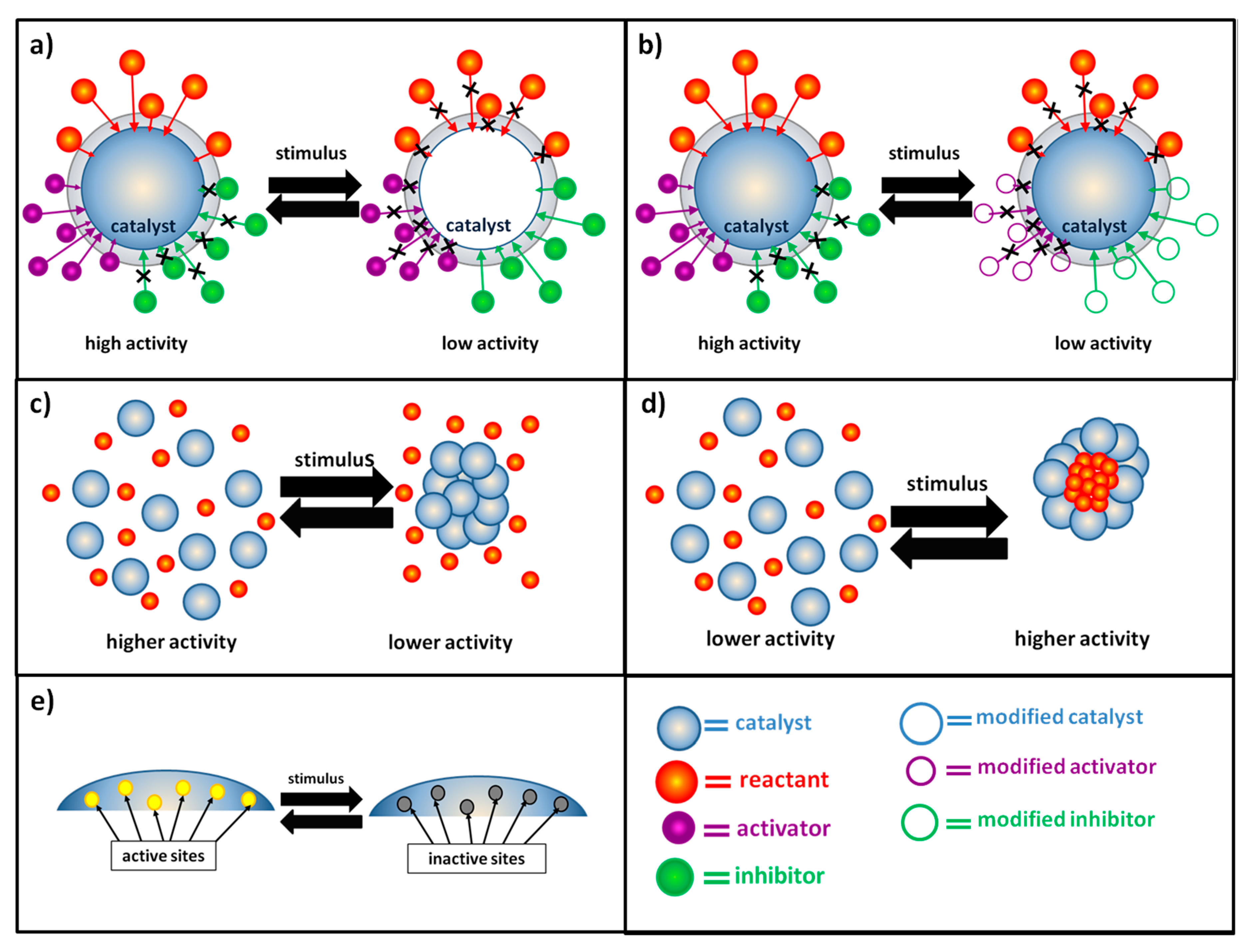
The most widespread strategy to obtain switchable catalysis is the preparation of systems able to respond to the application of an external stimulus by modifying the diffusion dynamics of different chemical species towards the catalyst surface. An enhancement of the catalytic activity and reaction rate is obtained when, after the stimulus, the diffusion of reactants or activators (i.e., the chemical species that promote the interaction between the catalyst and its substrates) is facilitated and the diffusion of inhibitors is obstructed. On the contrary, the reaction rate can be lowered and, eventually, the reaction can be stopped when the diffusion of reactants and activators is hindered and the diffusion of inhibitors is favored (
Figure 1a,b). Variation of the diffusion dynamics can be obtained both in cases where the external stimulus causes a direct modification of the catalytic system (generally metallic nanoparticles embedded in a stimuli-responsive hydrogel matrix or metallic nanoparticles functionalized with stimuli-responsive units) (
Figure 1a) or when the external stimulus modifies the properties of chemical species in the reaction medium (
Figure 1b). The first approach is the most utilized, and in the majority of cases, the external stimulus is used to modify the properties (hydrophilicity, polarity, solubility and volume) of the polymeric matrix with which the catalyst is combined. In both cases, different examples can be found using a variety of stimuli, especially temperature, pH or light. By using the same stimuli, it is possible to control the catalytic activity of the system also by means of variations of the aggregation state of the catalytic system. In most of the reported cases in the literature, a high catalytic activity is observed when the catalyst is homogeneously distributed inside the reaction medium in a non-aggregate state and a decrease of the reaction rate is obtained after its aggregation. This results from a surface effect: in the non-aggregate state catalyst surface is higher, and there are many more active sites accessible to the reactants (
Figure 1c). However, some interesting examples of reaction rate enhancement as a consequence of catalyst aggregation can be found. In particular, this is due to a confinement effect: thanks to the catalyst aggregation, preferential microenvironments for the occurrence of the reaction are formed. As a consequence of the catalyst aggregation, the reactants are concentrated and “trapped” in small regions, characterized by high catalytic activity (
Figure 1d). In most cases, it is difficult to distinguish whether the external stimulus modifies the diffusion dynamic of chemical species or the aggregation state of the catalytic system, often the two effects are combined and the overall result is a switchable catalysis based on switchable accessibility of catalytic active sites.
