Combined Cross-Linked Enzyme Aggregates as Biocatalysts
Abstract
:1. Introduction
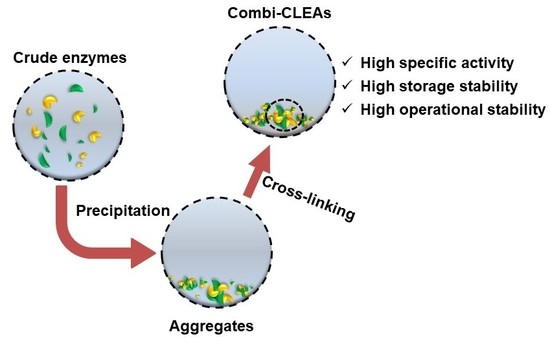
2. Combi-CLEA
2.1. Advantages of CLEAs and Combi-CLEAs
2.2. Factors Influencing CLEAs and Combi-CLEAs Preparation
2.2.1. Proportion of Enzymes
2.2.2. The Precipitants
2.2.3. The Cross-Linker
2.2.4. Effect of Temperature on the Cross-Linking
3. Applications of Combined CLEAs
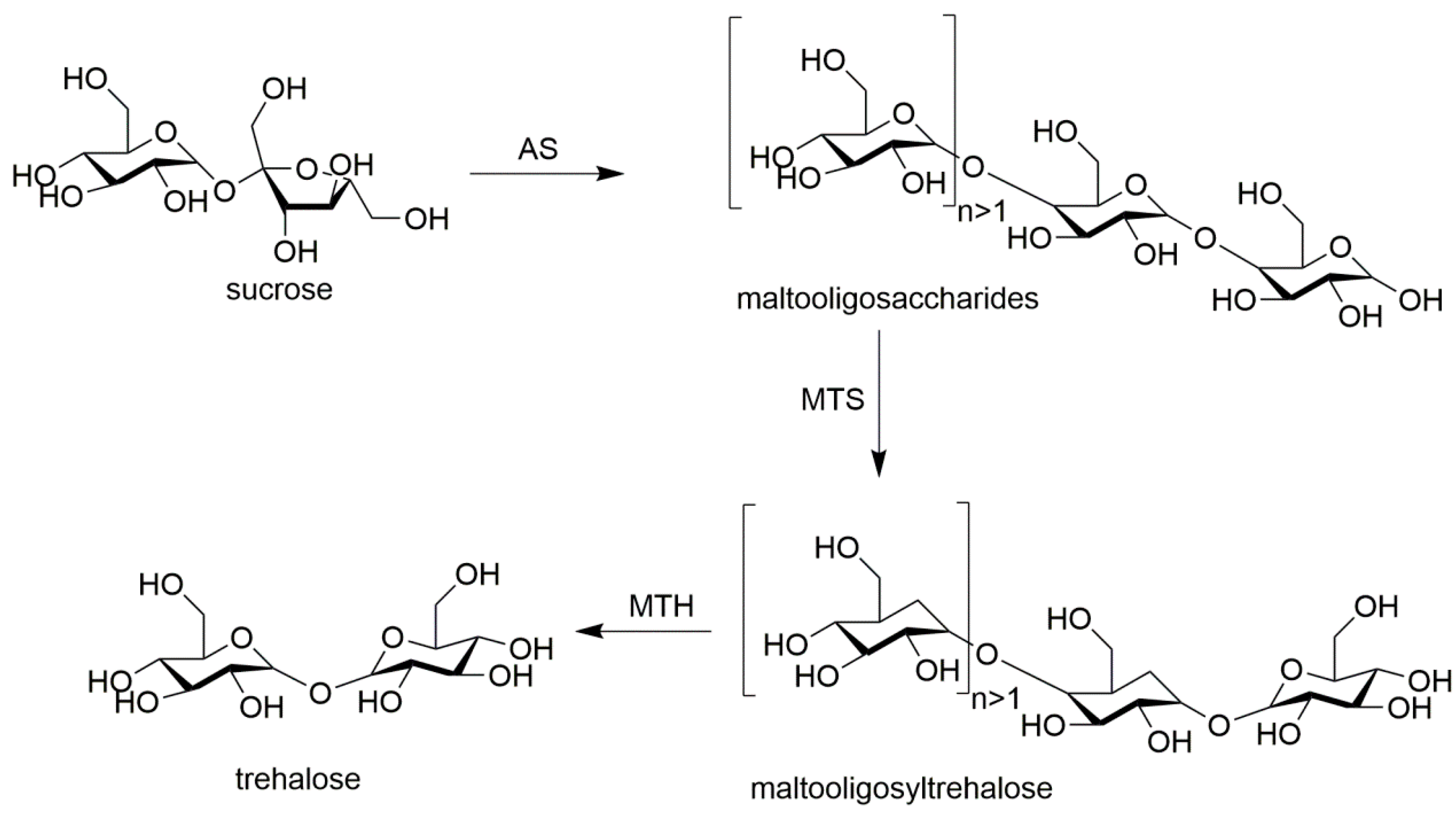
3.1. Amylosucrase, Maltooligosyltrehalose Synthase, and Maltooligosyltrehalose
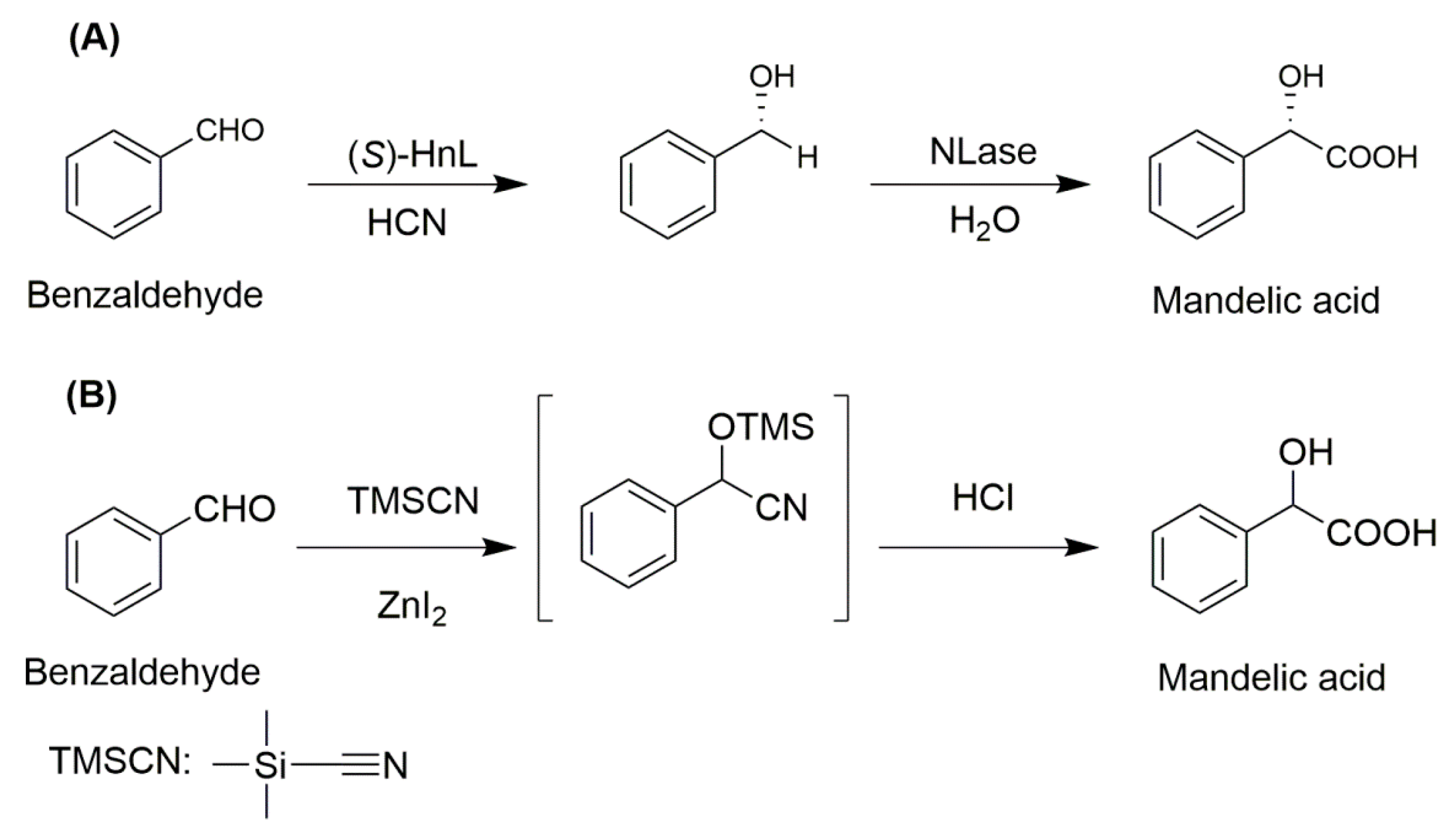
3.2. Hydroxynitrile Lyase and Nitrilase
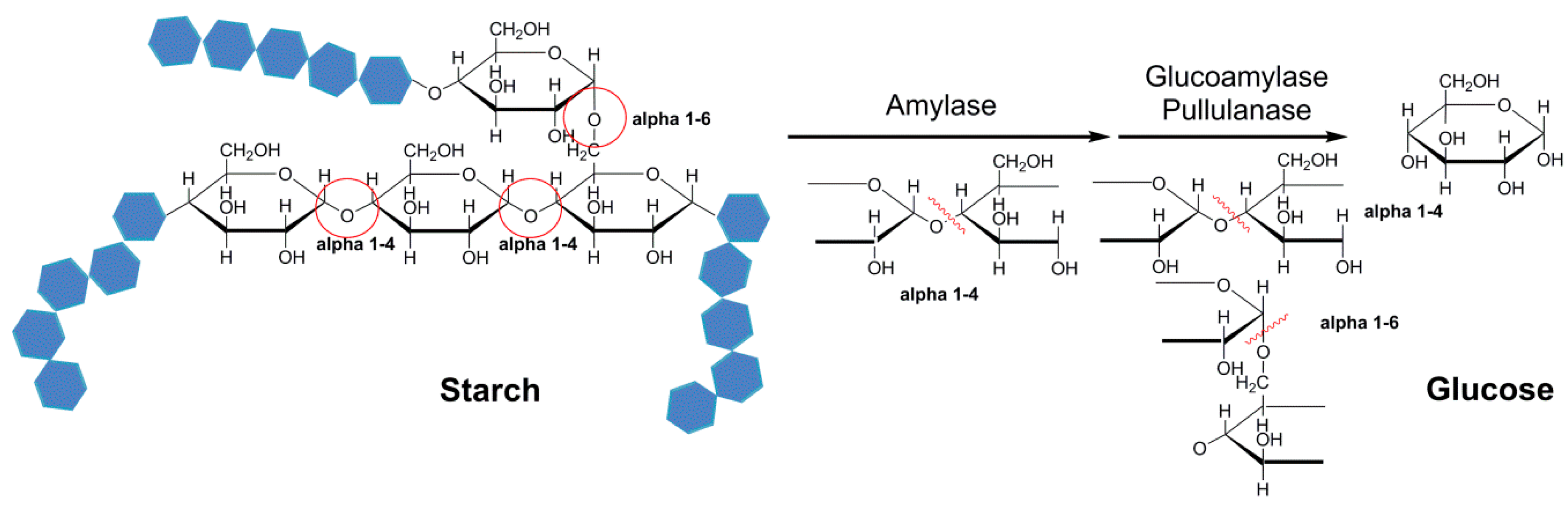
3.3. Amylase, Glucoamylase, and Pullulanase
3.4. l-Arabinosidase and d-Glycosidase
3.5. Aminopeptidase N and X-Prolyl-Dipeptidyl Aminopeptidase
3.6. Lipase and Protease
3.7. Eductase and Glucose Dehydrogenase
3.8. Peroxidase and Glucose Oxidase
3.9. Glucose Oxidase and Horseradish Peroxidase
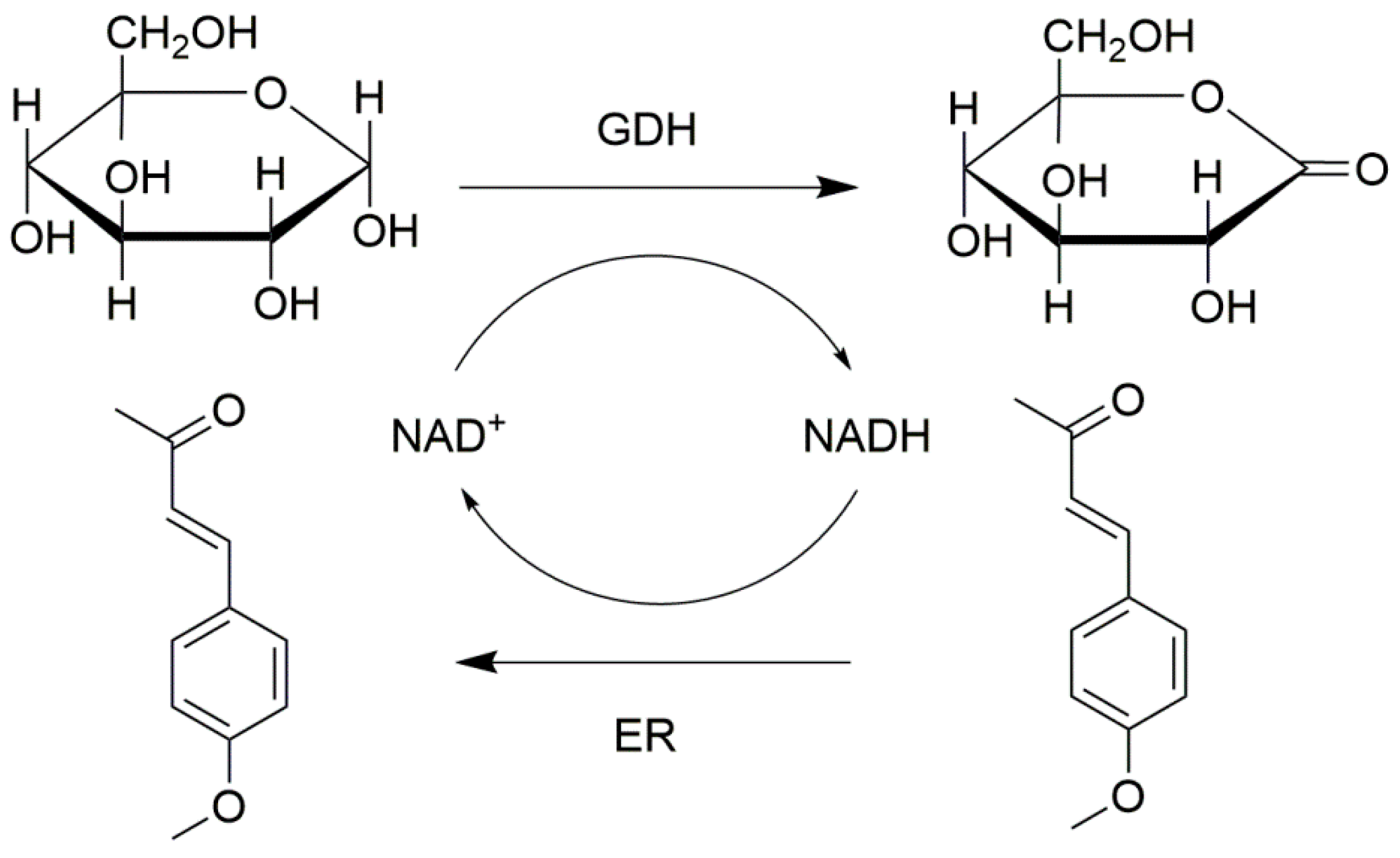
3.10. Alcohol Dehydrogenase and Glucose Dehydrogenase
3.11. Ketoreductase and Glucose Dehydrogenase
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wen, Y.; Xu, L.; Chen, F.; Gao, J.; Li, J.; Hu, L.; Li, J. Discovery of a novel inhibitor of NAD(P)+-dependent malic enzyme (ME2) by high-throughput screening. Acta Pharmacol. Sin. 2014, 35, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhao, T.; Zhou, Y.; Li, F.; Zou, Y.; Bai, S.; Wang, W.; Yang, L.; Wu, X. Optimization of enzyme-assisted extraction and characterization of collagen from Chinese sturgeon (Acipenser sturio Linnaeus) skin. Pharmacogn. Mag. 2013, 9, 32–37. [Google Scholar] [CrossRef]
- Yang, H.; Shen, Y.; Xu, Y.; Maqueda, A.S.; Zheng, J.; Wu, Q.; Tam, J.P. A novel strategy for the discrimination of gelatinous Chinese medicines based on enzymatic digestion followed by nano-flow liquid chromatography in tandem with orbitrap mass spectrum detection. Int. J. Nanomed. 2015, 10, 4947–4955. [Google Scholar] [CrossRef] [PubMed]
- Magne, V.; Amounas, M.; Innocent, C.; Dejean, E.; Seta, P. Enzyme textile for removal of urea with coupling process: Enzymatic reaction and electrodialysis. Desalination 2018, 144, 163–166. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Deng, Y.; Zhang, Y.; Xiao, J.; Huang, F.; Wei, W.; Zhang, M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with α-amylase. Food Chem. 2017, 221, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Agera, R.; Sharma, P.; Sunder, A.V.; Bajwa, H.; James, H.M.; Gaikaiwari, R.P.; Wangikar, P.P. Development of biotransformation process for asymmetric reduction with novel anti-Prelog NADH-dependent alcohol dehydrogenases. Process Biochem. 2018. [Google Scholar] [CrossRef]
- Zhang, L.; Singh, R.; Sivakumar, D.; Guo, Z.; Li, J.; Chen, F.; He, Y.; Guan, X.; Kang, Y.C.; Lee, J.K. An artificial synthetic pathway for acetoin, 2,3-butanediol, and 2-butanol production from ethanol using cell free multi-enzyme catalysis. Green Chem. 2017, 221, 636–643. [Google Scholar] [CrossRef]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.-P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of estrogens by laccase from Myceliophthora thermophila in fed-batch and enzymatic membrane reactors. J. Hazard. Mater. 