Coordinated “Naked” Pnicogenes and Catalysis †
Abstract
:1. Introduction
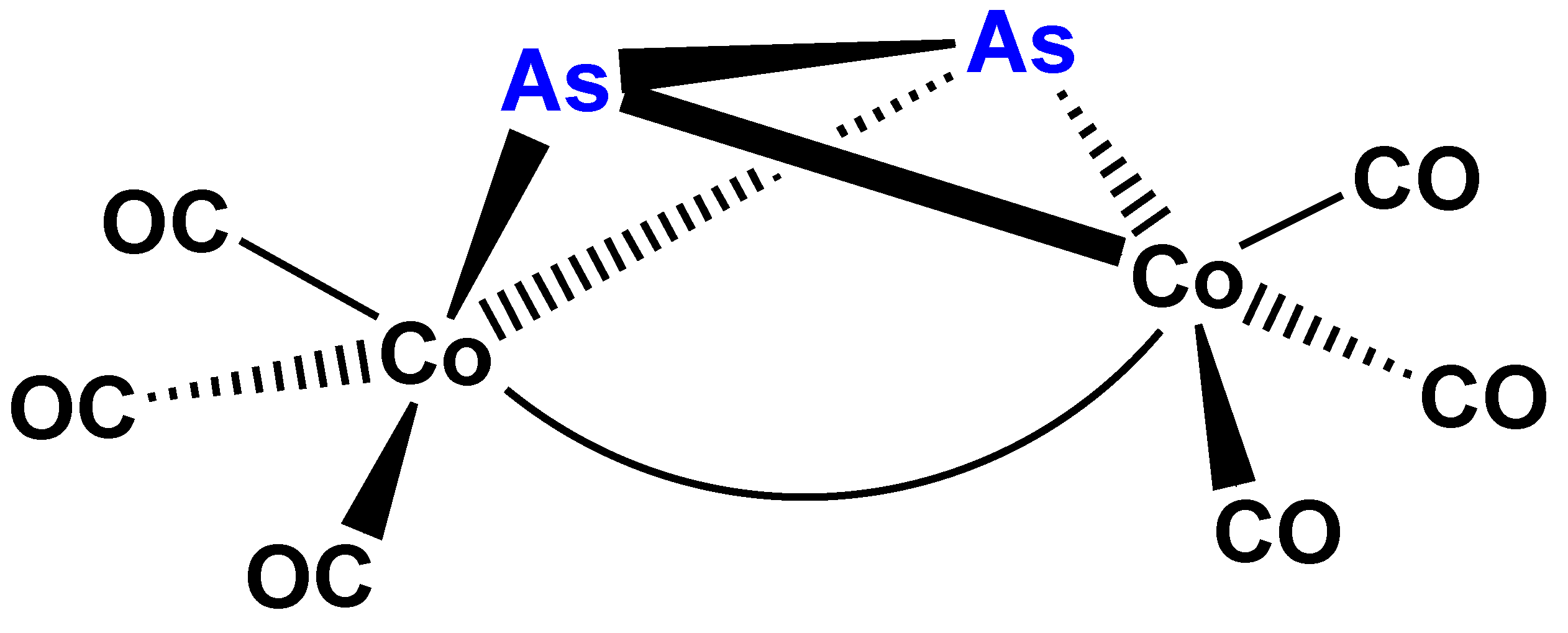
2. Isolobal Principle and Small Clusters
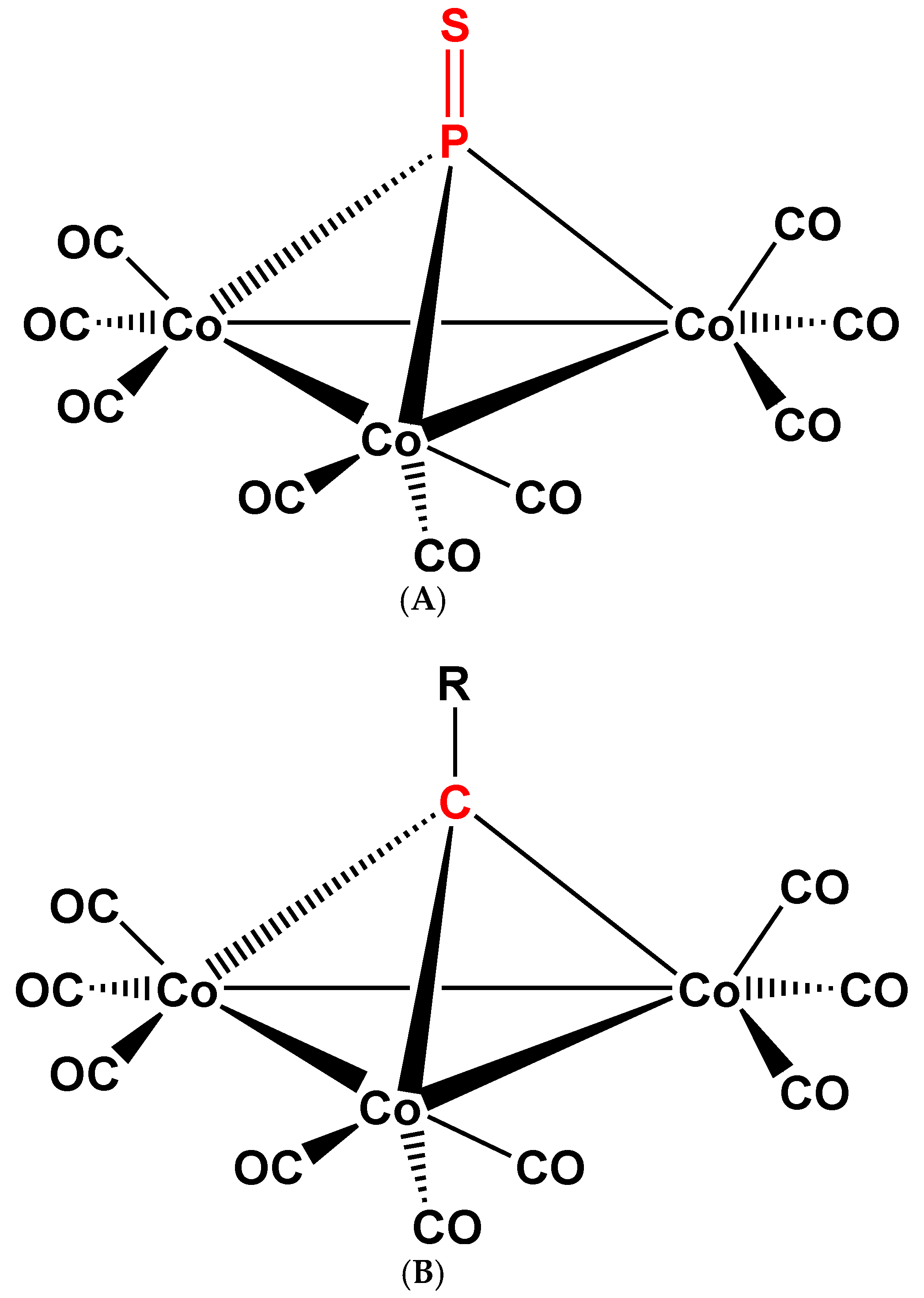
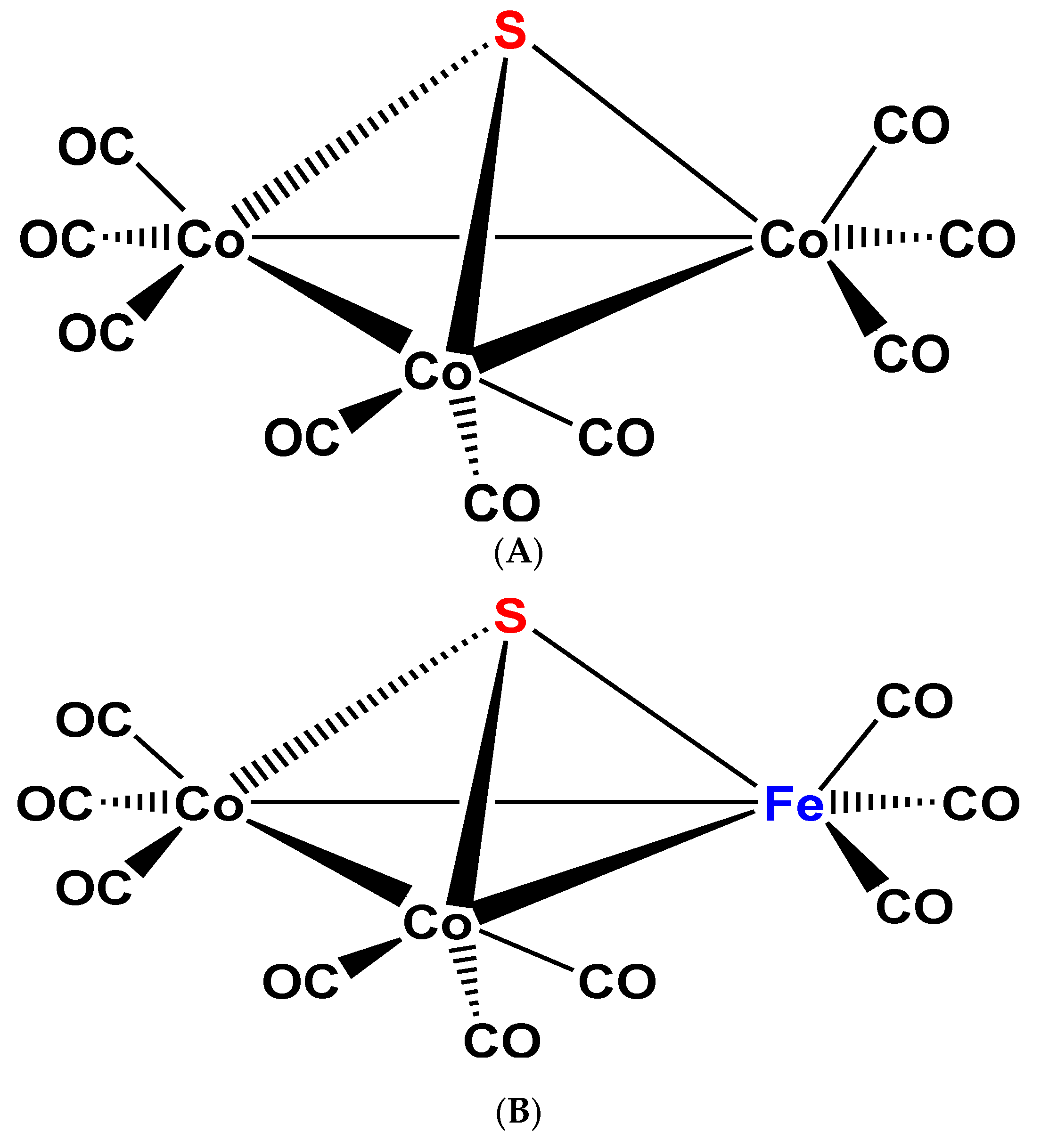
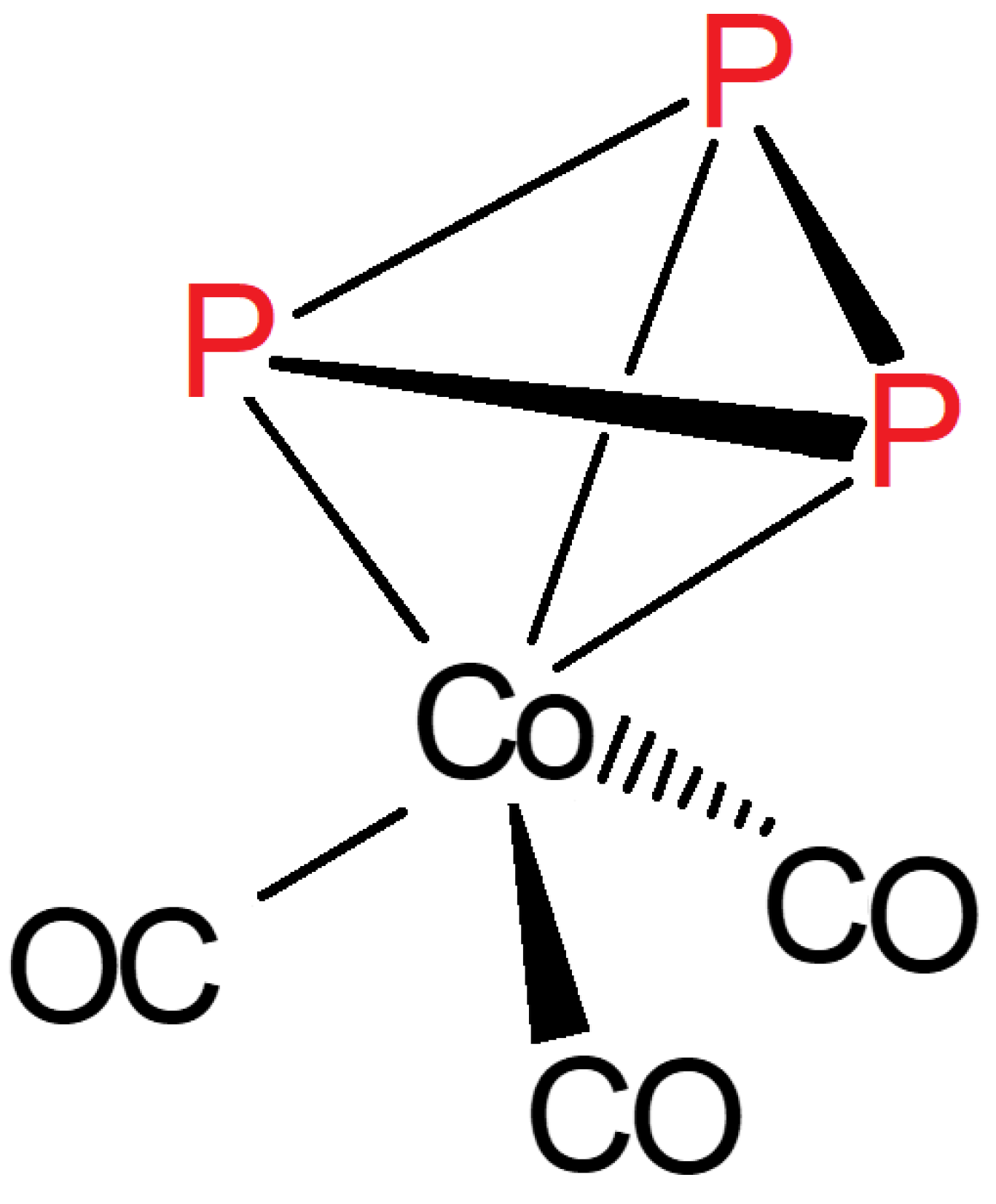
3. Substituting CO Ligands in Heteronuclear Clusters
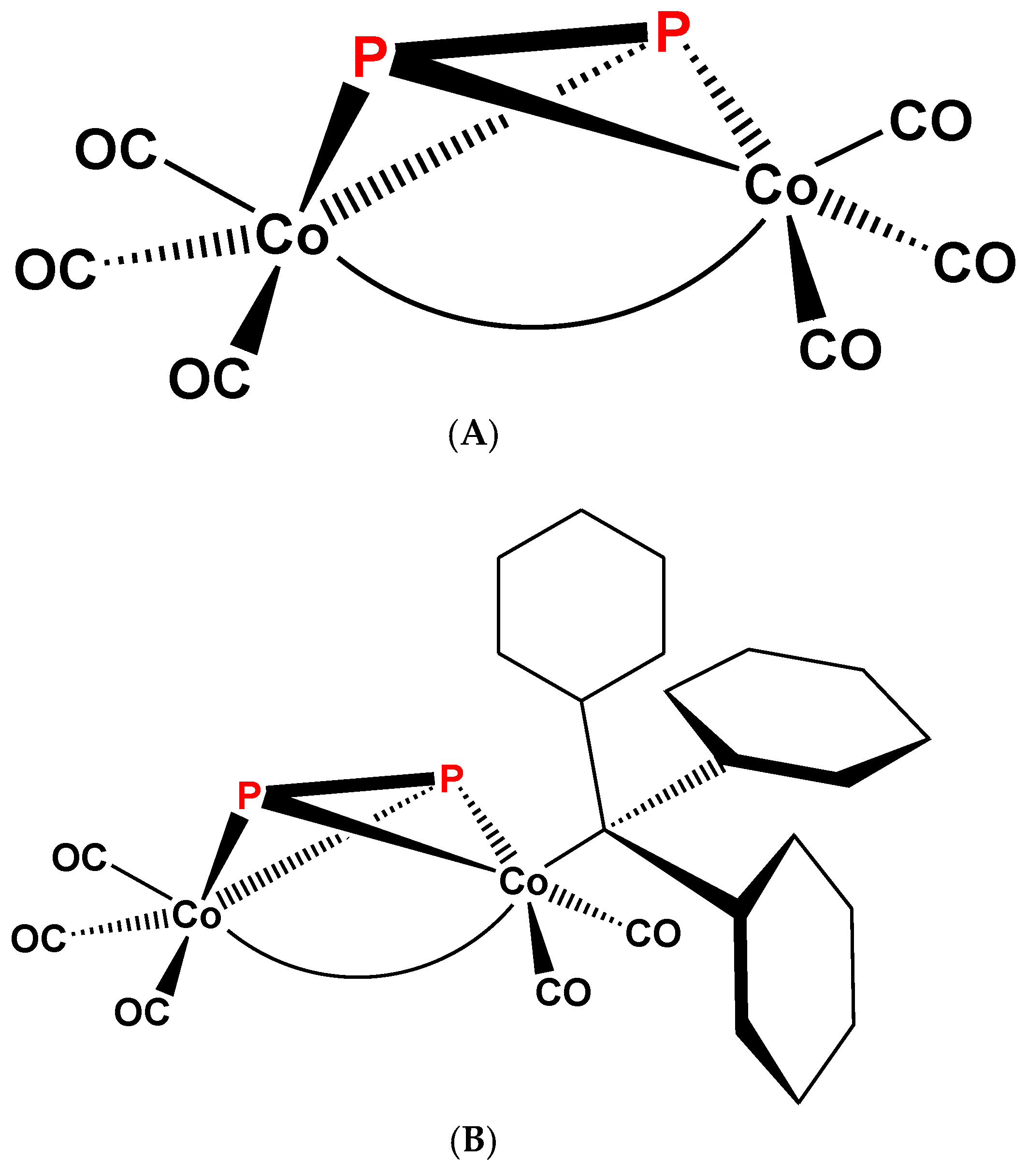
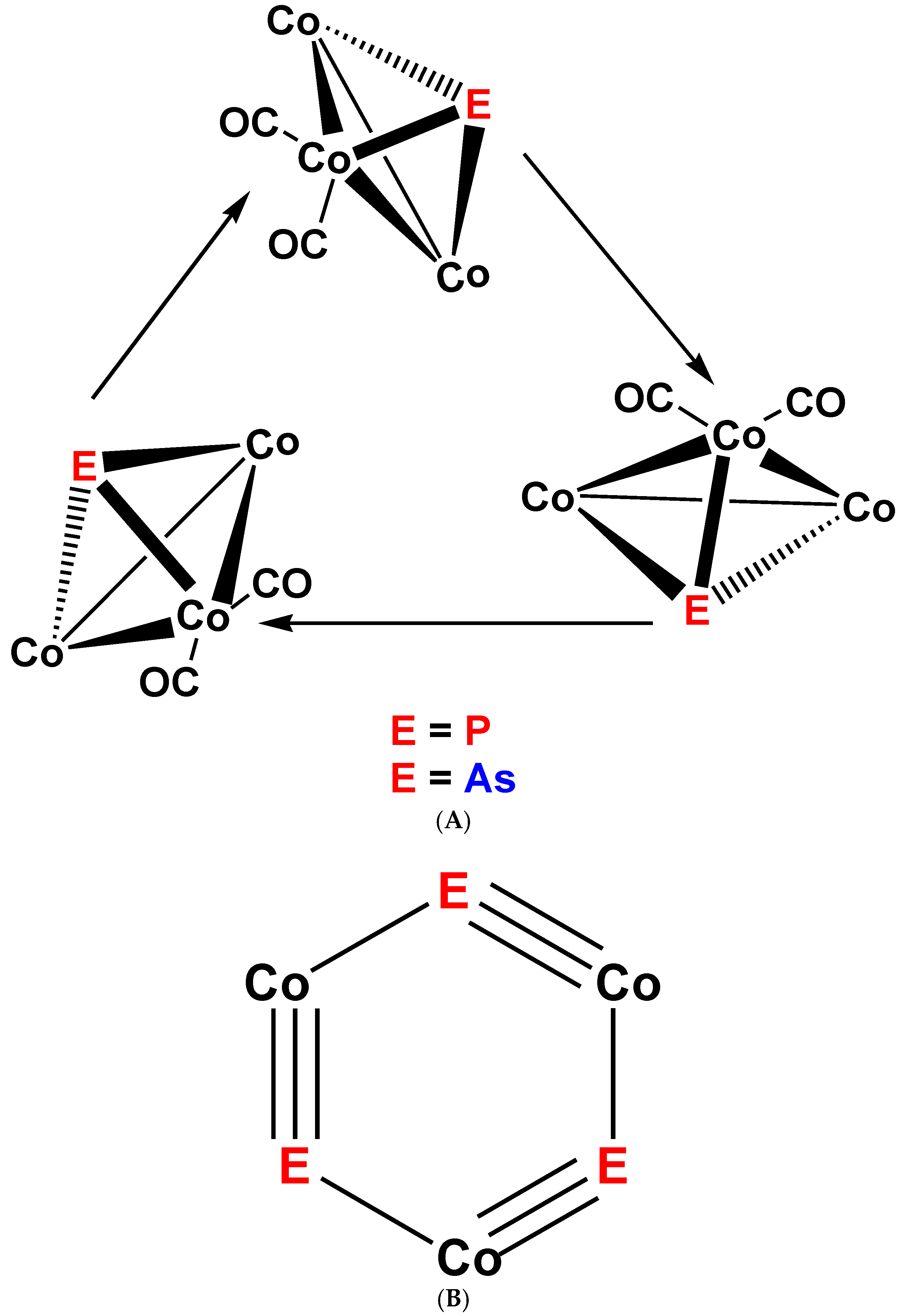
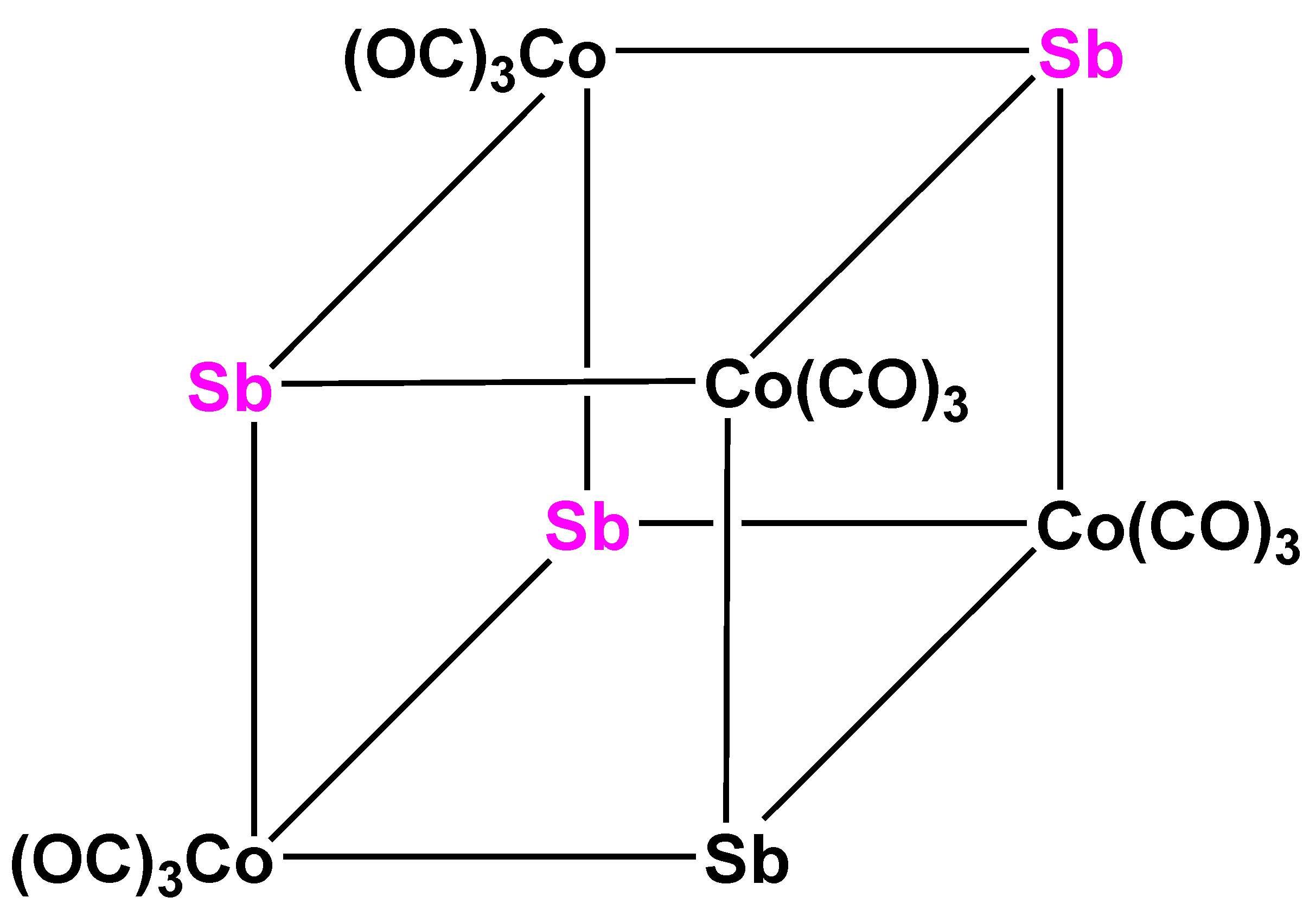
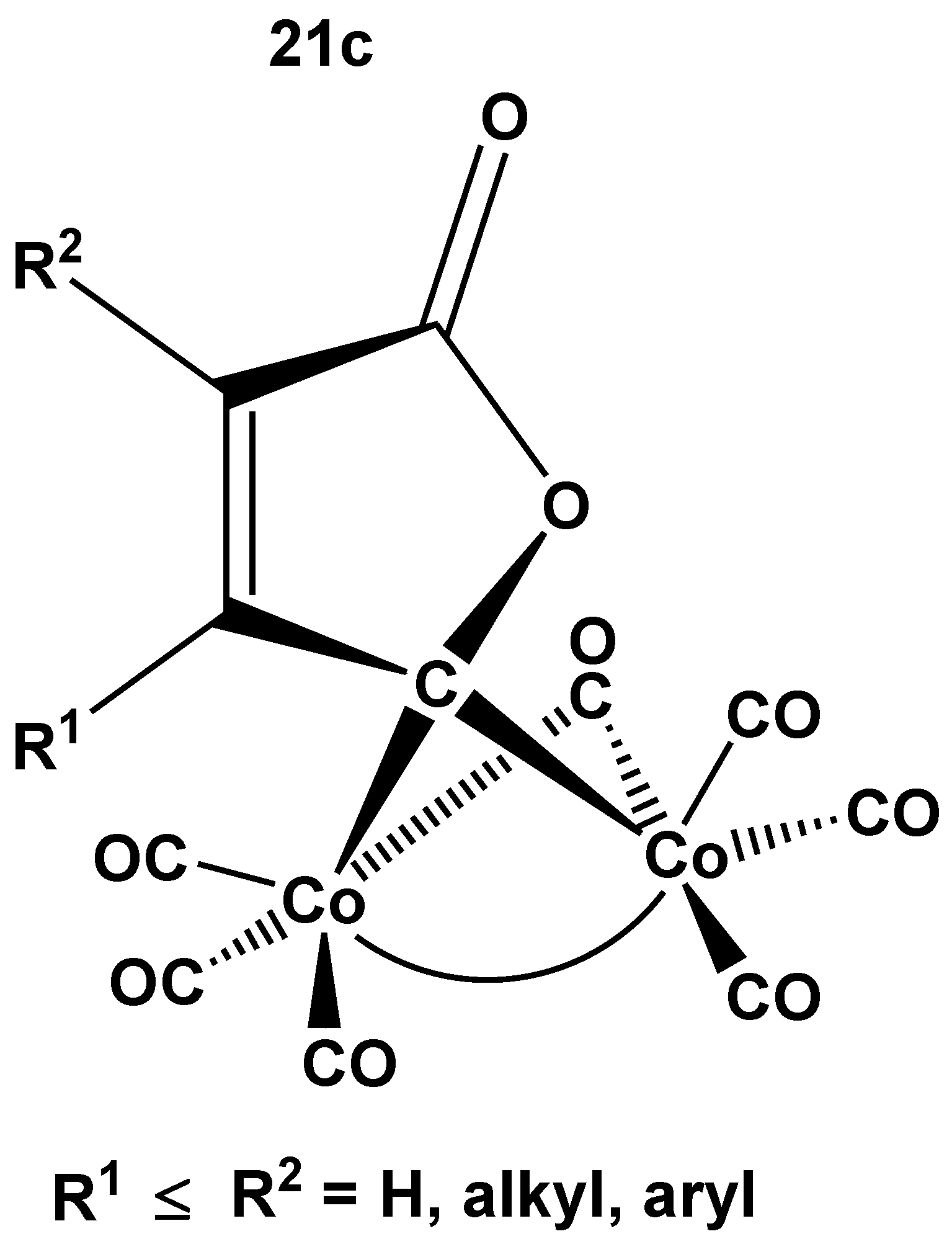
4. Pnicogen/Cobalt Clusters as Ligands
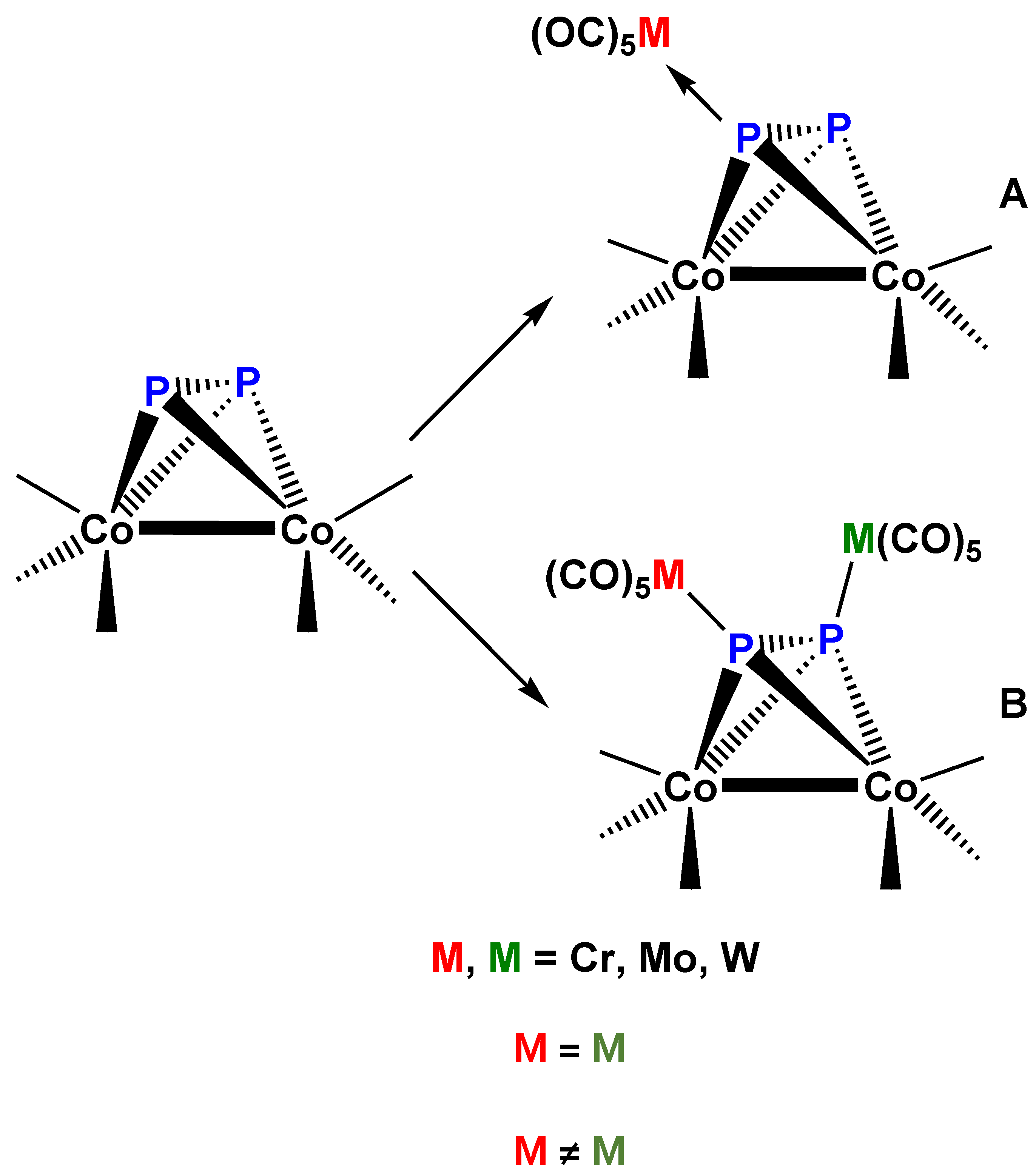
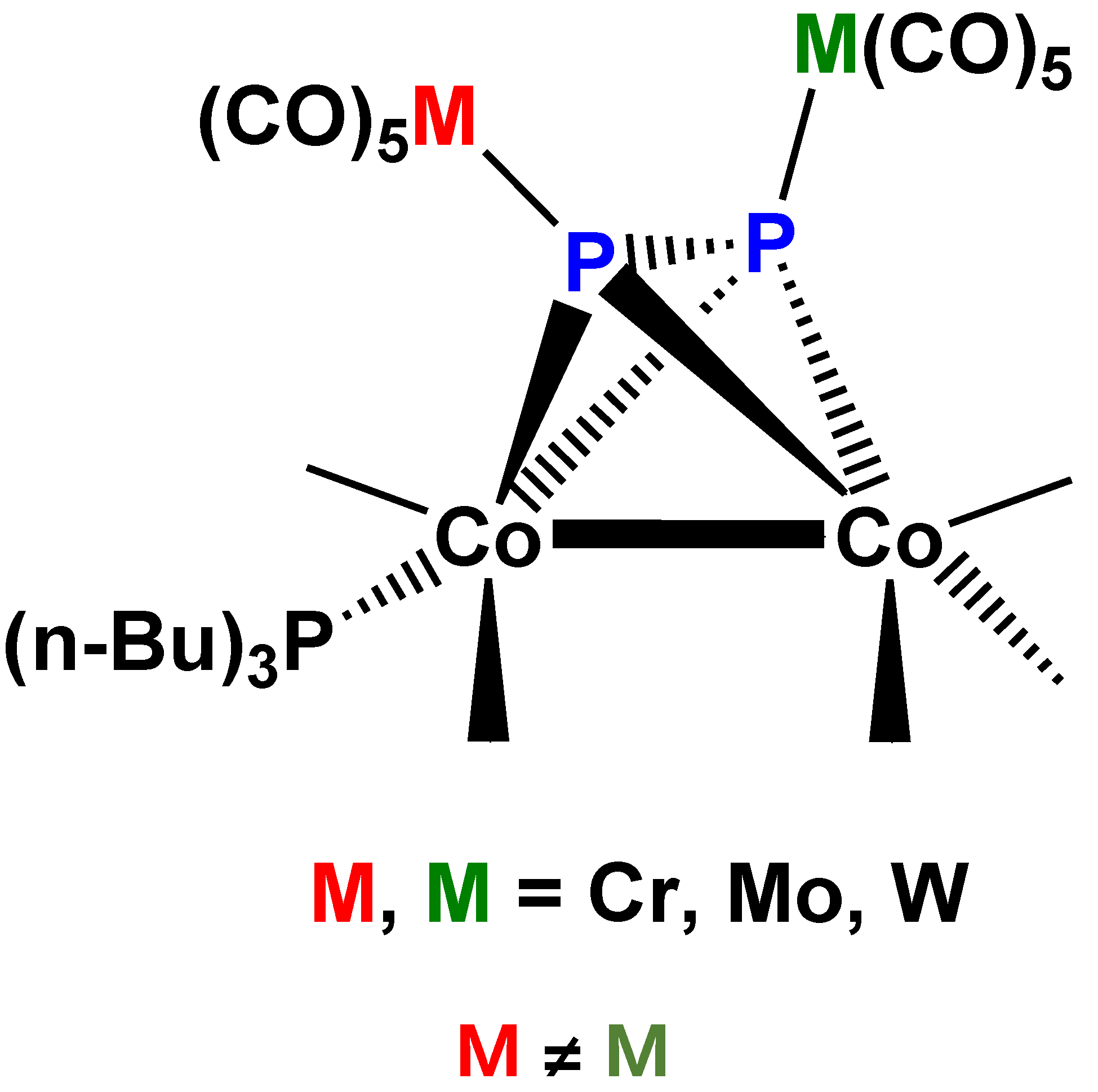
5. Interconversions of Pnicogen/Cobalt Clusters
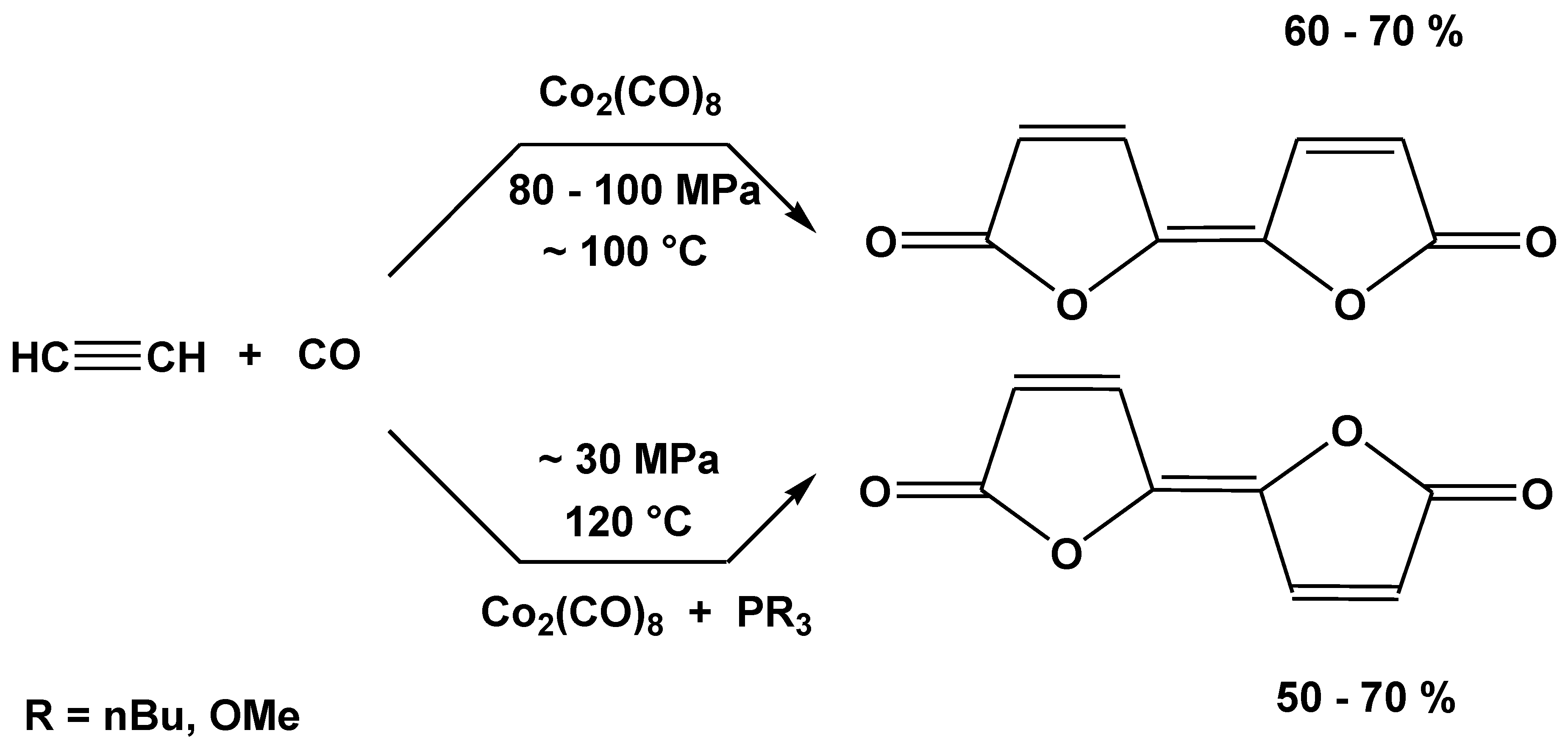
6. Outlook and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vízi-Orosz, A.; Pályi, G.; Markó, L. Phosphido cobalt carbonyl clusters: Co2(CO)6P2 and Co3(CO)9PS. J. Organomet. Chem. 1973, 60, C25–C26. [Google Scholar] [CrossRef]
- Vízi-Orosz, A.; Pályi, G.; Markó, L. (μ2-Diphosphorous)hexacarbonyldicobalt (Co–Co) and derivatives. In Organometallic Syntheses; King, R.B., Eisch, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 272–277. [Google Scholar]