2.1. Temperature-Controlled Switchable Catalysis
Temperature is the stimulus that has been most widely investigated, and different dynamic catalytic systems based on the employment of temperature-responsive polymers have been proposed. In most cases, a dynamic behavior is obtained by exploiting the capability of Poly(
N-IsoPropylAcrilAmide) PNIPAm to undergo a reversible phase transition at 32 °C [
19]. This temperature is called Lower Critical Solution Temperature (LCST). Below and above LCST, many polymer properties change: solubility in water, transparency, volume and morphology. In particular, at T < LCST, PNIPAm is hydrophilic, soluble in water and it is in a swollen state. It is in the form of coils and its solutions are transparent. On the contrary, at T > LCST, PNIPAm is hydrophobic, insoluble in water, and it is in a shrunken state. In this condition it forms globules, tends to precipitate and it generates turbid solutions. These properties can be exploited also in the field of catalysis: the catalyst activity can be switched “on” and “off” by adjusting the temperature below and above the LCST, changing the hydrophilicity of the polymeric matrix with which the catalyst is combined and converting it from an hydrophilic diffusion layer (easily accessible to polar molecules) to an hydrophobic diffusion layer (difficultly accessible to polar molecules). In addition, this behavior can be exploited to facilitate the recovery of the catalytic system, modifying the aggregation state of the polymeric matrix from a homogeneous state (when T < LCST) to a heterogeneous one (when T > LCST).
For example, Ballauff and coworkers [
20] reported the use of silver nanoparticles embedded in a polymeric network of PNIPAm cross-linked with
N,
N′-methylenebisacrylamide (BIS) attached to a colloidal core made of polystyrene (PS) to catalyze the reaction of reduction of 4-nitrophenol by NaBH
4 to 4-aminophenol in water. When the PS-PNIPAm-Ag system is at T < LCST, the PNIPAm chains are hydrophilic, the shell network is swollen, and the metallic nanoparticles are fully accessible to reactants. In these conditions, the reduction of 4-nitrophenol is well catalyzed. When temperature is enhanced to T > LCST, a significant decrease of the reaction rate is recorded. In these conditions, PNIPAm becomes hydrophobic, the polymeric network shrinks and the diffusion of the reactant within the network is hindered. As visible in
Figure 2, by increasing the temperature, the hydrodynamic radius of PS-PNIPAm-Ag particles (R
H) lowers, indicating that the PNIPAm layer gradually shrinks and this behavior affects the catalytic efficiency of the system. In fact, instead of a simple linear relationship between ln
k and T
−1, the change of rate constant (
k) with temperature can be divided in three regions. At low temperature (green square region in
Figure 2),
k exhibits a conventional Arrhenius-type dependence on T, since the PNIPAm network is totally swollen with water and AgNPs are fully accessible to reactants. When 25 °C < T < 32 °C (yellow square region in
Figure 2), the PNIPAm network begins to shrink and a diffusional barrier for reactants is formed around AgNPs, which turns down the reaction rate and this effect over-compensates for the thermodynamic increase of
k by the rise in temperature. This behavior is maintained until the LCST is reached, when
k acquires its minimum value. At T > LCST (red square region in
Figure 2), instead, the density of the polymer network is constant and the strong increase in
k with T dominates and the reaction rate rises again.