2012, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 39, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.Y.; Jiang, X.P.; Ling, X.M.; Xu, M.Q.; Zhu, Y.H.; Zhang, Y.W. Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J. Chem. Technol. Biotechnol. 2018, 93, 2351–2358. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, W.; Tao, Q.L.; Jiang, X.P.; Liu, C.H.; Zeng, S.; Zhang, Y.W. Immobilization of Lipase by Adsorption Onto Magnetic Nanoparticles in Organic Solvents. J. Nanosci. Nanotechnol. 2016, 16, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Pletschke, B.I. Strategic optimization of xylanase–mannanase combi-CLEAs for synergistic and efficient hydrolysis of complex lignocellulosic substrates. J. Mol. Catal. B Enzym. 2015, 115, 140–150. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Guzik, U.; Hupertkocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Zhang, D.; Jiang, S.; Shi, K.; Huang, Y.; Li, R.; Xu, Q. Methazolamide-loaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: Vitro and vivo study. J. Drug Target. 2014, 22, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, L.; Zhou, L.; Zhang, Z.; Cao, W.; Wu, Q. Effect of cell-penetrating peptide-coated nanostructured lipid carriers on the oral absorption of tripterine. Int. J. Nanomed. 2012, 7, 4581–4591. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Qi, W.; Su, R.; He, Z. Advances in carrier-bound and carrier-free immobilized nanobiocatalysts. Chem. Eng. Sci. 2015, 135, 21–32. [Google Scholar] [CrossRef]
- Xie, M.; Xu, Y.; Shen, H.; Shen, S.; Ge, Y.; Xie, J. Negative-charge-functionalized mesoporous silica nanoparticles as drug vehicles targeting hepatocellular carcinoma. Int. J. Pharm. 2014, 474, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, S.; Cao, J.; Ji, L.; He, Y.; Xi, J. Preparation and evaluation of a novel bioactive glass/lysozyme/PLGA composite microsphere. Drug Dev. Ind. Pharm. 2015, 41, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, Y.; Firempong, C.K.; Xu, X. Preparation, characterization and pharmacokinetic studies of linalool-loaded nanostructured lipid carriers. Pharm. Biol. 2016, 54, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.-J.; Chen, X.-L.; Du, L.; Li, F. Quercetin-loaded freeze-dried nanomicelles: Improving absorption and anti-glioma efficiency in vitro and in vivo. J. Control. Release 2016, 235, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Tao, Z.; Jia, L.; Luo, Y.-F.; Xu, J.; Chen, R.-H.; Ge, Z.-J.; Ma, T.-L.; Chen, H. Multifunctional nanocomposite based on halloysite nanotubes for efficient luminescent bioimaging and magnetic resonance imaging. Int. J. Nanomed. 2016, 11, 4765–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, M.; Xin, Y.; Li, R.; Ge, Y.; Feng, C.; Xu, X. Targeted Biomimetic Nanoparticles for Synergistic Combination Chemotherapy of Paclitaxel and Doxorubicin. Mol. Pharm. 2017, 14, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Firempong, C.K.; Wang, Y.; Xu, W.; Wang, M.; Cao, X.; Zhu, Y.; Tong, S.; Yu, J.; Xu, X. Ergosterol-loaded poly(lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta Pharmacol. Sin. 2017, 37, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Ngai, S.