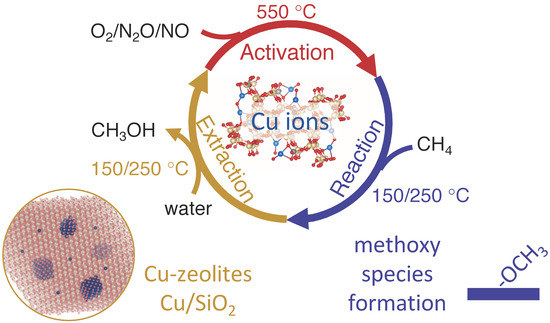
Copper-Modified Zeolites and Silica for Conversion of Methane to Methanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Methanol Production
2.2. Catalyst Characterisation
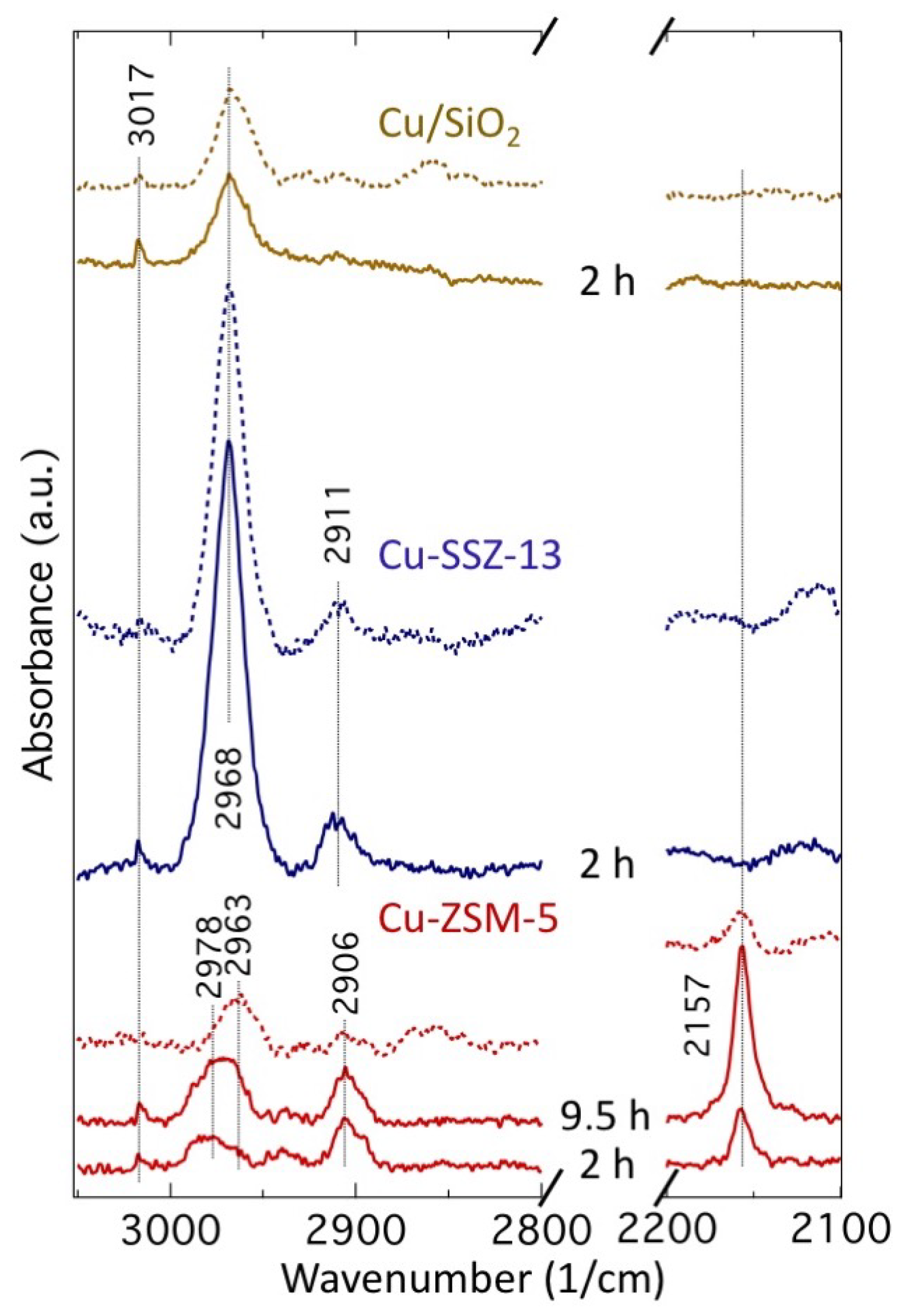
2.3. In Situ IR Study of Methane Oxidation and Water Extraction
2.4. Concluding Discussion
3. Experimental Section
3.1. Catalyst Preparation
3.2. Activity Test
3.3. Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldberg, I.; Rokem, J.S. Biology of Methylotrophs; Butterworth-Heinemann: Stoneham, MA, USA, 1991; p. 3. [Google Scholar]
- Woodland, M.P.; Dalton, H. Purification of Component A of the Soluble Methane Monooxygenase of Methylococcus capsulatus (Bath) by High-pressure Gel-permeation Chromatography. Anal. Biochem. 1984, 139, 459–462. [Google Scholar] [CrossRef]
- Ji, Y.; Mao, G.; Wang, Y.; Bartlam, M. Structural Insights into Diversity and N-alkane Biodegradation Mechanisms of Alkane Hydroxylases. Front. Microbiol. 2013, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Citek, C.; Gary, J.B.; Wasinger, E.C.; Stack, T.D. Chemical Plausibility of Cu-(III) with Biological Ligation in pMMO. J. Am. Chem. Soc. 2015, 137, 6991–6994. [Google Scholar] [CrossRef] [PubMed]
- Vanelderen, P.; Hadt, R.G.; Smeets, P.J.; Solomon, E.I.; Schoonheydt, R.A.; Sels, B.F. Cu-ZSM-5: A Biomimetic Inorganic Model for Methane Oxidation. J. Catal. 2011, 284, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Beznis, N.V.; Weckhuysen, B.M.; Bitter, J.H. Cu-ZSM-5 Zeolites for the Formation of Methanol from Methane and Oxygen: Probing the Active Sites and Spectator Species. Catal. Lett. 2010, 138, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Groothaert, M.H.; Smeets, P.J.; Sels, B.F.; Jacobs, P.A.; Schoonheydt, R.A. Selective Oxidation of Methane by the Bis(μ-oxo)dicopper Core Stabilized on ZSM-5 and Mordenite Zeolites. J. Am. Chem. Soc. 2005, 127, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Narsimhan, K.; Iyoki, K.; Dinh, K.; Román-Leshkov, Y. Catalytic Oxidation of Methane into Methanol over Copper-Exchanged Zeolites with Oxygen at Low Temperature. ACS Cent. Sci. 2016, 2, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Ipek, B.; Lobo, R.F. Catalytic Conversion of Methane to Methanol on Cu-SSZ-13 Using N2O as Oxidant. Chem. Commun. 2016, 52, 13401–13404. [Google Scholar] [CrossRef] [PubMed]
