Mixed Chlorometallate Ionic Liquids as C4 Alkylation Catalysts: A Quantitative Study of Acceptor Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Estimation of δi,cor at Infinite Dilution
2.2. AN Values and C4 Alkylation Performance
2.3. Effects of Metal Chloride (MCl) on the Acidity of the Chloroaluminate IL
2.4. The Speciation of Mixed Chlorometallate Ions
3. Materials and Methods
3.1. Preparation of Ionic Liquids
3.2. 31P NMR Spectroscopy
3.3. C4 Alkylation Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amarasekara, A.S. Acidic ionic liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.; Berton, P.; Kelley, S.P.; Aduri, P.; Katti, S.S.; Rogers, R.D. Group IIIA halometallate ionic liquids: Speciation and applications in catalysis. ACS Catal. 2017, 7, 7014–7028. [Google Scholar] [CrossRef]
- Radai, Z.; Kiss, N.Z.; Keglevich, G. An overview of the applications of ionic liquids as catalysts and additives in organic chemical reactions. Curr. Org. Chem. 2018, 22, 533–556. [Google Scholar] [CrossRef]
- Estager, J.; Holbrey, J.D.; Swadźba-Kwaśny, M. Halometallate ionic liquids -revisited. Chem. Soc. Rev. 2014, 43, 847–886. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Hogg, J.M.; Swadźba-Kwaśny, M. Lewis acidic ionic liquids. Top. Curr. Chem. 2017, 375, 1–40. [Google Scholar] [CrossRef]
- Chauvin, Y.; Hirschauer, A.; Olivier, H. Alkylation of isobutane with 2-butene using 1-butyl-3-methylimidazolium chloride-aluminium chloride molten salts as catalysts. J. Mol. Catal. 1994, 92, 155–165. [Google Scholar] [CrossRef]