-C.; Goh, B.-H.; Chan, K.-G.; Lee, L.-H.; Chuah, L.-H. Anticancer Activities of Surfactin and Potential Application of Nanotechnology Assisted Surfactin Delivery. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Deng, W.W.; Fu, M.; Wang, L.; Tong, S.S.; Wei, Y.W.; Xu, Y.; Su, W.Y.; Xu, X.M.; Yu, J.N. In vitro release and in vitro–in vivo correlation for silybin meglumine incorporated into hollow-type mesoporous silica nanoparticles. Int. J. Nanomed. 2012, 753. [Google Scholar] [CrossRef]
- Peng, W.; Jiang, X.; Zhu, Y.; Omari-Siaw, E.; Deng, W.; Yu, J.; Xu, X.; Zhang, W. Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharmacol. Sin. 2015, 36, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, L.; Liu, J.; Xie, M.; Shen, H.; Qi, X.; Yan, Y.; Ge, Y.; Jin, Y. Core–shell structured Fe3O4@TiO2-doxorubicin nanoparticles for targeted chemo-sonodynamic therapy of cancer. Int. J. Pharm. 2015, 486, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, F.; Zhang, X.-Y.; Li, Y.; Wang, X.-Y.; Xu, X.-M.; Zhang, Y.-W. Preparation of Magnetic Fe3O4@SiO2 Nanoparticles for Immobilization of Lipase. J. Nanosci. Nanotechnol. 2014, 14, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.-Y.; Jiang, X.-P.; Ye, J.-J.; Zhang, Y.-W.; Zhang, X.-Y. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulase. J. Nanopart. Res. 2015, 17, 8. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jiang, X.-P.; Li, Y.; Zeng, S.; Zhang, Y.-W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.-M.; Wang, X.-Y.; Ma, P.; Yang, Y.; Qin, J.-M.; Zhang, X.-J.; Zhang, Y.-W. Covalent Immobilization of Penicillin G Acylase onto Fe3O4@Chitosan Magnetic Nanoparticles. J. Microbiol. Biotechnol. 2016, 26, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.-L.; Li, Y.; Shi, Y.; Liu, R.-J.; Zhang, Y.-W.; Guo, J. Application of Molecular Imprinted Magnetic Fe3O4@SiO2 Nanoparticles for Selective Immobilization of Cellulase. J. Nanosci. Nanotechnol. 2016, 16, 6055–6060. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Li, X.Q.; Jiang, X.P.; Zhuang, M.Y.; Zhang, J.X.; Bao, C.H.; Zhang, Y.W. Preparation of Functionalized Graphene Oxide Nanocomposites for Covalent Immobilization of NADH Oxidase. Nanosci. Nanotechnol. Lett. 2016, 8, 164–167. [Google Scholar] [CrossRef]
- Gao, J.; Lu, C.-L.; Wang, Y.; Wang, S.-S.; Shen, J.-J.; Zhang, J.-X.; Zhang, Y.-W. Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer. Catalysts 2018, 8, 180. [Google Scholar] [CrossRef]
- Jiao, Z.; Chen, Y.; Wan, Y.; Zhang, H. Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int. J. Nanomed. 2011, 6, 2321–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, F.; Hu, K.; Yu, H.; Zhou, L.; Song, L.; Zhang, Y.; Shan, X.; Liu, J.; Gu, N. A Functional Iron Oxide Nanoparticles Modified with PLA-PEG-DG as Tumor-Targeted MRI Contrast Agent. Pharm. Res. 2017, 34, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Geng, L.; Ge, L.; Yu, P.; Duan, X.; Chen, J.; Chang, Y. Effect of iron liposomes on anemia of inflammation. Int. J. Pharm. 