- Campana, C.F.; Vízi-Orosz, A.; Pályi, G.; Markó, L.; Dahl, L.F. Structural characterization of Co2(CO)5[P(C6H5)3](μ-P2): A tricyclic complex containing bridging P2 ligand. Inorg. Chem. 1979, 18, 3054–3059. [Google Scholar] [CrossRef]
- Bor, G.; Markó, L.; Markó, B. Kobaltcarbonylderivate des Tetrachlorkohlenstoffs und organischer gem.-Trihalogen Verbindungen: Trikobaltenneacarbonylkohlenstoff Verbindungen. Chem. Ber. 1962, 95, 333–340. [Google Scholar] [CrossRef]
- Pályi, G.; Piacenti, F.; Markó, L. Methinyltricobalt enneacarbonyl compounds: Preparation, structure and properties. Inorg. Chim. Acta Rev. 1970, 4, 109–121. [Google Scholar] [CrossRef]
- Volpin, M.E.; Shur, V.B. Nitrogen fixation by transition metal complexes. Nature 1966, 209, 1236. [Google Scholar] [CrossRef]
- Allen, A.D.; Senoff, C.V. Nitrogenopentammineruthenium(II) complexes. Chem. Commun. 1965, 24, 621–622. [Google Scholar] [CrossRef]
- Brinzinger, H. Formation of ammonia by insertion of molecular nitrogen into metal-hydride bonds, III. Considerations on the properties of enzymatic nitrogen fixing systems and proposal of a general mechanism. Biochemistry 1966, 5, 3947–3950. [Google Scholar] [CrossRef]
- Newton, W.E. Chemical approaches to nitrogen fixation. Adv.Chem. [Bio. Chem.] 1980, 191, 351–377. [Google Scholar]