- Woertink, J.S.; Smeets, P.J.; Groothaert, M.H.; Vance, M.A.; Sels, B.F.; Schoonheydt, R.A.; Solomon, E.I. A [Cu2O]2+ Core in Cu-ZSM-5, the Active Site in the Oxidation of Methane to Methanol. Proc. Natl. Acad. Sci. USA 2009, 106, 18908–18913. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.J.; Groothaert, M.H.; Schoonheydt, R.A. Cu Based Zeolites: A UV-vis Study of the Active Site in the Selective Methane Oxidation at Low Temperatures. Catal. Today 2005, 110, 303–309. [Google Scholar] [CrossRef]
- Alayon, E.M.C.; Nachtegaal, M.; Bodi, A.; Ranocchiari, M.; van Bokhoven, J.A. Bis(μ-oxo) Versus Mono(μ-oxo)dicopper Cores in a Zeolite for Converting Methane to Methanol: An In Situ XAS and DFT Investigation. Phys. Chem. Chem. Phys. 2015, 17, 7681–7693. [Google Scholar] [CrossRef] [PubMed]
- Grundner, S.; Markovits, M.A.C.; Li, G.; Tromp, M.; Pidko, E.A.; Hensen, E.J.M.; Jentys, A.; Sanchez-Sanchez, M.; Lercher, J.A. Single-site Trinuclear Copper Oxygen Clusters in Mordenite for Selective Conversion of Methane to Methanol. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Hadt, R.G.; Vanelderen, P.; Sels, B.F.; Schoonheydt, R.A.; Solomon, E.I. [Cu2O]2+ Active Site Formation in Cu-ZSM-5: Geometric and Electronic Structure Requirements for N2O Activation. J. Am. Chem. Soc. 2014, 136, 3522–3529. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Vassilev, P.; Sanchez-Sanchez, M.; Lercher, J.A.; Hensen, E.J.M.; Pidko, E.A. Stability and Reactivity of Copper Oxo-clusters in ZSM-5 Zeolite for Selective Methane Oxidation to Methanol. J. Catal. 2016, 338, 305–312. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Zhao, Z.J.; Siahrostami, S.; Nørskov, J.K.; Studt, F. Monocopper Active Site for Partial Methane Oxidation in Cu-Exchanged 8MR Zeolites. ACS Catal. 2016, 10, 6531–6536. [Google Scholar] [CrossRef]
- Feig, A.L.; Lippard, S.J. Reactions of Non-Heme Iron(II) Centers with Dioxygen in Biology and Chemistry. Chem. Rev. 1994, 94, 759–805. [Google Scholar] [CrossRef]
- Dubkov, K.A.; Sobolev, V.I.; Talsi, E.P.; Rodkin, M.A.; Watkins, N.H.; Shteinman, A.A.; Panov, G.I. Kinetic Isotope Effects and Mechanism of Biomimetic Oxidation of Methane and Benzene on FeZSM-5 Zeolite. J. Mol. Catal. A Chem. 1997, 123, 155–161. [Google Scholar] [CrossRef]
- Tomkins, P.; Mansouri, A.; Bozbag, S.E.; Krumeich, F.; Park, M.B.; Alayon, E.M.; Ranocchiari, M.; van Bokhoven, J.A. Isothermal Cyclic Conversion of Methane into Methanol over Copper-Exchanged Zeolite at Low Temperature. Angew. Chem. Int. Ed. 2016, 55, 5467–5471. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.A.; Zhdanov, V.P.; Carlsson, P.A.; Grönbeck, H.; Hellman, A. Metal Dimer Sites in ZSM-5 Zeolite for Methane-to-Methanol Conversion from First-Principles Kinetic Modelling: Is the [Cu–O–Cu]2+ Motif Relevant for Ni, Co, Fe, Ag, and Au? Catal. Sci. Technol. 2017, 7, 1470–1477. [Google Scholar] [CrossRef]