- Cong, Y.; Liu, Y.; Hu, R. Isobutane/2-butene alkylation catalyzed by strong acids in the presence of ionic liquid additives. Pet. Sci. Technol. 2014, 32, 1981–1987. [Google Scholar] [CrossRef]
- Hu, P.; Wang, Y.; Meng, X.; Zhang, R.; Liu, H.; Xu, C.; Liu, Z. Isobutane alkylation with 2-butene catalyzed by amide-AlCl3-based ionic liquid analogues. Fuel 2017, 189, 203–209. [Google Scholar] [CrossRef]
- Huang, M.Y.; Wu, J.C.; Shieu, F.S.; Lin, J.J. Preparation of high energy fuel JP-10 by acidity-adjustable chloroaluminate ionic liquid catalyst. Fuel 2011, 90, 1012–1017. [Google Scholar] [CrossRef]
- Wang, L.; Zou, J.J.; Zhang, X.; Wang, L. Rearrangement of tetrahydrotricyclopentadiene using acidic ionic liquid: Synthesis of diamondoid fuel. Energy Fuels 2011, 25, 1342–1347. [Google Scholar] [CrossRef]
- Maase, M. Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T.T., Eds.; Wiley-VCH Verlags GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 7–23. ISBN 3527312390. [Google Scholar]
- Parshall, G.W. Catalysis in molten salt media. J. Am. Chem. Soc. 1972, 94, 8716–8719. [Google Scholar] [CrossRef]
- Kore, R.; Kelley, S.P.; Aduri, P.; Rogers, R.D. Mixed metal double salt ionic liquids comprised of [HN222]2[ZnCl4] and AlCl3 provide tunable Lewis acid catalysts related to the ionic environment. Dalton Trans. 2018, 47, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Zinurov, D.R.; Zinurov, R.R.; Akhmed’yanova, R.A.; Liakumovich, A.G. Skeletal isomerization of n-pentane in the presence of an AlCl3-based ionic liquid. Pet. Chem. 2010, 50, 376–380. [Google Scholar] [CrossRef]