- Hu, Y.; Ribbe, M.W. Nitrogenase and homologs. J. Biol. Inorg. Chem. 2015, 20, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Shilov, A.E. Intermediate complexes in chemical and biological nitrogen fixation. Pure Appl. Chem. 1992, 64, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Abel, E.W. The metal carbonyls. Quart. Rev. (Lond.) 1963, 17, 133–159. [Google Scholar] [CrossRef]
- Markó, L. Catalytic properties of the transition metal carbonyls and of their organic π-complexes. In Chemistry of Metal Carbonyls and Their Derivatives; Bor, G., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1966; pp. 138–170. (In Hungarian) [Google Scholar]
- Fischer, E.O. Coordination compounds of unsaturated hydrocarbons with metals. Chem. Soc. (Lond.) Spec. Publ. 1959, 13, 73–92. [Google Scholar]
- Sternberg, H.W.; Wender, I. Metal carbonyls and related compounds as catalytic intermediates in organic syntheses. Chem. Soc. (Lond.) Spec. Publ. 1959, 13, 35–55. [Google Scholar]
- Pauson, P.L. Hydrocarbon derivatives of transition metals. Endeavour 1962, 21, 175–182. [Google Scholar]
- Sternberg, H.W.; Greenfield, H.; Friedel, R.A.; Wotiz, J.H.; Markby, R.; Wender, I. A new type of metallo-organic complex derived from dicobalt octacarbonyl and acetylenes. J. Am. Chem. Soc. 1954, 76, 1457–1458. [Google Scholar] [CrossRef]