- Argauer, R.J.; Landolt, G.R. Crystalline Zeolite ZSM-5 and Method of Preparing the Same. U.S. Patent 3,702,886, 14 November 1972. [Google Scholar]
- Hadjiivanov, K. Advances in Catalysis; Academic Press: New York, NY, USA, 2014; Volume 57, p. 101. [Google Scholar]
- Oord, R.; Schmidt, J.E.; Weckhuysen, B.M. Methane-to-methanol Conversion over Zeolite Cu-SSZ-13, and Its Comparison with the Selective Catalytic Reduction of NOx with NH3. Catal. Sci. Technol. 2018, 8, 1028–1038. [Google Scholar] [CrossRef]
- Ipek, B.; Wulfers, M.J.; Kim, H.; Göltl, F.; Hermans, I.; Smith, J.P.; Booksh, K.S.; Brown, C.M.; Lobo, R.F. Formation of [Cu2O2]2+ and [Cu2O]2+ toward C-H Bond Activation in Cu-SSZ-13 and Cu-SSZ-39. ACS Catal. 2017, 7, 4291–4303. [Google Scholar] [CrossRef]
- Pappas, D.K.; Borfecchia, E.; Dyballa, M.; Pankin, I.A.; Lomachenko, K.A.; Martini, A.; Signorile, M.; Teketel, S.; Arstad, B.; Berlier, G.; et al. Methane to Methanol: Structure-Activity Relationships for Cu-CHA. J. Am. Chem. Soc. 2017, 139, 14961–14975. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, C.; Bordiga, S.; Salvalaggio, M.; Spoto, G.; Zecchina, A. XAFS, IR, and UV-Vis Study of the CuI Environment in CuI-ZSM-5. J. Phys. Chem. B 1997, 101, 344–360. [Google Scholar] [CrossRef]
- Groothaert, M.H.; Bokhoven, J.A.V.; Battiston, A.A.; Weckhuysen, B.M.; Schoonheydt, R.A. Bis(μ-oxo)dicopper in Cu-ZSM-5 and Its Role in the Decomposition of NO: A Combined in Situ XAFS, UV-Vis-Near-IR, and Kinetic Study. J. Am. Chem. Soc. 2002, 125, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.C.A.; Passos, F.B.; Schmal, M. Quantification of metallic area of high dispersed copper on ZSM-5 catalyst by TPD of H2. Catal. Commun. 2002, 3, 503–509. [Google Scholar] [CrossRef]
- Martini, A.; Borfecchia, E.; Lomachenko, K.A.; Pankin, I.A.; Negri, C.; Berlier, G.; Beato, P.; Falsig, H.; Bordiga, S.; Lamberti, C. Composition-driven Cu-speciation and reducibility in Cu-CHA zeolite catalysts: A multivariate XAS/FTIR approach to complexity. Chem. Sci. 2017, 8, 6836–6851. [Google Scholar] [CrossRef] [PubMed]
- International Zeolite Association Webpage. Available online: http://europe.izastructure.org (accessed on 14 March 2017).