- Stenzel, O.; Brüll, R.; Wahner, U.M.; Sanderson, R.D.; Raubenheimer, H.G. Oligomerization of olefins in a chloroaluminate ionic liquid. J. Mol. Catal. A Chem. 2003, 192, 217–222. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Meng, X.; Xu, C. Oligomerization of isobutene catalyzed by iron(III) chloride ionic liquids. Energy Fuels 2009, 23, 70–73. [Google Scholar] [CrossRef]
- Chauvin, Y.; Gilbert, B.; Guibard, I. Catalytic dimerization of alkenes by nickel complexes in organochloroaluminate molten salts. J. Chem. Soc. Chem. Commun. 1990, 1715–1716. [Google Scholar] [CrossRef]
- Gilbert, B.; Olivier-Bourbigou, H.; Favre, F. Chloroaluminate ionic liquids: From their structural properties to their applications in process intensification. Oil Gas Sci. Technol. 2007, 62, 745–759. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Liu, H.; Meng, X.; Xu, C.; Liu, Z.; Klusener, P.A.A. Quantitative characterization of lewis acidity and activity of chloroaluminate ionic liquids. Ind. Eng. Chem. Res. 2016, 55, 11878–11886. [Google Scholar] [CrossRef]
- Laurence, C.; Graton, J.; Gal, J.F. An overview of Lewis basicity and affinity scales. J. Chem. Educ. 2011, 88, 1651–1657. [Google Scholar] [CrossRef]
- Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. Determination of an acidic scale in room temperature ionic liquids. J. Am. Chem. Soc. 2003, 125, 5264–5265. [Google Scholar] [CrossRef] [PubMed]
- Zawodzinski, T.A.; Osteryoung, R.A. Donor-acceptor properties of ambient-temperature chloroaluminate melts. Inorg. Chem. 1989, 28, 1710–1715. [Google Scholar] [CrossRef]