Similar behaviors have been obtained by combining PNIPAm with other copolymers and other types of metallic nanoparticles. For example, Shi and coworkers showed that it is possible to prepare switchable catalysts by creating hydrogels of poly(glycidyl methacrylate) (PGMA) and PNIPAm (PGMA-
co-PNIPAm) and incorporating AuNPs [
21], stabilizing gold nanoparticles with PNIPAm-
b-P4VP (poly(4-vinyl pyridine) [
22] or supporting gold nanoparticles on PAA/P4PV/PEG/PNIPAm [
23] micelles. PNIPAm hydrogels can be combined also with catalysts different from metallic nanoparticles. For example, Huang et al. [
24] reported that it is possible to introduce glutathione peroxidase (GPx) active sites (functional monomer bis-(3-acryloyloxypropyl)-telluride) into a PNIPAm scaffold and obtain a smart microgel catalyst for the reduction of cumene hydroperoxide (CUOOH) by 3-carboxyl-4-nitrobenzenethiol. In this way, it is possible to create a dynamic artificial catalyst mimicking the activity of an antioxidative enzyme that plays a crucial role in the control of reactive oxygen species (ROS) in biological systems. In this regard, it is important to underline that PNIPAm undergoes a temperature-responsive behavior at a near-physiological temperature.
Actually, a similar behavior can also be obtained by employing other temperature-responsive polymers different from PNIPAm. For example, Liu et al. [
25] reported the use of AuNPs functionalized with citrate and a thermo-responsive hyperbranched polyethylenimine with isobutyramide (HPEI-IBAm) for the catalysis of the reduction of 4-nitrophenol by NaBH
4. The most interesting aspect of their work lies in the fact that with their system it is possible to modulate the LCSTs of the thermo-responsive AuNPs over a broader range (20–50 °C) of temperature. The same is true for the activation/deactivation of the catalyst. As in the above-cited examples, the activity of the catalyst is reduced by enhancing the temperature above the LCST of system, but different strategies have been identified to regulate the value of LCST: alteration of the molecular weight of the HPEI core, modification of the degree of substitution of the IBAm groups of the HPEI-IBAm polymers, or variation of solution pH.
All these systems show an inverse temperature response: their reactivity decreases when temperature increases. Cao and coworkers [
26], instead, proposed nanoreactors made of Ag nanoparticles and a functional polymer composite of poly(acrylamide) (PAAm) and poly(2-acrylamide-2-methylpropanesulfonic acid) (PAMPS) with positive responsive function. This means that its catalytic activity significantly increases with temperature enhancement. In particular, in addition to the thermodynamic enhancement of the reaction rate with temperature increase, an abrupt change is recorded in correspondence of a transition temperature (
Figure 3a). This effect is clearly evident when the catalytic activity of the Ag-PAAm-PAMPS system is compared with that of an analogous system made of Ag-PAAm. The catalytic activity of both the systems increases with temperature enhancement, but for Ag-PAAm-PAMPS a great increase of the % of reactant conversion occurs at about 25–35 °C. In fact, at 20 °C, Ag-PAAm-PAMPS demonstrates relatively low catalytic activity that is much smaller than that of Ag-PAAm; on the contrary, at 40 °C the reactivity of Ag-PAAm-PAMPS increases to a much higher level, which is close to that of Ag-PAAm. Interestingly, the temperature range in which this modification occurs (25–35 °C) is in agreement with the temperature range in which a dramatic enhancement of the hydrodynamic radius of Ag-PAAm-PAMPS is recorded (
Figure 3b). The authors suggested that thermal sensitivity derives from the PAAm-PAMPS interactions: at low temperature there are strong electrostatic interactions between the NH
3+ groups of PAAm and SO
3− groups of PAMPS; at high temperature, instead, a dissociation of the interpolymer complexation occurs (
Figure 3c).
As a consequence, at low temperatures (e.g., 20 °C), the PAA-PAMPS polymeric shell is compact and restricts the access of reactants to the encapsulated Ag NPs, leading to a low catalytic activity of the system. On the contrary, at relatively high temperatures (e.g., 40 °C), a significant catalytic activity can be achieved thanks to the dissociation of the interpolymer complexation, which causes relaxation and swelling of the shell network and allows reactants to access the encapsulated AgNPs. By lowering temperature below the transition temperature, it is possible to decrease again the reaction rate. Very recently, Borrmann et al. [
27] proposed another microgel catalyst characterized by a positive temperature response which has been successfully tested for the desymmetrization of meso-anhydrides in organic solvents.