- Pályi, G.; Váradi, G.; Markó, L. Stereochemistry of acetylenes coordinated to cobalt. In Stereochemistry of Organometallic and Inorganic Compounds; Bernal, I., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 1, pp. 358–410. [Google Scholar]
- Markó, L.; Bor, G.; Almásy, G. Tricobaltenneacarbonylsulphide. Chem. Ind. 1961, 1491–1492. [Google Scholar]
- Wei, C.H.; Dahl, L.F. Organometallic complexes, VI., Molecular structure of a tricyclic complex, tricobalt enneacarbonyl sulfide. Inorg. Chem. 1967, 6, 1229–1236. [Google Scholar] [CrossRef]
- Khattab, S.A.; Markó, L.; Bor, G. Sulphur containing metal carbonyls. VI., A mixed cobalt-iron carbonyl sulphide. J. Organomet. Chem. 1964, 1, 373–376. [Google Scholar] [CrossRef]
- Bor, G. Chemistry of Metal Carbonyls and Their Derivatives; Akadémiai Kiadó: Budapest, Hungary, 1966; pp. 155–159. (In Hungarian) [Google Scholar]
- Markó, L. Tetrahedral cobalt carbonyl clusters with heteroatoms. Gazz. Chim. Ital. 1979, 109, 247–253. [Google Scholar]
- Markó, L.; Ungváry, F.; Pályi, G.; Sisak, A.; Vízi-Orosz, A. Synthesis and catalytical applications of cobalt and iron carbonyls—Hydroformylation and related reactions. Magyar Kém. Folyóirat 1994, 100, 385–393. (In Hungarian) [Google Scholar]
