Water: Aspects of Emulsification. Langmuir 2004, 20, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, X.; Chen, Y.; Zhou, L.; He, Y.; Ma, L.; Gao, J. Pickering emulsion stabilized by lipase-containing periodic mesoporous organosilica particles: A robust biocatalyst system for biodiesel production. Bioresour. Technol. 2014, 153, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Batule, B.S.; Park, K.S.; Kim, M.I.; Park, H.G. Ultrafast sonochemical synthesis of protein-inorganic nanoflowers. Int. J. Nanomed. 2015, 10, 137–142. [Google Scholar] [CrossRef]
- Poojari, Y.; Clarson, S.J. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, H.; Zhou, L.; Cui, C.; Gao, J. Novelin SituBatch Reactor with a Facile Catalyst Separation Device for Biodiesel Production. Ind. Eng. Chem. Res. 2012, 51, 14935–14940. [Google Scholar] [CrossRef]
- Gu, H.; Jiang, Y.; Zhou, L.; Gao, J. Reactive extraction and in situ self-catalyzed methanolysis of germinated oilseed for biodiesel production. Energy Environ. Sci. 2011, 4, 1337–1344. [Google Scholar] [CrossRef]
- Kumar, D.; Nagar, S.; Bhushan, I.; Kumar, L.; Parshad, R.; Gupta, V.K. Covalent immobilization of organic solvent tolerant lipase on aluminum oxide pellets and its potential application in esterification reaction. J. Mol. Catal. B: Enzym. 2013, 87, 51–61. [Google Scholar] [CrossRef]
- Liu, L.; Hong, Y.; Ye, X.; Wei, L.; Liao, J.; Huang, X.; Liu, C. Biodiesel production from microbial granules in sequencing batch reactor. Bioresour. Technol. 2018, 249, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Process optimization for biodiesel production from waste cooking oil using multi-enzyme systems through response surface methodology. Renew. Energy 2017, 105, 465–472. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Muthukumar, K. Production of methyl ester from oedogonium sp. oil using immobilized isolated novel Bacillus sp. lipase. Energy Fuels 2012, 26, 6387–6392. [Google Scholar] [CrossRef]
- Zhou, Z.; Piepenbreier, F.; Marthala, V.R.R.; Karbacher, K.; Hartmann, M. Immobilization of lipase in cage-type mesoporous organosilicas via covalent bonding and crosslinking. Catal. Today 2015, 243, 173–183. [Google Scholar] [CrossRef]
- Wu, C.; Bai, S.; Ansorge-Schumacher, M.B.; Wang, D. Nanoparticle Cages for Enzyme Catalysis in Organic Media. Adv. Mater. 2011, 23, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Du, Y.; Zhou, L.; He, Y.; Ma, L.; Yin, L.; Kong, W.; Jiang, Y. Construction of biocatalytic colloidosome using lipase-containing dendritic mesoporous silica nanospheres for enhanced enzyme catalysis. Chem. Eng. J. 2017, 317, 175–186. [Google Scholar] [CrossRef]


| Factor | Name | Unit | Low Level (−) | High Level (+) | −alpha | +alpha |
|---|---|---|---|---|---|---|
| r | Molar ratio of methanol to seed acid | 3 | 7 | 1 | 9 | |
| M | CALB@PE dosage | mg | 80 | 140 | 50 | 170 |
| T | Temperature | °C | 30 | 45 | 22.5 | 52.5 |
| t | Time | h | 8 | 12 | 6 | 14 |
| No. | r (Molar Ratio of Methanol to Seed Acid) | M (CALB@PE Dosage, mg) | T (Temperature, °C) | t (Time, h) | Yield % |
|---|---|---|---|---|---|
| 1 | 3 | 80 | 30 | 8 | 65.80 |
| 2 | 7 | 80 | 30 | 12 | 69.00 |
| 3 | 5 | 170 | 37.5 | 10 | 88.51 |
| 4 | 7 | 80 | 45 | 8 | 58.24 |
| 5 | 5 | 110 | 37.5 | 14 | 95.50 |
| 6 | 9 | 110 | 37.5 | 10 | 59.12 |
| 7 | 7 | 140 | 30 | 8 | 72.69 |
| 8 | 7 | 140 | 45 | 8 | 72.01 |
| 9 | 5 | 110 | 37.5 | 6 | 71.80 |
| 10 | 5 | 110 | 37.5 | 10 | 94.10 |
| 11 | 3 | 80 | 45 | 12 | 80.00 |
| 12 | 3 | 140 | 30 | 12 | 87.00 |
| 13 | 3 | 80 | 30 | 12 | 79.00 |
| 14 | 1 | 110 | 37.5 | 10 | 70.86 |
| 15 | 5 | 110 | 37.5 | 10 | 94.53 |
| 16 | 7 | 80 | 45 | 12 | 69.00 |
| 17 | 5 | 110 | 37.5 | 10 | 93.21 |
| 18 | 7 | 140 | 30 | 12 | 76.00 |
| 19 | 5 | 110 | 37.5 | 10 | 94.70 |
| 20 | 3 | 80 | 45 | 8 | 60.00 |
| 21 | 3 | 140 | 45 | 8 | 74.00 |
| 22 | 5 | 110 | 52.5 | 10 | 71.26 |
| 23 | 7 | 140 | 45 | 12 | 82.92 |
| 24 | 5 | 110 | 37.5 | 10 | 95.39 |
| 25 | 7 | 80 | 30 | 8 | 67.00 |
| 26 | 5 | 110 | 37.5 | 10 | 93.80 |
| 27 | 5 | 50 | 37.5 | 10 | 66.50 |
| 28 | 5 | 110 | 22.5 | 10 | 71.30 |
| 29 | 3 | 140 | 30 | 8 | 75.00 |
| 30 | 3 | 140 | 45 | 12 | 95.00 |
| Terms | F-Value | p-Value (Prob > F) | Value | Analysis |
|---|---|---|---|---|
| Model | 829.88 | <0.0001 | significant | |
| r (Molar ratio of methanol to seed acid) | 1318.57 | <0.0001 | ||
| M (CALB@PE dosage) | 406.71 | <0.0001 | ||
| T (Temperature) | 759.10 | <0.0001 | ||
| t (Time) | 347.97 | <0.0001 | ||
| Lack of Fit | 0.47 | 0.8537 | not significant | |
| R2 | 0.9987 | |||
| Adjusted R2 | 0.9975 | |||
| Predicted R2 | 0.9954 | |||
| Adequate Precision | 85.028 | |||
| Coefficient of variation % | 0.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, X.; Jiang, Y.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production. Catalysts 2018, 8, 587. https://doi.org/10.3390/catal8120587
Wang L, Liu X, Jiang Y, Zhou L, Ma L, He Y, Gao J. Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production. Catalysts. 2018; 8(12):587. https://doi.org/10.3390/catal8120587
Chicago/Turabian StyleWang, Lihui, Xinlong Liu, Yanjun Jiang, Liya Zhou, Li Ma, Ying He, and Jing Gao. 2018. "Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production" Catalysts 8, no. 12: 587. https://doi.org/10.3390/catal8120587
APA StyleWang, L., Liu, X., Jiang, Y., Zhou, L., Ma, L., He, Y., & Gao, J. (2018). Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production. Catalysts, 8(12), 587. https://doi.org/10.3390/catal8120587