2013, 454, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Lou, J.; Jiang, R.; Fang, Z.; Zhao, X.; Niu, Y.; Zou, S.; Zhang, M.; Gong, A.; Wu, C. Hyaluronic acid-functionalized bismuth oxide nanoparticles for computed tomography imaging-guided radiotherapy of tumor. Int. J. Nanomed. 2017, 12, 5973–5992. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Morsy, R.; Elzawawy, N.A.; Fareed, M.F.; Bedaiwy, M.Y. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): A novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed. 2017, 12, 6059–6073. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, L.; Congyan, L.; Zhang, Z.; Zhou, L.; Qu, D. Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int. J. Nanomed. 2013, 4339–4350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Jiang, S.; Liu, Z.; Gu, W.; Yu, H.; Li, Y. Porous starch based self-assembled nano-delivery system improves the oral absorption of lipophilic drug. Int. J. Pharm. 2013, 444, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Qu, Y.; Gao, T.; Ge, Y.; Feng, C.; Xu, X. Simultaneous delivery of anti-miR21 with doxorubicin prodrug by mimetic lipoprotein nanoparticles for synergistic effect against drug resistance in cancer cells. Int. J. Nanomed. 2017, 12, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, W.F.; Zhou, J.Y.; Xu, X.M.; Li, F.Q. Triggering effect of N-acetylglucosamine on retarded drug release from a lectin-anchored chitosan nanoparticles-in-microparticles system. Int. J. Pharm. 2013, 449, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lv, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Tischer, W.; Kasche, V. Immobilized enzymes: Crystals or carriers? Trends Biotechnol. 1999, 17, 326–335. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Emilia Abraham, T. Strategies in Making Cross-Linked Enzyme Crystals. Chem. Rev. 2004, 104, 3705–3722. [Google Scholar] [CrossRef] [PubMed]
- Quiocho, F.A.; Richards, F.M. Intermolecular cross linking of a protein in the crystallinestate: Carboxypeptidasw-A. Proc. Natl. Acad. Sci. USA 1964, 52, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Nayhouse, M.; Christofides, P.D.; Orkoulas, G. Protein Crystal Shape and Size Control in Batch Crystallization: Comparing Model Predictive Control with Conventional Operating Policies. Ind. Eng. Chem. Res. 2014, 53, 5002–5014. [Google Scholar] [CrossRef]
- Liu, J.J.; Ma, C.Y.; Hu, Y.D.; Wang, X.Z. Effect of seed loading and cooling rate on crystal size and shape distributions in protein crystallization—A study using morphological population balance simulation. Comput. Chem. Eng. 2010, 34, 1945–1952. [Google Scholar] [CrossRef]
- Velascolozano, S.; Lópezgallego, F.; Mateosdíaz, J.C.; Favelatorres, E. Cross-linked enzyme aggregates (CLEA) in enzymeimprovement—A review. Biocatalysis 2016, 1, 166–177. [Google Scholar] [CrossRef]
- Clair, N.L.S.; Navia, M.A. Cross-linked enzyme crystals as robust biocatalysts. J. Am. Chem. Soc. 1992, 114, 7314–7316. [Google Scholar]
- Zelinski, T.; Waldmann, H. Cross-Linked Enzyme Crystals(CLECs): Efficient and Stable Biocatalysts for Preparative Organic Chemistry. Angew. Chem. Int. Ed. Engl. 1997, 36, 722–724. [Google Scholar] [CrossRef]