- Issleib, K. Coordination chemistry of tetravalent phosphorous. Pure Appl. Chem. 1975, 44, 237–267. [Google Scholar] [CrossRef]
- Lang, H.; Zsolnai, L.; Huttner, G. Diphosphorous, -P=P-, as 8-electron ligand. Angew. Chem. Int. Ed. 1983, 22, 976–977. [Google Scholar]
- Caporali, M.; Gonsalvi, L.; Rossin, A.; Petruzzini, M. P4-activation by late transition metal complexes. Chem. Rev. 2010, 110, 4178–4235. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, K.H. Transition metal complexes of the pnictide elements. Coord. Chem. Rev. 2018, 376, 114–195. [Google Scholar] [CrossRef]
- Foust, A.S.; Campana, C.F.; Sinclair, J.D.; Dahl, L.F. Preparation and structural characterization of the mono(triphenylphosphine) and bis(triphenylphosphine) substituted derivatives of Co2(CO)6(μ-As2)—Effect of phosphine ligand substitution on the Co2As2 metal cluster system. Inorg. Chem. 1979, 18, 3047–3054. [Google Scholar] [CrossRef]
- Elian, M.; Chen, M.M.-L.; Mingos, D.M.P.; Hoffmann, R. Comparative bonding study of conical fragments. Inorg. Chem. 1976, 15, 1148–1155. [Google Scholar] [CrossRef]
- De Kock, R.L.; Fehlner, T.P. On the validity of the isolobal principle. Polyhedron 1982, 1, 521–523. [Google Scholar] [CrossRef]