- Scherrer, P. Bestimmung der Größe und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen Math. Phys. Klasse 1918, 3, 98–100. [Google Scholar]
- The Farrel Lytle Database. Available online: http://ixs.csrri.iit.edu/database/data/Farrel_Lytle_data/ (accessed on 18 May 2017).
- Manzanares, C.; Brock, A.; Peng, J.; Blunt, V.M. Vibrational Spectroscopy of C-H Bonds of Methane and Tetramethylsilane in Liquid Argon Solutions. Chem. Phys. Lett. 1993, 207, 159–166. [Google Scholar] [CrossRef]
- Kung, M.C.; Lin, S.S.Y.; Kung, H.H. In situ Infrared Spectroscopic Study of CH4 Oxidation Over Co-ZSM-5. Top. Catal. 2012, 55, 108–115. [Google Scholar] [CrossRef]
- Wood, B.R.; Reimer, J.A.; Bell, A.T.; Janicke, M.T.; Ott, K.C. Methanol Formation on Fe/Al-MFI via the Oxidation of Methane by Nitrous Oxide. J. Catal. 2004, 225, 300–306. [Google Scholar] [CrossRef]
- Campbell, S.M.; Jiang, X.Z.; Howe, R.F. Methanol to Hydrocarbons: Spectroscopic Studies and the Significance of Extra-Framework Aluminium. Microporous Mesoporous Mater. 1999, 29, 91–108. [Google Scholar] [CrossRef]
- Wang, X.; Arvidsson, A.A.; Cichocka, M.O.; Zou, X.; Martin, N.M.; Nilsson, J.; Carlson, S.; Gustafson, J.; Skoglundh, M.; Hellman, A.; et al. Methanol Desorption from Cu-ZSM-5 Studied by In Situ Infrared Spectroscopy and First-Principles Calculations. J. Phys. Chem. C 2017, 121, 27389–27398. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Kantcheva, M.M.; Klissurski, D.G. IR Study of CO Adsorption on Cu-ZSM-5 and CuO/SiO2 Catalysts: δ and π Components of the Cu+-CO Bond. J. Chem. Soc. Faraday Trans. 1996, 92, 4595–4600. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J.A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Permenov, D.G.; Radzig, V.A. Mechanisms of Heterogeneous Processes in the System SiO2 + CH4: I. Methane Chemisorption on a Reactive Silica Surface. Kinet. Catal. 2004, 45, 14–23. [Google Scholar] [CrossRef]
- Chen, L.; Lin, L.; Xu, Z.; Zhang, T.; Liang, D. Interaction of Methane with Surfaces of Silica, Aluminas and HZSM-5 Zeolite. A Comparative FT-IR Study. Catal. Lett. 1995, 35, 245–258. [Google Scholar] [CrossRef]
- Kazansky, V.B. State and Properties of Ion-exchanged Cations in Zeolites: 2. IR Spectra and Chemical Activation of Adsorbed Methane. Kinet. Catal. 2014, 55, 737–747. [Google Scholar] [CrossRef]
- Kazanskii, V.; Serykh, A.; Bell, A. Diffuse-reflectance IR Spectra of Methane Adsorbed on NaZSM-5 and HZSM-5 zeolites. Kinet. Catal. 2002, 43, 419–426. [Google Scholar] [CrossRef]