- Roessl, U.; Nahálka, J.; Nidetzky, B. Carrier-free immobilized enzymes for biocatalysis. Biotechnol. Lett. 2010, 32, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.L.; Chen, D.W.; Peng, C.; Yan, Y.J. Conformation and Activity of Sol-Gels Encapsulated Cross-Linked Enzyme Aggregates of Lipase from Burkholderia cepacia. Adv. Mater. Res. 2011, 291–294, 614–620. [Google Scholar] [CrossRef]
- Goetze, D.; Foletto, E.F.; da Silva, H.B.; Silveira, V.C.C.; Dal Magro, L.; Rodrigues, R.C. Effect of feather meal as proteic feeder on combi-CLEAs preparation for grape juice clarification. Process Biochem. 2017, 62, 122–127. [Google Scholar] [CrossRef]
- Min, H.K.; Park, S.; Yong, H.K.; Won, K.; Sang, H.L. Immobilization of formate dehydrogenase from Candida boidinii through cross-linked enzyme aggregates. J. Mol. Catal. B Enzym. 2013, 97, 209–214. [Google Scholar] [CrossRef]
- Matijošytė, I.; Arends, I.W.C.E.; Vries, S.D.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Sangeetha, K.; Abraham, T.E. Preparation and characterization of cross-linked enzyme aggregates (CLEA) of Subtilisin for controlled release applications. Int. J. Biol. Macromol. 2008, 43, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Cerdobbel, A.; De, W.K.; Desmet, T.; Soetaert, W. Sucrose phosphorylase as cross-linked enzyme aggregate: Improved thermal stability for industrial applications. Biotechnol. J. 2010, 5, 1192–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.Y.; Li, X.F.; Lou, W.Y.; Zong, M.H. Cross-linked enzyme aggregates of Mung bean epoxide hydrolases: A highly active, stable and recyclable biocatalyst for asymmetric hydrolysis of epoxides. J. Biotechnol. 2013, 166, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, E.; Grill, B.; Raghoebar, Z.; Pelt, S.V.; Sheldon, R.; Steiner, K.; Glieder, A.; Winkler, M. Production of hydroxynitrile lyase from D. tyermanii (DtHNL) in Komagataella phaffii and its immobilization as CLEA to generate a robust biocatalyst. Chembiochem 2017, 19, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; Giordano, R.D.L.C.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Subramanian, S. Carrier-free co-immobilization of xylanase, cellulase and β-1,3-glucanase as combined cross-linked enzyme aggregates (combi-CLEAs) for one-pot saccharification of sugarcane bagasse. RSC Adv. 2016, 6, 32849–32857. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Q.; Sun, L.; Li, Q.; Zhou, L.; Chen, Q.; Li, S.; Yu, M.; Li, W. Magnetic Combined Cross-Linked Enzyme Aggregates of Ketoreductase and Alcohol Dehydrogenase: An Efficient and Stable Biocatalyst for Asymmetric Synthesis of (R)-3-Quinuclidinol with Regeneration of Coenzymes In Situ. Catalysts 2018, 8, 334. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Junghanns, C.; Demarche, P.; Moreira, M.T.; Feijoo, G.; Lema, J.M.; Agathos, S.N. Combined cross-linked enzyme aggregates from versatile peroxidase and glucose oxidase: Production, partial characterization and application for the elimination of endocrine disruptors. Bioresour. Technol. 2011, 102, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-H.; Jung, J.-H.; Seo, D.-H.; Ha, S.-J.; Kweon, D.-K.; Park, C.-S. One-pot bioconversion of sucrose to trehalose using enzymatic sequential reactions in combined cross-linked enzyme aggregates. Bioresour. Technol. 2013, 130, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Jian, D.C.; Shi, R.J. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates. Environ. Sci. Pollut. Res. 2017, 24, 7035–7041. [Google Scholar] [CrossRef] [PubMed]