- Hoffmann, R. Building bridges between inorganic and organic chemistry. Angew. Chem. Int. Ed. 1982, 21, 711–724. [Google Scholar] [CrossRef]
- Vízi-Orosz, A. Phosphido cobalt carbonyl clusters Pn[Co(CO)3]4-n (n = 1, 2, 3). J. Organomet. Chem. 1976, 111, 61–64. [Google Scholar] [CrossRef]
- Vízi-Orosz, A.; Galamb, V.; Pályi, G.; Markó, L.; Bor, G.; Natile, G. AsCo3(CO)9, its cyclic trimer, As3Co9(CO)24 and the phosphorous-containing analog P3Co9(CO)24. J. Organomet. Chem. 1976, 107, 235–240. [Google Scholar] [CrossRef]
- Goldberg, K.I.; Hoffmann, D.M.; Hoffmann, R. Existence of binuclear π-bonded dinitrogen complexes. Inorg. Chem. 1982, 21, 3863–3868. [Google Scholar] [CrossRef]
- Bán, M.I.; Dömötör, G.; Vízi-Orosz, A.; Pályi, G. Electronic structure of (μ2-X2)Co2(CO)6 (X = CH, N, P, As) complexes. Atti Accad. Sci. Bologna Cl. Sci. Fis. 1983, 271, 217–225. [Google Scholar]
- Foust, A.S. Synthetic and Structural Studies of the Coordinative Versatility of Pnicogen Atoms in Metal Carbonyl Compounds. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 1970. [Google Scholar]
- Gall, R.S.; Foust, A.S.; Pollick, P.J.; Woiciki, A.; Dahl, L.F. In Proceedings of the Abstracts, American Crystallogrphic Association, Meeting, Berkeley, CA, USA, 24–28 March 1974.