- Koronaios, P.; King, D.; Osteryoung, R.A. Acidity of neutral buffered 1-ethyl-3-methylimidazolium chloride−AlCl3 ambient-temperature molten salts. Inorg. Chem. 1998, 37, 2028–2032. [Google Scholar] [CrossRef]
- Estager, J.; Oliferenko, A.A.; Seddon, K.R.; Swadźba-Kwaśny, M. Chlorometallate(III) ionic liquids as Lewis acidic catalysts—A quantitative study of acceptor properties. Dalton Trans. 2010, 39, 11375–11382. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W.; Men, S.; Clarke, C.J.; Licence, P. Acidity and basicity of halometallate-based ionic liquids from X-ray photoelectron spectroscopy. RSC Adv. 2013, 3, 9436–9445. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Kou, Y. Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, R.; Meng, X.; Liu, H.; Xu, C.; Liu, Z. Structural and spectroscopic characterizations of amide-AlCl3-based ionic liquid analogues. Inorg. Chem. 2016, 55, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, R.; Xu, C.; Su, H. Alkylation of isobutene with 2-butene using composite ionic liquid catalysts. Appl. Catal. A Gen. 2008, 346, 189–193. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Sun, H.; Hu, R. Effects of catalyst composition on the ionic liquid catalyzed isobutane/2-butene alkylation. J. Mol. Catal. A Chem. 2015, 398, 133–139. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, R.; Hu, R. Reaction mechanism of ionic liquid catalyzed alkylation: Alkylation of 2-butene with deuterated isobutene. J. Mol. Catal. A Chem. 2016, 421, 29–36. [Google Scholar] [CrossRef]