- Park, M.B.; Ahn, S.H.; Mansouri, A.; Ranocchiari, M.; van Bokhoven, J.A. Comparative Study of Diverse Copper Zeolites for the Conversion of Methane into Methanol. ChemCatChem 2017, 9, 3705–3713. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local Environment and Nature of Cu Active Sites in Zeolite-Based Catalysts for the Selective Catalytic Reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Yumura, T.; Hirose, Y.; Wakasugi, T.; Kuroda, Y.; Kobayashi, H. Roles of Water Molecules in Modulating the Reactivity of Dioxygen-Bound Cu-ZSM-5 toward Methane: A Theoretical Prediction. ACS Catal. 2016, 6, 2487–2495. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Sot, P.; Nachtegaal, M.; Ranocchiari, M.; van Bokhoven, J.A.; Mesters, C. Direct Stepwise Oxidation of Methane to Methanol over Cu-SiO2. ACS Catal. 2018, 8, 5721–5731. [Google Scholar] [CrossRef]
- Keil, F.J. Methanol-to-hydrocarbons: Process Technology. Microporous Mesoporous Mater. 1999, 29, 49–66. [Google Scholar] [CrossRef]
- Shishkin, A.; Kannisto, H.; Carlsson, P.A.; Härelind, H.; Skoglundh, M. Synthesis and Functionalization of SSZ-13 as an NH3-SCR Catalyst. Catal. Sci. Technol. 2014, 4, 3917–3926. [Google Scholar] [CrossRef]
- Zones, S.I. Zeolite SSZ-13 and Its Method of Preparation. U.S. Patent 4,544,538, 1 October 1985. [Google Scholar]
- Velin, P.; Stenman, U.; Skoglundh, M.; Carlsson, P.A. Portable Device for Generation of Ultra-pure Water Vapor Feeds. Rev. Sci. Instrum. 2017, 88, 115102. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rivallan, M.; Thibault-Starzyk, F.; Travert, A.; Meunier, F.C. Effective Bulk and Surface Temperatures of the Catalyst Bed of FT-IR Cells Used for in situ and operando Studies. Phys. Chem. Chem. Phys. 2013, 15, 7321–7327. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Martin, N.M.; Nilsson, J.; Carlson, S.; Gustafson, J.; Skoglundh, M.; Carlsson, P.-A. Copper-Modified Zeolites and Silica for Conversion of Methane to Methanol. Catalysts 2018, 8, 545. https://doi.org/10.3390/catal8110545
Wang X, Martin NM, Nilsson J, Carlson S, Gustafson J, Skoglundh M, Carlsson P-A. Copper-Modified Zeolites and Silica for Conversion of Methane to Methanol. Catalysts. 2018; 8(11):545. https://doi.org/10.3390/catal8110545
Chicago/Turabian StyleWang, Xueting, Natalia M. Martin, Johan Nilsson, Stefan Carlson, Johan Gustafson, Magnus Skoglundh, and Per-Anders Carlsson. 2018. "Copper-Modified Zeolites and Silica for Conversion of Methane to Methanol" Catalysts 8, no. 11: 545. https://doi.org/10.3390/catal8110545