2.2. Switchable Catalysis Based on Variation of pH, Ionic Strength or Other Chemical Stimuli
Similarly, pH-responsive polymers can be employed to functionalize metallic nanoparticles and obtain systems whose catalytic activity can be switched on and off by adjusting pH or ionic strength of the reaction medium. Generally speaking, pH-responsive polymers are polyelectrolytes with weakly ionizable groups in their structure, which either accept or release protons in response to modification of environmental pH. Both weak polyacids and polybases exist. Polyacids accept protons at low values of pH and release them at neutral or high values of pH, acquiring negative charges that create electrostatic repulsion and cause swelling of the polymer network. Examples are poly(acrylic acid) (PAA) or poly(methacrylic acid) (PMMA). In the case of polybases, instead, they are protonated and uncharged at high values of pH and they are positively ionized in neutral and acidic conditions. In this case, the positive charges inside the polymeric network at pH values lower than the polymer pk
a cause electrostatic repulsion and polymer swelling. Examples are poly(4 or 2-vinylpyridine) (P4VP or P2VP) and poly(1-vinylimidazole)(PVIm). Also, in this case, the most frequently employed strategy for the control of the dynamic behavior of the system catalytic activity is based on the control of the swelling degree of the polymeric network used to functionalize the catalyst itself. When the polymeric network is in a swollen state, the reactants are free to reach the catalyst surface; on the contrary, when the polymeric network is in a shrunken state, reactant diffusion is hindered, and the reaction is less catalyzed. An example is the work of Xiao et al. [
28], who reported the preparation of Au@PVP hybrid nanogels for the pH-responsive catalysis of the reduction of 4-nitrophenol by NaBH
4. Actually, this reaction is normally affected by pH variations; in particular, during the process of reduction, sodium metaborate is produced, causing an increase in pH, and when it reaches high values, such as 9–10, 4-nitrophenolate ions can be produced [
29]. Therefore, it is necessary to consider that a lowering of pH by itself favors the reaction of reduction and that 4-nitrophenolate can react differently from 4-nitrophenol. All these aspects make the study of reaction kinetics variation difficult, but the authors showed that the system is characterized by a pH-responsive swelling-deswelling behavior (variations of hydrodynamic radius), which is able to influence also the catalytic activity of the system.
When pH-responsive polymers are involved, it is possible to achieve a dynamic control on their behavior also by varying the ionic strength of the reaction medium. An example was proposed by Yang and co-workers [
30]. In this case, the reduction of 4-nitrophenol by NaBH
4 is catalyzed by Ag@air@PMAA nanorattles. The system is composed of a core made of AgNPs, surrounded by an empty layer of air, which serves as microreactor, and a stimuli-responsive PMMA layer (
Figure 4a). At pH 9, all the PMMA chains are deprotonated and negatively charged: they repel each other and the polymer shell is in a swollen state, which promotes the diffusion of reactants inside the air cavity, where the reaction is efficiently catalyzed. By adding NaCl inside the reaction medium, Na
+ ions are released and interact with the COO
− groups of the PMAA chains. In this way, the above-cited electrostatic repulsion is reduced, the polymeric structure collapses and the diffusion of reactants towards the Ag core is hindered. The result is a decreased catalytic activity in the system, similar to the one that can be obtained by changing the solution pH. In addition, by modifying the ionic strength of the solution it is possible not only to switch the catalysis between an “on” and “off” state, but also to control, dynamically, the reaction rate in a continuous way (
Figure 4b).
Zhang and coworkers suggested that the capability of the polymeric matrix to pass from an hydrophilic to an hydrophobic state according to the external pH can also be exploited to facilitate the separation and reuse of the whole catalytic system [
31,
32], similarly to what happens when pH-sensitive organic capping agents are used to functionalize metallic nanoparticles [
33]. In fact, besides the modification of the capability of permeation of reactants through the matrix toward the catalyst surface, the variable degree of hydrophilicity of the polymeric network of the composite systems enables to modify the aggregation state of the polymeric matrix in which the catalysts are embedded. In particular, they showed that it is possible to obtain efficient recyclable catalytic systems for the Suzuki and Heck reactions in the case of iminodiacetic (IDA)-functionalized Pd nanoparticles embedded in the shell layer of core-shell PS-PMMA [
31] or PS-PGMA [
32] systems. In fact it is possible to disperse PS-
co-PMAA-IDA-Pd in basic reaction medium (pH > 10) and cause it to precipitate by lowering the pH below 5. In basic environments, the system behaves as a homogeneous catalyst with reactants that freely reach the catalyst surface, so it is characterized by a very high catalytic activity. By lowering the pH, the polymeric matrix becomes more hydrophobic, the diffusion of reactant is hindered, and the catalytic activity of the whole system is reduced. In addition, in acidic environments, the polymers tend to segregate from water, the system precipitates and becomes a heterogeneous catalyst, which can be easily removed from the solution. Subsequently, it can be resuspended in a fresh reaction medium and efficiently reused once pH has been adjusted at values higher than 10. They showed that the same system can be reused 4 times without any loss of activity. A similar behavior is ascribed also to the PS-
co-PGMA-IDA-Pd system, even if in this case the pH values for the phase transition are 3 and 11. Also Pd NPs supported on PS-co-PAEMA-co-PMMA core/shell NPs [
34] behave in a similar way.