- Cui, J.; De With, J.; Klusener, P.A.A.; Su, X.; Meng, X.; Zhang, R.; Liu, Z.; Xu, C.; Liu, H. Identification of acidic species in chloroaluminate ionic liquid catalysts. J. Catal. 2014, 320, 26–32. [Google Scholar] [CrossRef]
- Currie, M.; Estager, J.; Licence, P.; Men, S.; Nockemann, P.; Seddon, K.R.; Swadźba-Kwaśny, M.; Terrade, C. Chlorostannate(II) ionic liquids: Speciation, lewis acidity, and oxidative stability. Inorg. Chem. 2013, 52, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, V. The Donor-Acceptor Approach to Molecular Interactions; Plenum Press: New York, NY, USA; London, UK, 1978; pp. 25–35. ISBN 978-1-4615-8827-6. [Google Scholar]
- Turner, R.W.; Amma, E.L. Crystal and molecular structure of metal ion-aromatic complexes. I. The cuprous ion-benzene complex, C6H6·CuAlCl4. J. Am. Chem. Soc. 1963, 85, 4046–4047. [Google Scholar] [CrossRef]
- Roebuck, A.; Evering, B. Isobutane-olefin alklation with inhibited aluminum chloride catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 76–82. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Z.; Han, B.X.; Gao, H.X.; Liu, Z.M.; Jiang, T.; Huang, J. Desulfurization of flue gas: SO2 absorption by an ionic liquid. Angew. Chem. Int. Ed. 2004, 43, 2415–2417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J. Lewis acidity and basicity of mixed chlorometallate ionic liquids: Investigations from surface analysis and Fukui function. Molecules 2018, 23, 2516. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.L.T.; Korth, W.; Aschauer, S.; Jess, A. Alkylation of isobutane with 2-butene using ionic liquids as catalyst. Green Chem. 2009, 11, 1961–1967. [Google Scholar] [CrossRef]