- Tandjaoui, N.; Tassist, A.; Abouseoud, M.; Couvert, A.; Amrane, A. Preparation and characterization of cross-linked enzyme aggregates (CLEAs) of Brassica rapa peroxidase. Biocatal. Agric. Biotechnol. 2015, 4, 208–213. [Google Scholar] [CrossRef]
- Dalal, S.; Kapoor, M.; Gupta, M.N. Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J. Mol. Catal. B Enzym. 2007, 44, 128–132. [Google Scholar] [CrossRef]
- Andreazza, R.; Pieniz, S.; Okeke, B.; Camargo, F.A. Evaluation of copper resistant bacteria from vineyard soils and mining waste for copper biosorption. Braz. J. Microbiol. 2011, 42, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pchelintsev, N.A.; Youshko, M.I.; Švedas, V.K. Quantitative characteristic of the catalytic properties and microstructure of cross-linked enzyme aggregates of penicillin acylase. J. Mol. Catal. B Enzym. 2009, 56, 202–207. [Google Scholar] [CrossRef]
- Richards, F.M.; Knowles, J.R. Glutaraldehyde as a protein cross-linkage reagent. J. Mol. Biol. 1968, 37, 231–233. [Google Scholar] [CrossRef]
- Dartiguenave, M.C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Wine, Y.; Cohenhadar, N.; Freeman, A.; Frolow, F. Elucidation of the mechanism and end products of glutaraldehyde crosslinking reaction by X-ray structure analysis. Biotechnol. Bioeng. 2010, 98, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Van Langen, L.M.; Van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, F.; Chen, F.; Wang, M.; Li, H.; Zeng, Z.; Xie, T.; Chen, Z. A facile technique to prepare cross-linked enzyme aggregates using p-benzoquinone as cross-linking agent. Korean J. Chem. Eng. 2011, 28, 1090–1095. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Cabana, H.; Ahamed, A.; Leduc, R. Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour. Technol. 2011, 102, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Kunjukunju, S.; Roy, A.; Shekhar, S.; Kumta, P.N. Cross-linked enzyme aggregates of alginate lyase: A systematic engineered approach to controlled degradation of alginate hydrogel. Int. J. Biol. Macromol. 2018, 115, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Mehde, A.A.; Mehdi, W.A.; Özacar, M.; Özacar, Z.Z. Evaluation of different saccharides and chitin as eco-friendly additive to improve the magnetic cross-linked enzyme aggregates (CLEAs) activities. Int. J. Biol. Macromol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, L.; Chen, D.; Wang, Y.; Zhang, J.; Shao, L. Co-expression of the recombined alcohol dehydrogenase and glucose dehydrogenase and cross-linked enzyme aggregates stabilization. Bioresour. Technol. 2016, 224, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Torabizadeh, H.; Tavakoli, M.; Safari, M. Immobilization of thermostable α-amylase from Bacillus licheniformis by cross-linked enzyme aggregates method using calcium and sodium ions as additives. J. Mol. Catal. B Enzym. 2014, 108, 13–20. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. ChemInform Abstract: Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. Cheminform 2013, 4, 1583–1600. [Google Scholar] [CrossRef]