pH (as well as temperature) variations can be used also to enhance the reaction rate by inducing reactant confinement within polymeric matrix enriched of catalytic NPs. For example, Wang et al. [
35] reported that it is possible to catalyze C-C Cross-Coupling reactions with Pd NPs embedded into PNIPAm-
co-PMACHE hydrogels. By increasing the temperature of the system above the LCST of PNIPAm or by adjusting the pH above 10 or below 4 (PMACE swells at the pH 4–10 and deswells out of this range), it is possible to make the hydrogel shrink, creating a hydrophobic environment ideal for hosting hydrophobic molecules. In addition, since Pd NPs are embedded into the hydrogel, when it shrinks, Pd catalysts are enriched and concentrated in a small volume (
Figure 5). This fact, together with encapsulation of hydrophobic reactants and their concentration in a smaller volume rich of catalytic sites, makes it possible to obtain higher reaction rates.
The enhancement of the reaction rate during hydrogel deswelling has been proved for the Suzuki and the Heck reactions, but a similar behavior was obtained by Zhang and coworkers [
36] during the preparation of a composite catalyst for aerobic alcohol oxidation made of Au NPs embedded into PNIPAm-
co-PMACHE hydrogel. In both the cases, the reversible swelling/deswelling of the hydrogel can be exploited also to obtain an easy separation and reuse of the catalytic system.
Variations of pH can be used also to create preferential channels to facilitate the diffusion of reactants towards the catalyst surface. The Shi group proposed an example [
37]: core-shell-corona Au-micelle composites containing a PS core, a hybrid shell of P4P/Au/PEG and a PEG (polyethylene glycol) corona. At low values of pH (e.g., pH = 2), PEG and P4VP are hydrophilic and the hybrid shell is in a swollen state. This means that Au NPs are accessible to reactants and a good catalytic activity can be reached. By increasing pH, the catalytic activity can be further enhanced. In fact, at pH≥7, P4P become hydrophobic and collapses to the PS core, while PEG maintains its hydrophilic behavior. The hydrophilic PEG chains connect the PS core and pass through the collapsed hybrid shell, creating preferential channels for the diffusion of small hydrophilic reactants.
Dynamic catalysis can be obtained by switching the hydrophilicity/hydrophobicity of the systems by means of other chemical stimuli. For example, Byun et al. [
38] have proposed a CO
2-switchable catalyst for the photodegradation of organic dyes and photoredox reactions of organic water-soluble molecules under visible light illumination. The photocatalyst is a tertiary amine tethered conjugated polymer (P-BT-DEA), which exhibits a reversible hydrophilicity triggered by CO
2. In fact, diethylamine (DEA) at the terminal of the polymer is able to react with CO
2 dissolved in water producing the bicarbonate salt, which boosts the water compatibility of the polymeric chain, leading to good polymer dispersion in water. The adsorbed CO
2 can be removed through N
2 bubbling and the elimination of the bicarbonate salt causes precipitation of the hydrophobic polymer. As a consequence, in the presence of CO
2 it is possible to use the system to efficiently catalyze the photodegradation of different organic dyes (rhodamine B, methylene blue and crystal violet) in water and various organic transformation reactions (e.g., photooxydation of 2-furoic acid, photoreduction of 4-nitrophenol and coupling of caffeine and aryldiazoniumtetrafluoroborate); on the contrary, when N
2 is purged, it displaces CO
2, the polymer becomes hydrophobic, it precipitates and its catalytic activity is reduced.
Another example of CO
2-switchable polymer was reported by Zhang et al. [
39] for the 4-nitrophenol reduction by NaBH
4 in the presence of AuNPs functionalized with thiol-terminated PDEAEMA (poly(
N,
N-diethylaminoethylmethacrylate)). Without CO
2, the PDEAEMA polymer is poorly soluble in water and polymeric chains are hydrophobic, they tend to contract, entangle and strongly interact with each other. As a consequence, AuNPs tend to aggregate and form a low-density polymer network floating on the surface of the reaction medium, which is difficultly accessible to reactants. This fact decreases the catalytic capability of the system. On the contrary, after CO
2 bubbling, water becomes a relatively good solvent for PDEAEMA: thanks to the formation of ammonium bicarbonates originated from the protonation of the amine groups by carbonic acid, the polymer chains switch from hydrophobic to hydrophilic and become more extended in solution. Hence, PDEAEMA-AuNPs become well dispersed in water, forming a homogeneous colloidal solution, in which all AuNPs are easily accessible to the reactants. This fact results in an enhancement of the reaction rate.