- Dahl, L.F.; Foust, A.S. Organometallic pnicogen complexes, V., Preparation, structure and bonding of the tetrameric antimony-cobalt cluster system, Co4(CO)12Sb4: The first known (main group element)(metal carbonyl) cubane type structure. J. Am. Chem. Soc. 1970, 92, 7337–7341. [Google Scholar] [CrossRef]
- Norman, N.C.; Webster, P.M.; Farrugia, L.J. Further synthetic and structural studies on cobalt carbonyl containing antimony complexes. J. Organomet. Chem. 1992, 430, 205–219. [Google Scholar] [CrossRef]
- Etzrodt, G.; Boese, R.; Schmid, G. Heteronucleare Clustersysteme, XVI., Darstellung und Untersuchung von Tris(tetracarbonylcobaltio)bismutan—Ein Beitrag zur Frage der Existenz Bismuthaltiger Nonacarbonyltricobalt Clusterverbindungen. Chem. Ber. 1979, 112, 2574–2580. [Google Scholar] [CrossRef]
- Kruppa, W.; Blaser, D.; Boese, R.; Schmid, G. Heteronuclear cluster systems, XX [1] μ3-Bismutio-cyclo-tris(tricarbonyliridium) (3Ir-Ir), BiIr3(CO)9—Synthesis and structural investigation of a novel iridium cluster. Z. Naturforsch. B 1982, 37, 209–213. [Google Scholar] [CrossRef]
- Martinengo, S.; Ciani, G. Bismuth-cobalt heteronuclear carbonyl cluster compounds. Synthesis and X-ray characterization of the neutral [BiCo3(CO)9] and of the paramagnetic anion [Bi2Co4(CO)11]−. J. Chem. Soc. Chem. Commun. 1987, 20, 1589–1591. [Google Scholar] [CrossRef]
- Bán, M.I.; Dömötör, G.; Vízi-Orosz, A.; Pályi, G. Electronic structures of the hetreonuclear cobalt clusters Pn[Co(CO)3]4-n. J. Mol. Struct. THEOCHEM 1986, 31, 193–196. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. Side-on binding of the nitrogen molecule to first-row transition metal dimers. J. Chem. Phys. 1991, 95, 364–372. [Google Scholar] [CrossRef]
- Seyferth, D.; Henderson, R.S. Phosphaacetylenehexacarbonyldicobalt complexes—New cluster Lewis bases. J. Organomet. Chem. 1978, 162, C35–C38. [Google Scholar] [CrossRef]
- Seyferth, D.; Merola, J.S. Arsaacetylenes, RC≡As, as ligands in dicobalt hexacarbonyl complexes: Novel main group element—Transition metal hybrid cluster compounds. J. Am. Chem. Soc. 1978, 100, 6783–6784. [Google Scholar] [CrossRef]
- Seyferth, D.; Merola, J.S.; Henderson, R.S. Organocobalt cluster complexes, 34., Preparation properties and chemical reactivity of phospha- and arsaacetylenedicobalt hexacarbonyl complexes, (RCM)Co2(CO)6 (M = P, As). Organometallics 1982, 1, 859–866. [Google Scholar] [CrossRef]
- Bán, M.I.; Dömötör, G.; Vízi-Orosz, A.; Pályi, G. Electronic structures, bonding properties and photoelectron spectra of (μ2-diphosphorous)- and (μ2-1-alkyl-2-phosphaacetylene)hexacarbonyldicobalt (Co–Co). J. Mol. Struct. THEOCHEM 1990, 209, 399–410. [Google Scholar] [CrossRef]
- Bönnemann, H.; Brinkmann, R. Single-step cobalt-catalyzed synthesis of pyridines. Synthesis 1975, 9, 600–602. [Google Scholar] [CrossRef]
- Bönnemann, H. Cobalt-catalyzed pyridine syntheses from alkynes and nitriles. Angew. Chem. Int. Ed. 1978, 17, 505–515. [Google Scholar] [CrossRef]
- Bönnemann, H. Organocobalt compounds in the synthesis of pyridines—An example of structure-effectivity relationships in homogeneous catalysis. Angew. Chem. Int. Ed. 1985, 24, 248–262. [Google Scholar] [CrossRef]
- Falbe, J. Synthesen mit Kohlenmonoxid; Springer: Berlin/Heidelberg, Germany, 1967. [Google Scholar]
- Beller, M.; Cornils, B.; Frohning, C.D.; Kohlpainter, C.W. Progress in hydroformylation and carbonylation. J. Mol. Catal. A 1995, 104, 17–85. [Google Scholar] [CrossRef]
- Klinger, R.J.; Chen, M.J.; Rathke, J.W.; Kramarz, K.W. Effect of phosphines on the thermodynamics of the cobalt-catalyzed hydroformylation system. Organometallics 2007, 26, 352–357. [Google Scholar] [CrossRef]
- Falbe, J. (Ed.) New Syntheses with Carbon Monoxide; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bor, G.; Markó, L. Monosubstituted derivative of dicobalt octacarbonyl with triphenylphosphine: Co2(CO)7P(C6H5)3. Chem. Ind. (Lond.) 1963, 22, 912–913. [Google Scholar]
- Piacenti, F.; Bianchi, M.; Benedetti, E.; Frediani, P. Hydroformylation of propene in the presence of Co2(CO)6[P(C4H9)3]2. J. Organomet. Chem. 1970, 23, 257–264. [Google Scholar] [CrossRef]
- Szabó, P.; Fekete, L.; Nagy-Magos, Z.; Bor, G.; Markó, L. Phosphorous containing metal carbonyls, III., Monosubstituted derivatives of dicobalt octacarbonyl with phosphines and phosphites. J. Organomet. Chem. 1968, 12, 245–248. [Google Scholar] [CrossRef]
- Bartik, T.; Krümmling, T.; Happ, B.; Seiker, A.; Markó, L.; Boese, R.; Ugo, R.; Zucchi, C.; Pályi, G. Intermediates and isomers in the substitution of cobalt carbonyl hydride with thertiary phosphorous ligands. Catal. Lett. 1993, 19, 383–389. [Google Scholar] [CrossRef]
- Galamb, V.; Pályi, G.; Cser, F.; Furmanova, M.G.; Struchkov, Y.T. Stable alkylcobalt carbonyls—[(alkoxycarbonyl)methyl]cobalt tetracarbonyl compounds. J. Organomet. Chem. 1981, 209, 183–195. [Google Scholar] [CrossRef]
- Galamb, V.; Pályi, G.; Ungváry, F.; Markó, L.; Boese, R.; Schmid, G. (η1-Benzyl)cobalt, (η3-benzyl)cobalt and (η1-phenylacetyl)cobalt carbonyls. J. Am. Chem. Soc. 1986, 108, 3344–3351. [Google Scholar] [CrossRef]
- Váradi, G.; Horváth, I.T.; Palágyi, J.; Bak, T.; Pályi, G. On the reactivity of acetylenes coordinated to cobalt, IV., Influence of tertiary phosphorous compounds on the catalytic synthesis of bifurandione. J. Mol. Catal. 1980, 9, 457–460. [Google Scholar] [CrossRef]
- Pályi, G.; Váradi, G.; Horváth, I.T. Activation of carbon monoxide and acetylenes by cobalt carbonyls. J. Mol. Catal. 1981, 13, 61–70. [Google Scholar] [CrossRef]
- Pályi, G.; Váradi, G.; Vízi-Orosz, A.; Markó, L. Reactivity of μ2-acetylenes coordinated to cobalt: Stereochemistry of the formation of the butene-2-olide-4 complexes Co2(CO)7(RR’C4O2). J. Organomet. Chem. 1975, 90, 85–91. [Google Scholar] [CrossRef]
- Váradi, G.; Vecsei, I.; Ötvös, I.; Pályi, G. Reactivity of acetylenes coordinated to cobalt, 2., Regiospecifity of carbonylation of internal acetylenes. J. Organomet. Chem. 1979, 182, 415–423. [Google Scholar] [CrossRef]
- Bán, M.I.; Révész, M.; Bálint, I.; Váradi, G.; Pályi, G. A CNDO/2 study of (μ2--acetylene)hexacarbonyldicobalt (Co–Co) complexes. J. Mol. Struct. THEOCHEM 1982, 88, 357–370. [Google Scholar] [CrossRef]