- Reshmi, R.; Sugunan, S. Improved biochemical characteristics of crosslinked β-glucosidase on nanoporous silica foams. J. Mol. Catal. B Enzym. 2013, 85, 111–118. [Google Scholar] [CrossRef]
- Šulek, F.; Fernández, D.P.; Knez, Ž; Habulin, M.; Sheldon, R.A. Immobilization of horseradish peroxidase as crosslinked enzyme aggregates (CLEAs). Process Biochem. 2011, 46, 765–769. [Google Scholar] [CrossRef]
- Liao, Q.; Du, X.; Jiang, W.; Tong, Y.; Zhao, Z.; Fang, R.; Feng, J.; Tang, L. Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1: Preparation, characterization and application as a biocatalyst. J. Biotechnol. 2018, 272–273, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Kim, J.; Lee, J.; Jia, H.; Na, H.B.; Youn, J.K.; Kwak, J.H.; Dohnalkova, A.; Grate, J.W.; Wang, P. Crosslinked enzyme aggregates in hierarchically-ordered mesoporous silica: A simple and effective method for enzyme stabilization. Biotechnol. Bioeng. 2007, 96, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Paradiso, M.; Wallacher, D.; Brandt, A.; Hartmann, M. Formation of Cross-Linked Chloroperoxidase Aggregates in the Pores of Mesocellular Foams: Characterization by SANS and Catalytic Properties. Chemsuschem 2010, 2, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Rajendhran, J.; Gunasekaran, P. Application of cross-linked enzyme aggregates of Bacillus badius penicillin G acylase for the production of 6-aminopenicillanic acid. Lett. Appl. Microbiol. 2007, 44, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, A.; Cabana, H.; Jones, J.P. Laccase-Based CLEAs: Chitosan as a Novel Cross-Linking Agent. Enzyme Res. 2011, 2011, 376015. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Wang, B.; Tan, J.; Zhu, L.; Li, L. Immobilized multienzymatic systems for catalysis of cascade reactions. Process Biochem. 2016, 51, 1193–1203. [Google Scholar] [CrossRef]
- Mateo, C.; Chmura, A.; Rustler, S.; Rantwijk, F.V.; Stolz, A.; Sheldon, R.A. Synthesis of enantiomerically pure (S)-mandelic acid using an oxynitrilase–nitrilase bienzymatic cascade: A nitrilase surprisingly shows nitrile hydratase activity. Tetrahedron Asymmetry 2006, 17, 320–323. [Google Scholar] [CrossRef]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- Stressler, T.; Ewert, J.; Eisele, T.; Fischer, L. Cross-linked enzyme aggregates (CLEAs) of PepX and PepN–production, partial characterization and application of combi-CLEAs for milk protein hydrolysis. Biocatal. Agric. Biotechnol. 2015, 4, 752–760. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Yusof, F.; Shah, H.; Jami, M.S.; Khanahmadi, S. Development of an immobilized biocatalyst with lipase and protease activities as a multipurpose cross-linked enzyme aggregate (multi-CLEA). Process Biochem. 2015, 50, 2144–2157. [Google Scholar] [CrossRef]