An alternative approach to regulating the catalytic activity of the system by controlling directly the reaction medium consists of the modification of the dimension of inhibitors. Grzybowski and co-workers [
40] recently proposed AuNPs functionalized with
N,
N,
N-trimethyl(11-mercaptoundecyl) ammonium chloride (TMA) or 11-mercaptoundecanoic acid (MUA) for the catalysis of the reduction of 4-nitrophenol by NaBH
4. AuNPs functionalized with TMA ligand are positively charged, while AuNPs functionalized with MUA ligand are characterized by a negative surface charge. The catalytic activity of both the types of AuNPs depends not only on the properties of the charged ligand shell, but also on the size of counterions surrounding the charged end-groups. In particular, the authors showed that large counterions can decrease or completely hinder the particle catalytic activity, while small counterions allow the reactant to reach the catalyst surface. Therefore, by exchanging the surrounding counterions, it is possible to dynamically switch between “on” and “off” active states. For example, they compare the catalytic activity of positively charged Au-TMA NPs combined with different counterions: Cl
− (both from the TMA-Cl and TBA-Cl salts) and SDS
−. As shown in
Figure 6, the reaction can be catalyzed when the small Cl
− counterion is considered, while when a bigger counterion (such as SDS
−) is used, the reaction is stopped, since these ions behave as a barrier, hindering the diffusion of reactants toward the catalyst surface. A similar behavior is exhibited by Au-MUA NPs, even if in this case the catalytic activity is controlled by the size of positively charged counterions.
This study also shows how it is possible to perform a dynamic experiment controlling catalysis by switching counterions. Initially, Au-TMA NPs are surrounded by SDS
− counterions and they do not show any catalytic activity. When an equivalent amount of CTAB is added to the reaction medium, the positively charged CTA
+ aggregate into micelles combining with negatively charged SDS
−. In this way big SDS
− counterions are replaced by much smaller Br
− coming from CTAB and in these conditions, Au-TMA NPs become catalytically active. Catalysis can be again switched “off” by the addition of SDS and then “on” again by adding CTAB (
Figure 7).
Reversible catalytic activity has also been obtained by modifying chemically the oxidation state of redox-responsive polymers. Elbert et al. [
41] reported that it is possible to reversibly modulate the catalytic activity of Grubbs Second Generation catalyst for the ROMP (ring-opening metathesis polymerization) reaction of nerbornene when it is immobilized on the surface of SiO
2 NPs functionalized with poly (vinylferrocene). In fact, when poly(vinylferrocene) is oxidized, it increases its hydrophilicity and the polymeric network swells [
42] creating a thicker shell layer that hinders the diffusion of apolar molecules (such as nerbornene) toward the catalyst. As a consequence, after oxidation of the poly(vinylferrocene), the ROMP reaction can be not catalyzed by the immobilized Grubbs Second Generation catalysts, which are not accessible to norbonene monomers. After the reduction of poly(vinylferrocene), the catalytic activity of the system can be restored.
2.3. Light-Controlled Switchable Catalysis
Light is particularly attractive as an alternative stimulus, as it can be delivered to nearly every place and at any time. It is noninvasive and can be precisely controlled with an appropriate source, offering excellent temporal and spatial resolution. Furthermore, using light of predetermined wavelengths allows the selective excitation and subsequent reactivity of specific molecular units [
43].
Most of the photo-switchable systems reported in literature are based on the use of azobenzene units embedded in different matrices, the same happens in the field of responsive catalysis. Azobenzene is a photo-responsive molecule, which undergoes a
trans-
cis isomerization when it is illuminated with UV light and reverts back to the
trans isomer when it is illuminated with visible light (or when it is heated). The isomerization entails an obvious variation of molecule geometry and volume, as well as modification of color and enhancement of dipole moment. All these properties have been widely exploited for the preparation of different types of smart materials, and it is possible to find in the literature interesting examples related to smart catalysis. Lawrence et al. [
44] proposed the use of peptide-ligand-capped Au NPs with azobenzene units integrated into the biomolecular ligand for the switchable catalysis of the reduction of 4-nitrophenol by NaBH
4. Illumination of the system with UV light causes the isomerization of the azobenzene units of the ligand from the
trans to the
cis form. This molecular switching process is propagated through the peptide structure on the nanoparticle surface and a global rearrangement of the ligand layer is obtained (
Figure 8). These alterations of the molecular conformation at the AuNPs surface produce changes in the catalytic activity, since they can modify the number of the exposed active sites on the metal surface (
trans configuration lead to a low exposition of the metal surface, while
cis configuration enables an enhanced exposition of the metal surface toward the reactants). In particular, when azobenzene units are in the
cis state, the peptide ligand shell is more open, and reactants can reach the catalyst surface more easily. In this condition, the reaction occurs quickly. On the contrary, when azobenzene moieties are in the
trans configuration, the peptide chains collapse over the nanoparticle surface, hindering reactant diffusion and lowering the reaction rate. A new enhancement of the catalytic activity can be reached by simply illuminating the system with UV light.