- Müller, M.; Vahrenkamp, H. Darstellung von Mehrkernkomplexen aus As2Co2(CO)6. J. Organomet. Chem. 1983, 252, 95–104. [Google Scholar] [CrossRef]
- Di Varia, M.; Ghilardi, C.A.; Midollini, S.; Sacconi, L. cyclo-Triphosphorus (Δ-P3) as ligand in cobalt and nickel complexes with 1,1,1-tris(diphenylphosphinomethyl)ethane. Formation and structure. J. Am. Chem. Soc. 1978, 100, 2550–2551. [Google Scholar] [CrossRef]
- Váradi, G.; Vízi-Orosz, A.; Vastag, S.; Pályi, G. Preparation and infrared spectra of monosubstituted PR3 and P(OR)3 (R = alkyl, aryl) derivatives of (μ2-L)2Co2(CO)6 compounds. J. Organomet. Chem. 1976, 108, 225–233. [Google Scholar] [CrossRef]
- Vízi-Orosz, A.; Galamb, V.; Ötvös, I.; Pályi, G.; Markó, L. The behaviour of the AsCo3(CO)9 cluster as a ligand for Cr, Mo, W and Fe carbonyls and AlCl3. Transit. Met. Chem. 1979, 4, 415–423. [Google Scholar] [CrossRef]
- Lang, H.; Huttner, G.; Sigwarth, B.; Jibril, I.; Zsolnai, L.; Orama, O. μ3-P und μ3-As verbrückte Cluster als Liganden. J. Organomet. Chem. 1986, 304, 137–155. [Google Scholar] [CrossRef]
- Vízi-Orosz, A.; Pályi, G.; Markó, L.; Boese, R.; Schmid, G. Reaction of coordinated diphosphorous with metal carbonyl fragments. Crystal structure of [(CO)5Cr][(CO)5W]P2Co2(CO)6. J. Organomet. Chem. 1985, 288, 179–187. [Google Scholar] [CrossRef]
- Douglas, A.E.; Rao, K.S. A new band system of the P2 molecule analogous to the Lyman-Birge-Hopfield bands of N2. Can. J. Phys. 1958, 36, 565–570. [Google Scholar] [CrossRef]
- Malisch, W.; Panster, P. Tris[dicarbonyl(η-cyclopentadienyl)ferrio]stibane—A 5B element base with total transition metal substitution. Angew. Chem. Int. Ed. 1976, 15, 618–619. [Google Scholar] [CrossRef]
- Schmid, G.; Kempny, H.P. The use of elemental phosphorus as ligand in iron carbonyls. Z. Anorg. Allg. Chem. 1977, 432, 160–166. [Google Scholar] [CrossRef]
- Huttner, G.; Sigwarth, B.; Scheidsteger, O.; Zsolnai, L.; Orama, O. Diarsenic, As2, as four-, six- or eight-electron donor ligand. Organometallics 1985, 4, 326–332. [Google Scholar] [CrossRef]
- Scherer, O.J. Phosphorous, arsenic, antimony and bismuth multiply bonded systems with low coordination number—Their roles as complex ligands. Angew. Chem. Int. Ed. 1985, 24, 924–943. [Google Scholar] [CrossRef]
- Lang, H.; Huttner, G.; Zsolnai, L.; Mohr, G.; Sigwarth, B.; Weber, U.; Orama, O.; Jibril, I. Diphosphor, Phosphor-, Arsen- und Antimonatome als Clusterbaugruppen. J. Organomet. Chem. 1986, 304, 157–179. [Google Scholar] [CrossRef]
- Di Varia, M.; Stoppioni, P.; Peruzzini, M. Naked phosphorous atoms and units in transition metal compounds. Polyhedron 1987, 6, 351–382. [Google Scholar]
- Barr, A.M.; Kerlogue, M.D.; Norman, N.C.; Webster, P.M.; Farrugia, L.J. The synthetic and structural characterization of some organotransition metal complexes incorporating antimony. Polyhedron 1989, 8, 2495–2505. [Google Scholar] [CrossRef]
- Scherer, O.J. Complexes with substituent-free acyclic and cyclic phosphorous, arsenic, antimony and bismuth ligands. Angew. Chem. Int. Ed. 1990, 29, 1104–1122. [Google Scholar] [CrossRef]
- Seyferth, D. Chemistry of carbon-functional alkylidynetricobalt nonacarbonyl cluster complexes. Adv. Organomet. Chem. 1976, 14, 97–144. [Google Scholar]
- Chini, P.; Longoni, G.; Albano, V.G. High nuclearity metal carbonyl clusters. Adv. Organomet. Chem. 1976, 14, 285–344. [Google Scholar]
- Váradi, G.; Pályi, G. (μ2-Dihaloacetylene)hexacarbonyldicobalt (Co–Co) compounds and their reaction with cobalt carbonyls. Inorg. Chim. Acta 1976, 20, L33–L34. [Google Scholar] [CrossRef]
- Váradi, G.; Galamb, V.; Palágyi, J.; Pályi, G. On the reactivity of acetylenes coordinated to cobalt, V., Unexpected formation of trinuclear carbyne derivatives from acetylene mono- and dicarboxylic acid esters. Inorg. Chim. Acta 1981, 53, L29–L30, L222. [Google Scholar]
- Harley, A.D.; Guskey, G.J.; Geoffroy, G.L. Interconversion of phosphido-bridged polynuclear cobalt carbonyl complexes—Clevage of the phosphido bridge during hydroformylation catalysis. Organometallics 1983, 2, 53–59. [Google Scholar] [CrossRef]
- Vízi-Orosz, A.; Galamb, V.; Pályi, G.; Markó, L. Transformations of the heteronuclear clusters En[Co(CO)3]4-n (E = P, As; n = 1-3) when treated with soft Lewis acids and bases. J. Organomet. Chem. 1981, 216, 105–111. [Google Scholar] [CrossRef]
- Scheer, M.; Friedrich, G.; Schuster, K. [Cr(CO)5PCl3]—A P1 building block for the formation of complexes with cyclo-Px (X = 3,5) ligands. Angew. Chem. Int. Ed. 1993, 32, 593–594. [Google Scholar] [CrossRef]
- Barr, M.E.; Adams, B.R.; Weller, R.R.; Dahl, L.F. Synthesis and structural-bonding analysis of (η5-C5H4Me)4Fe4(CO)6P8 and (η5-C5H4Me)4Fe6(CO)13P8, two unprecedented transition-metal complexes containing cage-like P8 subunit of Hittorf’s monoclinic phosphorous allotrope. J. Am. Chem. Soc. 1991, 113, 3052–3060. [Google Scholar] [CrossRef]
- Fremoni, C.; Bussoli, G.; Ciabatti, I.; Ermini, M.; Hayatifar, M.; Iapalucci, M.C.; Zacchini, S. Interstitial bismuth atoms in icosahedral rhodium cages: Syntheses and molecular structures of the [Bi@Rh12(CO)27Bi2]3−, [(Bi@Rh12(CO)26)2Bi]5−, [Bi@Rh14(CO)27Bi2]3− and [Bi@Rh17(CO)33Bi2]4− carbonyl clusters. Inorg. Chem. 2017, 56, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Sloveichik, G. Future of Ammonia Production: Improvement of Haber-Bosch Process or Electrochemical Synthesis. Available online: https://nh3fuelassociation.org/2017/10/01/future-of-ammonia-production-improvement-of-haber-bosch-process-or-electrochemical-synthesis/ (accessed on 18 September 2018).
- Nishibayashi, Y. Development of the catalytic nitrogen fixation using transition metal-dinitrogen complexes under mild reaction conditions. Dalton Trans. 2018, 47, 11290–11297. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vízi-Orosz, A.; Berzeviczy, G.; Pályi, G. Coordinated “Naked” Pnicogenes and Catalysis. Catalysts 2018, 8, 583. https://doi.org/10.3390/catal8120583
Vízi-Orosz A, Berzeviczy G, Pályi G. Coordinated “Naked” Pnicogenes and Catalysis. Catalysts. 2018; 8(12):583. https://doi.org/10.3390/catal8120583
Chicago/Turabian StyleVízi-Orosz, Anna, Gergely Berzeviczy, and Gyula Pályi. 2018. "Coordinated “Naked” Pnicogenes and Catalysis" Catalysts 8, no. 12: 583. https://doi.org/10.3390/catal8120583