- Li, H.; Xiao, W.; Xie, P.; Zheng, L. Co-immobilization of enoate reductase with a cofactor-recycling partner enzyme. Enzyme Microb. Technol. 2018, 109, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Yang, K.L. Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions. Enzyme Microb. Technol. 2017, 100, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Su, E.; Tian, Y.; Wei, D. Combined cross-linked enzyme aggregates (combi-CLEAs) for efficient integration of a ketoreductase and a cofactor regeneration system. J. Biotechnol. 2014, 184, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Sirimanne, S.R.; Patterson, D.G., Jr. A one-pot synthesis of (±)-(ring 13C6)-mandelic acid. J. Label. Compd. Radiopharm. 1993, 33, 725–731. [Google Scholar] [CrossRef]
- Gao, J.; Wang, A.R.; Jiang, X.P.; Zhang, J.X.; Zhang, Y.W. Preparation of Expoxy-Functionalized Magnetic Nanoparticles for Immobilization of Glycerol Dehydrogenase. J. Nanosci. Nanotechnol. 2018, 4852–4857. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, K.; Martínez-Gil, A.; Moreno-Simunovic, Y.; Illanes, A.; Wilson, L. Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates. Molecules 2016, 21, 1485. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Zhang, Y.W.; Nguyen, N.P.; Jeya, M.; Lee, J.K. Covalent immobilization of β-1,4-glucosidase from Agaricus arvensis onto functionalized silicon oxide nanoparticles. Appl. Microbiol. Biotechnol. 2010, 89, 337–344. [Google Scholar] [CrossRef] [PubMed]






| Enzymes | Factors | References | |||
|---|---|---|---|---|---|
| Enzymes Proportion | Precipitants | Cross-Linker | Cross-Linking Temperature | ||
| Glucose oxidase and versatile peroxidase | 10:7 (mass) | Polyethylene glycol | Glutaraldehyde | 30 °C | [71] |
| Amylosucrase, maltooligosyltrehalose synthase and maltooligosyltrehalose trehalohydrolase | 16:1:1 (mass) | Acetone | Glutaraldehyde | 4 °C | [72] |
| Xylanase and mannanase | 1:1 (mass) | Acetone | Glutaraldehyde | 37 °C | [14] |
| Lipase, α-amylase and phospholipase A2 | NA | Dimethoxyethane | Glutaraldehyde | 4 °C | [76] |
| Amylase, glucoamylase and pullulanase | 3:3:1 (activity) | Ammonium sulfate | Glutaraldehyde | 35 °C | [101] |
| α-l-arabinosidase and β-d-glucosidase | NA | Ammonium sulfate | Glutaraldehyde | 4 °C | [102] |
| X-prolyl-dipeptidyl aminopeptidase and general aminopeptidase N | 1:1 (mass) | Ammonium sulfate | Glutaraldehyde | Ice | [103] |
| Lipase and protease | NA | Ammonium sulfate | Glutaraldehyde | 4 °C | [104] |
| Eductases and glucose dehydrogenase | 1.1:5 (activity) | Ammonium sulfate | Oxidized dextran | 4 °C | [105] |
| Glucose oxidase and horseradish peroxidase | 150:1 (mass) | Acetonitrile | Glutaraldehyde | NA | [106] |
| Ketoreductase and D-glucose dehydrogenase | 1:1(mass) | 1,2-Dimethoxyethane | Glutaraldehyde | 20 °C | [107] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.-Q.; Wang, S.-S.; Li, L.-N.; Gao, J.; Zhang, Y.-W. Combined Cross-Linked Enzyme Aggregates as Biocatalysts. Catalysts 2018, 8, 460. https://doi.org/10.3390/catal8100460
Xu M-Q, Wang S-S, Li L-N, Gao J, Zhang Y-W. Combined Cross-Linked Enzyme Aggregates as Biocatalysts. Catalysts. 2018; 8(10):460. https://doi.org/10.3390/catal8100460
Chicago/Turabian StyleXu, Meng-Qiu, Shuang-Shuang Wang, Li-Na Li, Jian Gao, and Ye-Wang Zhang. 2018. "Combined Cross-Linked Enzyme Aggregates as Biocatalysts" Catalysts 8, no. 10: 460. https://doi.org/10.3390/catal8100460