Another interesting approach was proposed by Szewczyk et al. [
45]. The catalytic system is composed of gold nanoparticles whose surface has been functionalized with a ligand shell made of PEG chains alternated with PEG chains containing azobenzene units and a catalytic site (AzoPro) at the end (AzoPro50@AuNPs). In this case, the molecular catalyst is immobilized on catalytically idle nanoparticle surface and the considered reaction is the aldol reaction between
p-nitrobenzaldehyde and cyclohexanone. When the system is illuminated with UV light, the azobenzene units inside the ligand layer switch to their
cis form and undergo significant geometric restructuration. This fact leads to a modification of the catalyst (AzoPro) position and orientation in space, making it less accessible to reactants, which causes a significant decrease of the catalytic activity (
Figure 9a). When the system is irradiated with visible light, the azobenzene units reisomerize into the
trans form and the reaction rate can be enhanced again (
Figure 9b). Actually, because of the need to reorganize the spatial disposition of the ligand shell, it is not possible to completely restore the fast kinetics achievable directly under visible illumination, thus, a non-totally switchable behavior can be obtained.
Azobenzene derivatives (4,4′-diazene-1,2-diyldibenzoic acid, H
2AzDC) have recently been used to functionalize Metal-Organic Frameworks (MOF) and create a photoswitchable catalyst for the Knoevenagel condensation reaction [
46]. The mechanism at the base of this photoswitchable behavior has been ascribed to the fact that H
2AzDC located on the MOF pore wall isomerizes under UV illumination from the
trans to the
cis form, which is characterized by a higher steric hindrance. This isomerization causes a reduction of the volume in which reactants can diffuse, a decrease of the storage capacity of guest molecules inside the MOF and a modification of the capability of coordination of the catalyst (for example, according to DFT calculations, each
trans-H
2AzDC can be surrounded by 4 benzaldehyde molecules, while each
cis-H
2AzDC can accommodate only 3 benzaldehyde molecules). All these aspects lead to a drastic decrease of the reaction rate under UV illumination. Interestingly, the authors reported photoswitching not only of catalytic activity (high catalytic activity in dark, low catalytic activity under UV illumination), but also of catalytic selectivity (under UV illumination, the reaction occurs only with small reactants).
Light can be used as a stimulus to regulate the catalytic activity of the considered system to modify not only the ligands on catalysts, but also chemical species contained inside the reaction medium. Prins and co-workers [
47] proposed a dynamic photo-switchable system composed of gold nanoparticles functionalized with a monolayer of C9-thiols terminated with a 1,4,7-triazacyclononane (TACN)•Zn
2+ headgroup, which is able to catalyze the transphosphorylation of 2-hydroxypropyl-4-nitrophenylphospate (HPNPP, a model substrate for RNA-hydrolysis). If a light-sensitive chemical substrate that is able to modify its affinity for the catalytic system upon photoisomerization is introduced inside the reaction medium, it is possible to use illumination to control the occurrence of the reaction. In this case, the stimulus does not have a direct effect on the catalyst, but on a molecule in the reaction medium, which acts as inhibitor. In the cited example, 4-(phenylazo)-benzoic acid, which combines a photoresponsive azobenzene and a carboxylic group negatively charged at pH 7, was chosen as inhibitor. The
trans isomer has a higher affinity for the considered catalyst in comparison to the
cis one. In fact, the increase in polarity of azobenzene upon
trans-
cis isomerization reduces favorable hydrophobic interaction between 4-(phenylazo)-benzoic acid and the apolar part of the TACN monolayer. This means that when the inhibitor is in its
trans form, it has a high affinity for the catalyst, to which it is bound thanks to hydrophobic interaction. This fact significantly reduces the efficiency of the catalyst. Upon illumination with UV light, it is possible to make 4-(phenylazo)-benzoic acid isomerize to its
cis form, which has a low affinity for the catalyst. In this condition the inhibitor exerts a minor competition for the interaction with the catalyst and the reaction can be catalyzed more efficiently (
Figure 10a). By illuminating the system with visible light again, it is possible to slow down the reaction and control the oscillations of the catalyst between states of high or low activity (
Figure 10b).
Light can also be utilized to control the state of aggregation of the catalyst. Wei et al. [
48] synthesized AuNPs functionalized with a mixture of “background” alkane amines and photo-switchable azobenzene-terminated alkane thiols that can be used for the catalysis of the hydrosilylation reaction of 4-methoxybanzaldeydhe. In the absence of light, the NPs remain unaggregated, exposing a large surface area that promotes the efficient catalysis of the reaction. Upon irradiation with UV light, the NPs aggregate, reducing the solvent-exposed surface area and effectively switching the catalysis off. The catalytic activity is regained upon visible irradiation and NP dispersion (
Figure 11).