- Bui, T.L.T.; Korth, W.; Jess, A. Influence of acidity of modified chloroaluminate based ionic liquid catalysts on alkylation of iso-butene with butene-2. Catal. Commun. 2012, 25, 118–124. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Liu, H.; Smith, D.S.; Hanson, J.C.; Osterhout, D.; Ciraolo, M.; Grey, C.P.; Martin, J.D. Sorptive reconstruction of the CuAlCl4 framework upon reversible ethylene binding. J. Am. Chem. Soc. 2003, 125, 11065–11079. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Leafblad, B.R.; Sullivan, R.M.; Boyle, P.D. α- and β-CuAlCl4: Framework construction using corner-shared tetrahedral metal−halide building blocks. Inorg. Chem. 1998, 37, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.W.; Amma, E.L. Metal ion-aromatic complexes. III. The crystal and molecular structure of C6H6·CuAlCl4. J. Am. Chem. Soc. 1966, 88, 1877–1882. [Google Scholar] [CrossRef]
- Yoo, K.; Naboodiri, V.V.; Varma, R.S. Ionic liquid-catalyzed alkylaton of isobutane with 2-butene. J. Catal. 2004, 222, 511–519. [Google Scholar] [CrossRef]
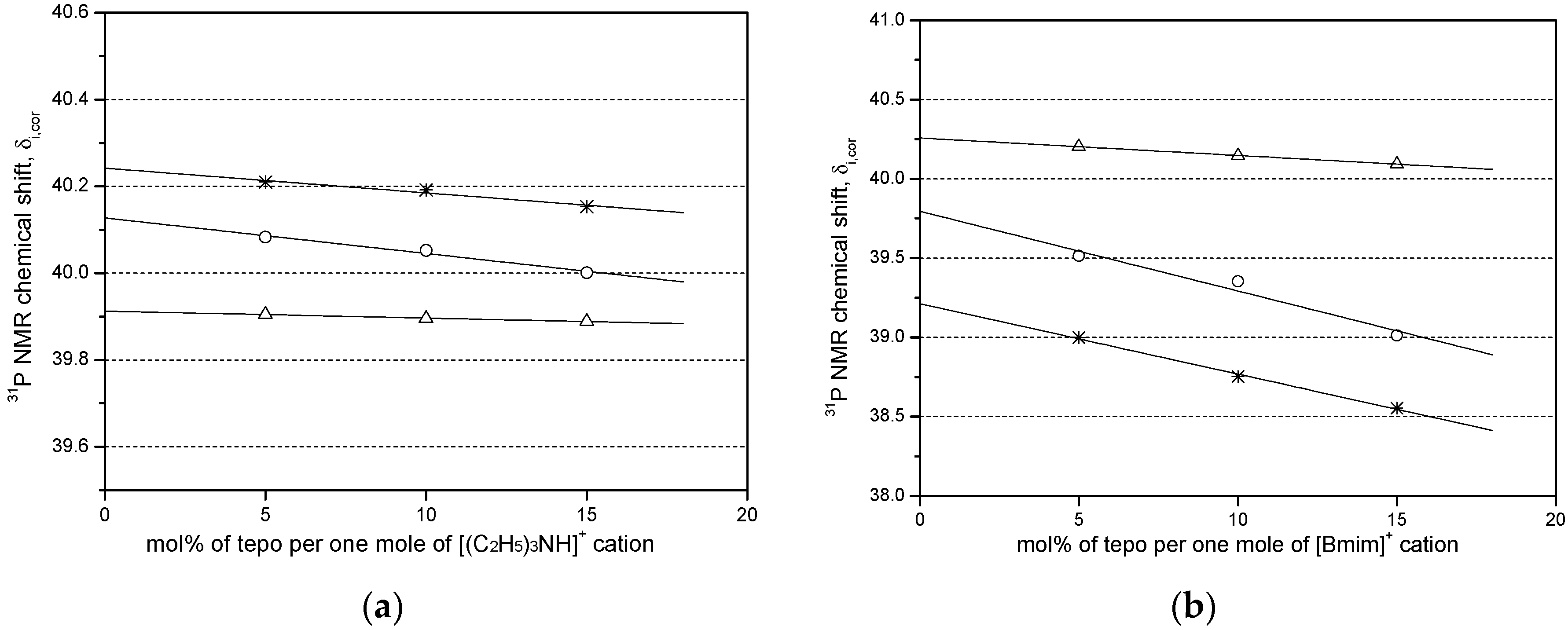
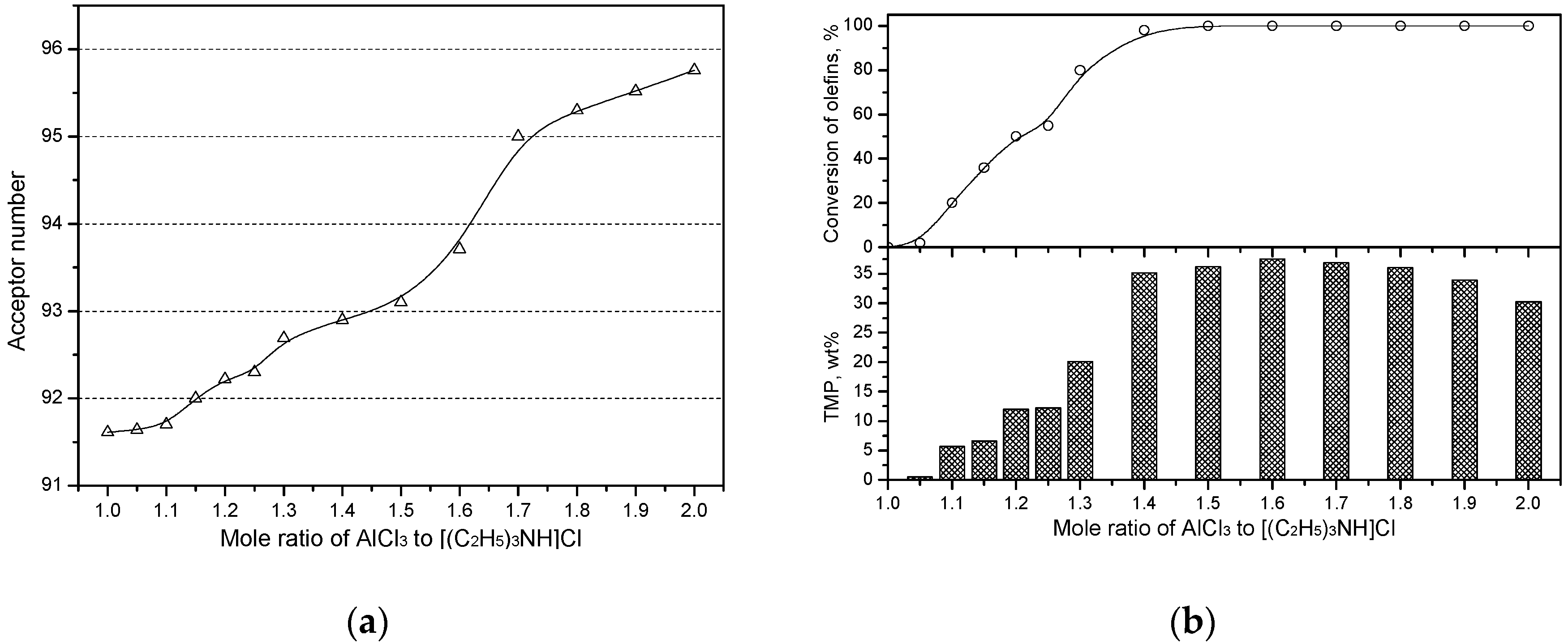
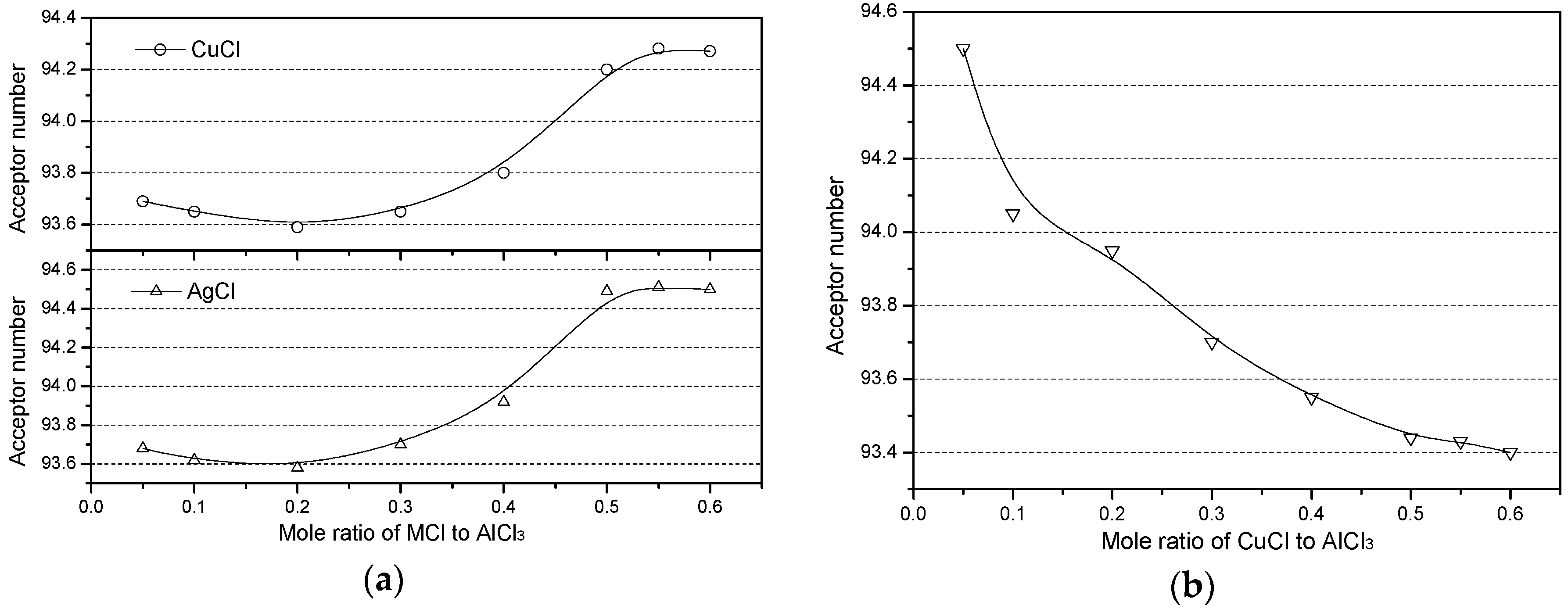
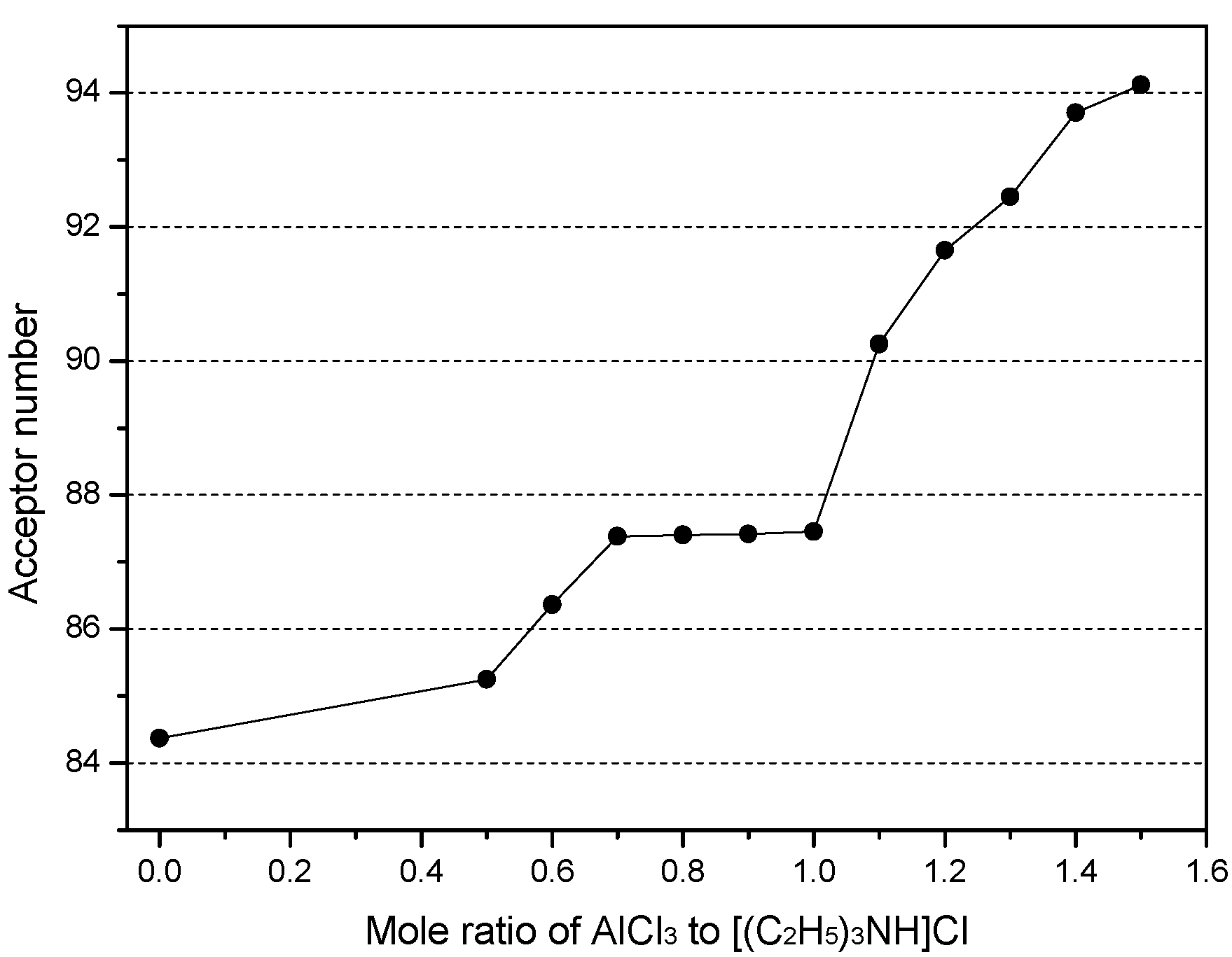



| Entry | Ionic Liquids 1 | m | δi,cor | R2 |
|---|---|---|---|---|
| 1 | [(C2H5)3NH]Cl−AlCl3 | −0.0016 | 39.9123 | 0.9591 |
| 2 | [(C2H5)3NH]Cl−AlCl3−CuCl | −0.0082 | 40.1273 | 0.9611 |
| 3 | [(C2H5)3NH]Cl−AlCl3−AgCl | −0.0057 | 40.2422 | 0.9631 |
| 4 | [Bmim]Cl−AlCl3 | −0.0109 | 40.2572 | 0.9983 |
| 5 | [Bmim]Cl−AlCl3−CuCl | −0.0502 | 39.7951 | 0.9203 |
| 6 | [Bmim]Cl−AlCl3−AgCl | −0.0444 | 39.2126 | 0.9921 |