The ability to switch catalysis “on” and “off” derives from light-stimulated reversible aggregation of the functionalized AuNPs. In particular, when AuNPs are illuminated with UV light, the azobenzene units isomerize from trans to cis configuration and develop electric dipoles. In non-polar solvent, like toluene (which is used in this reaction), these dipoles translate into attractive forces between NPs, leading to the formation of aggregates. After illumination with visible light, azobenzene units undergo a cis-trans isomerization and aggregates dissolve. The authors reported that it is possible to perform three subsequent catalytic cycles, but beyond this point not all NPs aggregate upon UV illumination and catalysis cannot be completely switched off.
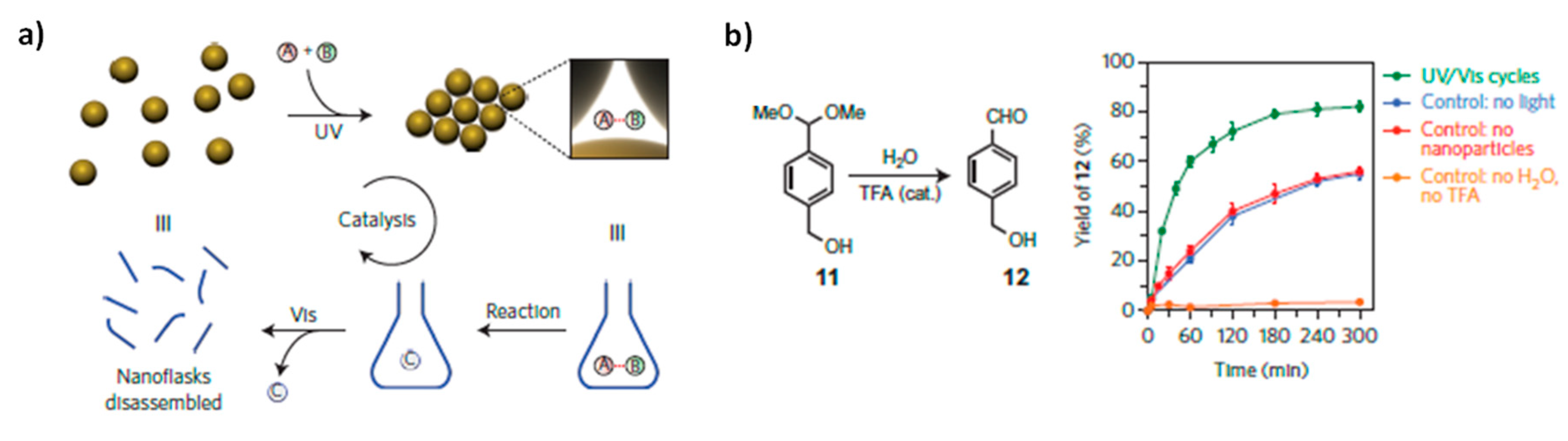
On the contrary, Klajn and coworkers [
49] reported that it is possible to enhance the reaction rate by inducing catalyst aggregation, by trapping reactants within dynamically self-assembled nanoflasks. The proposed catalytic system is composed of NPs (Au, Fe
3O
4 or SiO
2) functionalized with azobenzene containing ligands. When suspended in apolar solvent (e.g., toluene) and illuminated with UV light, they self-assemble as a result of the attractive dipole-dipole interactions between
cis-azobenzene moieties on different NPs. Supracrystals are formed: the NPs are closely packed, leaving small, essentially polar (
cis-azobenzene rich) cavities, which are ideal for the trapping of small polar molecules contained inside the apolar solvent. If pairs of molecules that can potentially react with each other are simultaneously trapped, the confinement inside the cavities of the supracrystal accelerates the reaction between them. Then, once the product has formed, the aggregate can be disintegrated by visible light irradiation, the product can be released, recovered and the whole cycle can be repeated (
Figure 12a). The acid-catalyzed hydrolysis of an acetal to its aldehyde in water-saturated toluene has been studied and it has been verified that in the presence AuNPs aggregate (formed after UV illumination) the reaction proceeds several time faster than in the absence of NPs or in presence of NPs but in absence of UV illumination (AuNPs are not aggregated) (
Figure 12b).
Interestingly, the authors demonstrated that this trapping process can also alter the chemical reactivity of the guest species and the reaction stereoselectivity. For example, in the case of anthracene dimerization, if the reaction occurs in the absence of NP aggregates, almost exclusively the anti-product can be obtained; vice-versa, in the presence of nanoflasks, the syn-isomer is the main product. Probably, inside the NPs aggregates, anthracene pre-organizes itself via hydrogen-bonding with cis-azobenzene units of the NPs and this fact leads to a high yield of the otherwise unstable product. Therefore, this trapping strategy can be employed not just to accelerate a reaction, but also to modify its stereoselectivity. Furthermore, by functionalizing the NPs with azobenzene units and additional ligands, it could be possible to make the trapping selective for the recognition of particular chemical species or achieve also reaction enantioselectivity.