| 7 | [(C2H5)3NH]Cl−CuCl | −0.0361 | 35.9313 | 0.9998 |
| 8 | [(C2H5)3NH]Cl−ZnCl2 | −0.0316 | 36.3523 | 0.9877 |
| Entry | Catalysts | AN | TMP 1, wt.% | Calculated RON 2 |
|---|---|---|---|---|
| 1 | [(C2H5)3NH]Cl−AlCl3 | 93.71 | 37.5 | 88.6 |
| 2 | [(C2H5)3NH]Cl−AlCl3−CuCl | 94.22 | 78.2 | 99.0 |
| 3 | [(C2H5)3NH]Cl−AlCl3−AgCl | 94.49 | 80.5 | 99.6 |
| 4 | [Bmim]Cl−AlCl3 | 94.52 | 43.7 | 90.6 |
| 5 | [Bmim]Cl−AlCl3−CuCl | 93.44 | 74.8 | 98.3 |
| 6 | [Bmim]Cl−AlCl3−AgCl | 92.07 | 76.5 | 98.9 |
| 7 | [Bmim]Cl−AlCl3−ZnCl2 | 92.61 | 71.6 | 97.5 |
| 8 | benzene−CuAlCl4 | 91.08 | 68.7 | 97.0 |
| 9 | ether−CuClAl4 | 91.95 | 69.1 | 97.2 |
| 10 | CH3COOH | 55.68 | none | - |
| 11 | CF3SO3H | 126.21 | 28.2 | 84.9 |
| 12 | [(C2H5)3NH]Cl−CuCl | 84.37 | none | - |
| 13 | [(C2H5)3NH]Cl−ZnCl2 | 85.36 | none | - |
| 14 | CF3COOH | 115.14 | 34.0 | 86.4 |
| 15 | CH3COOOH | 70.25 | none | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Liu, Y.; Wang, J. Mixed Chlorometallate Ionic Liquids as C4 Alkylation Catalysts: A Quantitative Study of Acceptor Properties. Catalysts 2018, 8, 498. https://doi.org/10.3390/catal8110498
Pang X, Liu Y, Wang J. Mixed Chlorometallate Ionic Liquids as C4 Alkylation Catalysts: A Quantitative Study of Acceptor Properties. Catalysts. 2018; 8(11):498. https://doi.org/10.3390/catal8110498
Chicago/Turabian StylePang, Xiaoying, Ying Liu, and Juanfang Wang. 2018. "Mixed Chlorometallate Ionic Liquids as C4 Alkylation Catalysts: A Quantitative Study of Acceptor Properties" Catalysts 8, no. 11: 498. https://doi.org/10.3390/catal8110498





